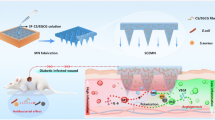
Abstract
With increased diabetes incidence, diabetic wound healing is one of the most common diabetes complications and is characterized by easy infection, chronic inflammation, and reduced vascularization. To address these issues, biomaterials with multifunctional antibacterial, immunomodulatory, and angiogenic properties must be developed to improve overall diabetic wound healing for patients. In our study, we prepared porous poly (L-lactic acid) (PLA) nanofiber membranes using electrospinning and solvent evaporation methods. Then, sulfated chitosan (SCS) combined with polydopamine-gentamicin (PDA-GS) was stepwise modified onto porous PLA nanofiber membrane surfaces. Controlled GS release was facilitated via dopamine self-polymerization to prevent early stage infection. PDA was also applied to PLA nanofiber membranes to suppress inflammation. In vitro cell tests results showed that PLA/SCS/PDA-GS nanofiber membranes immuomodulated macrophage toward the M2 phenotype and increased endogenous vascular endothelial growth factor secretion to induce vascularization. Moreover, SCS-contained PLA nanofiber membranes also showed good potential in enhancing macrophage trans-differentiation to fibroblasts, thereby improving wound healing processes. Furthermore, our in vitro antibacterial studies against Staphylococcus aureus indicated the effective antibacterial properties of the PLA/SCS/PDA-GS nanofiber membranes. In summary, our novel porous PLA/SCS/PDA-GS nanofiber membranes possessing enhanced antibacterial, anti-inflammatory, and angiogenic properties demonstrate promising potential in diabetic wound healing processes.
Similar content being viewed by others
Background
According to 2019 International Diabetes Federation data, an estimated 463 million adults had diabetes, and the numbers are expected to increase to over 700 million by 2045 [1.
Preparation and characterization of PLA nanofiber membranes
PLA was dissolved in DCM/DMF mixtures (volume ratios of 9/1, 8/2, and 7/3) at 10 wt% and the solution transferred to a 5 mL syringe for electrospinning. The parameters were as follows: flow rate = 1 mL/h, collecting distance = 20 cm, and voltage = 20 kV.
Porous PLA fiber membrane morphologies were observed using scanning electron microscopy (SEM, Prox, Phenom) at 10 kV accelerating voltage. Samples were sputter-coated with gold for 40 s. Fiber diameters and distributions were measured using SEM images by ImageJ (win64, National Institutes of Health, USA), and more than 100 fibers were randomly were analyzed per sample.
Preparation and characterization of PLA-based nanofiber membranes
To prepare PLA/SCS nanofiber membranes, PLA nanofiber membranes were immersed in SCS solution (10 mg/mL) for 1 h and dried overnight in a fume hood.
To prepare PLA/SCS/PDA nanofiber membranes, PLA/SCS nanofiber membranes were immersed in Tris-buffer (pH 8), dopamine hydrochloride (10 mg/mL) was then added into the solution. After that, the mixed solutions were reacted overnight with stirring.
To prepare PLA/SCS/PDA-GS nanofiber membranes, the prepared PLA/SCS nanofiber membranes were mixed with the Tris-buffer, dopamine hydrochloride and GS were added into the solution and reacted overnight with stirring.
Wettability tests
Porous PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes with a dimension of 2 × 2 mm were subjected to the contact angle test. The wettability of the prepared samples was determined using a water contact angle instrument (OCA25, Dataphysics, Germany). Three parallel tests were measured on each nanofiber membrane groups.
Swelling tests
Equilibrated swelling ratios (ESRs) of porous PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were measured in phosphate buffered saline (PBS) solution. The nanofiber membranes were first weighed (dry weight (M0)) and then transferred into PBS at 37 ℃. The nanofiber membranes were removed carefully at different time points and weighed after removing the excess solution (MW) until the swelling equilibrium was reached.
ESRs were calculated as follows:
where M0 and Mw = membrane weights under dry and swollen conditions, respectively. Three repeated measurements were performed.
In vitro GS release
PLA/SCS/PDA-GS nanofiber membranes were used in the in vitro GS release study. The prepared nanofiber membranes were immersed in Dulbecco's phosphate-buffered saline (DPBS) medium at 37 ℃ and agitated at 90 rpm. At different time points, half the supernatant was collected and the same volume of fresh DPBS was added. After 7 days of release, the released GS amounts were determined using UV-Vis spectrophotometry (ZF-20D, Shanghai, China), as described elsewhere [35].
In vitro cell culture
Mouse macrophages cell line (Raw 264.7) and human umbilical vein endothelial cells (HUVECs) were purchased from the Procell Life Science&Technology Company (Wuhan, China). Raw 246.7 cells and HUVECs were cultured with DMEM high-glucose. All medium supplemented with 10% FBS and 1% penicillin/streptomycin (P/S, Beyotime). Moreover, all cells were cultured in a humid incubator at 37 °C and a CO2 level of 5%.
In vitro cytotoxicity assays
The morphologies of Raw264.7 cells on porous PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were determined using the Calcein-AM and propidium iodide (PI) stains, which labeled live and dead cells, respectively. At each incubation time point, the culture medium was removed and the cells were washed with three times in DPBS. Calcein-AM and PI in DPBS were then added to the 24-well plates and incubated for 15 min at room temperature. Fluorescence images were recorded using a laser scanning microscope (DFC7000 GT DMi8, Leica, Germany).
Nanofiber membrane cytotoxicity was quantitatively analyzed using CCK8 assays (Do**do Molecular Technologies, Inc.) according to the manufacturer’s instruction. Briefly, porous PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were placed in 24-well plates, 5 × 104 Raw264.7 cells were then seeded onto the prepared nanofiber membranes. After 1 and 3 days of culture, the culture medium was removed, 10% CCK8/culture medium was added, the cells were incubated for 2 h, and the absorbance was measured at 450 nm on a microplate reader (Infinite M200, Tecan, USA). PLA nanofiber membranes acted as controls, and the cell viability was expressed as percentages relative to the control group.
Real-time polymerase chain reaction (RT-PCR)
To determine the influence of the PLA-based nanofiber membrane on macrophage angiogenic and immune responses, RT-PCR was performed. For PLA-based samples, Raw 264.7 cells (5 × 104) were seeded on PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes and cultured for 1 and 3 days. And gene expression data were acquired.
In vitro antibacterial culture
The antibacterial activities of porous PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were studied using the Gram-positive bacteria Staphylococcus aureus (S. aureus) (ATCC 6538). Same volume of Staphylococcus aureus-containing suspensions were spread on the agar surface for inoculation. Zones of inhibition (ZOI), shake-flask culture, and bacterial live/dead staining tests were carried out to determine the anti-adhesive and bactericidal functions of nanofiber membranes toward S. aureus.
ZOI assays
S. aureus suspension (1.0 × 106 CFU/mL) were uniformly spread onto nutrient agar, afterward, the prepared PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were placed on the Petri dishes and incubated at 37 ºC for 24 h. ZOIs were measured using a perpendicular caliper. Three parallel samples were measured to assess the antimicrobial activity of the nanofiber membrane.
Bacteria killing tests
Bacteria killing properties were studied by immersing the nanofiber memberanes into bacterial solutions in shake-flask tests. After immersing for 15 min, bacterial solutions had homogeneous coated the membranes, which were placed onto solid agar and incubated, after which bacterial numbers were counted. The survival rate was defined as the percentage of bacteria relative to the initial total number in the suspension.
Live/dead bacterial staining
S. aureus suspensions (1.0 × 105 CFU/mL) were seeded onto the PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes and co-cultured for 24 h. Samples were then stained using a live/dead BacLight bacterial viability kit (L-7012, Invitrogen) according to the manufacturer’s instructions. Bacterial morphology was observed by a fluorescence microscopy (Zeiss, Germany).
Statistical analysis
The One-way ANOVA with Tukey’s correction (compare among groups) and Student’s t-tests (between two groups) were used to calculate statistical significance. A value of p < 0.05 was considered to be statistically significant. All the data are presented as the mean ± standard deviation (SD).
Results and discussion
SCS synthesis and characterization
To analyze CS sulfation, FTIR and 1H NMR were measured (Fig. 1a, b). The peak around 3422 cm−1 was belonged to the overlap** of the stretching vibration of O–H and the stretching vibration of N–H groups of chitosan. The peak at 895 cm−1 was the stretching vibration peak of C-O. And the peak around 1600 cm−1 in chitosan was attributed to the bending vibration peak of protonated amino group. When compared with CS, the FTIR spectra of SCS showed the new bands at approximately 810 cm−1 and 1230 cm−1, which were attributed to C-O-S and O = S = O group stretching vibrations, respectively. Moreover, peaks at 3.3 ppm and 4.6 ppm in SCS 1H NMR spectra of SCS confirmed that the sulfonation had occurred positions in C2 and C6 of CS. These data suggest successful CS sulfation.
a CS and SCS FTIR spectra; b SCS 1H NMR spectrum; c HIF-1α and d VEGF gene expression in Raw.264.7 cells in SCS/culture medium for 24 h; relative gene expression of e, g TNF-α, IL-1β, IL-6 and f, h IL-4, IL-10, Arg-1 of Raw 264.7 cells in SCS/culture medium for 24 h and 48 h of culture, respectively
Angiogenesis is a complex and coordinated process involving multiple-factors [36, 37]. It remains a challenge to induce vascularization in engineered tissues by delivering just one growth factor (e.g., VEGF or PDGF-BB). As a key upstream transcription factor, HIF-1α plays important roles in bone tissue engineering via VEGF [38, 39] and stromal cell-derived factor 1 (SDF-1) generation to upregulate the VEGF-induced vascularization and SDF-1 induced progenitor cells recruitment [40, 41]. HIF-1α and VEGF gene expression levels in Raw 264.7 cells were significantly increased after 24 h of SCS treatment (Fig. 1c, d). The pro-angiogenic potential of SCS in human umbilical vein endothelial cells (HUVECs) was also evaluated using capillary tube formation assays (Fig. 2). The exogenous VEGF- (6 ng/mL) treated group was used as a positive control. Capillary-like networks were formed on VEGF- and SCS-treated HUVECs after 3 h of treatment, whereas few capillary tubes were observed in the control group (***p < 0.001). All branch points, loops per field, and total capillary tube length per field were increased in VEGF and SCS-treated groups in the first 6 h. However, at 9 h, capillary-like networks no longer increased in the VEGF treated group, but more capillary-like networks were formed in the SCS treated group. Thus, SCS alone promoted capillary tube formation without exogenous VEGF addition, with higher enhanced capabilities when compared with the VEGF-treated group. Moreover, SCS effects were was longer than VEGF effects, which could be attributed to the short half-life of VEGF. Therefore, SCS alone promoted angiogenesis in HUVECs and induced pro-angiogenic factor expression in macrophages without exogenous VEGF addition. When compared with VEGF, SCS was easily available, cheap, had low toxicity, and was stable. In the future, SCS may be used as a VEGF substitute in clinical settings.
Representative bright light images showing the capillary networks after 3 h, 6 h, 9 h of (a, e, i) Control, (b, f, j) VEGF and (c, g, k) SCS treatment, respectively; quantitative analysis of sprouted HUVECs on (d) number of branch points per field, (h) the number of loops per field and (l) total capillary tube length per field
To study the influence of SCS on the macrophage polarization, pro-inflammatory and anti-inflammatory gene expression was investigated using RT-PCR. After 1 day of SCS treatment, M1 phenotype-related genes (IL-1β and IL-6) were decreased, whereas the M2 phenotype-related gene IL-4 was increased. Interestingly, after SCS incubation for 3 days, both M1 (TNF-α, IL-1β, and IL-6) and M2 phenotype-related genes (IL-4, IL-10, and Arg-1) were dramatically increased. As Shen et al. reported, some macrophages participate in proliferation processes during diabetic wound healing, and M1/M2 double-positive macrophages can benefit macrophage trans-differentiation to fibroblasts [21]. Therefore, SCS appears to regulate macrophages toward an anti-inflammatory phenotype and potentially promote macrophage trans-differentiation into fibroblasts.
Synthesis and characterization of PLA-based nanofiber membranes
Our preliminary data showed that the mixture solvent ratios play the leading role in PLA nanofiber porous structures of PLA nanofibers when compared with the PLA concentrations, electric field intensity, and liquid flow rates (data not shown). Hence, the effects of solvent ratios on porous PLA nanofiber morphologies and corresponding diameter distribution were investigated. In the present work, a mixture of solvents with ratios of 7:3, 8:2 and 9:1 was performed. The SEM images showed that when DCM: DMF ratio varied from 7:3 to 9:1, PLA nanofiber porous structures were formed and nanofiber diameters increased stepwise (Fig. 3). Moreover, PLA nanofiber diameters were more uniform in the DCM: DMF 8:2 ratio when compared with 9:1 ratio. Upon organic solvent evaporation, pores were formed on PLA nanofiber surfaces, and owing to evaporation rate differences between DCM and DMF, increased DCM ratios enhanced the pore forming rates. Thus, the DCM: DMF ratios of 8:2 was selected as the optimal parameter to prepare electrospun porous PLA nanofiber membranes and used for subsequent studies.
Morphological changes in PLA nanofiber membranes after SCS, PDA, and GS modification were measured using SEM. After SCS modification, relatively smooth surfaces and increased PLA nanofiber diameters were observed, and the porous PLA nanofiber structures had disappeared (Fig. 4a, b). The diameter of PLA nanofiber was increased from 590 ± 120 nm to PLA/SCS (800 ± 300 nm), PLA/SCS/PDA (830 ± 170 nm) and PLA/SCS/PDA-GS (970 ± 230 nm). With self-aggregated PDA on the PLA/SCS nanofiber membrane surface, granular-like morphology was formed (Fig. 4c). Also, PLA/SCS nanofiber roughness and diameters were increased with the addition of GS into dopamine before the self-aggregation of PDA (Fig. 4(d)). PLA hydrophilicity was improved after SCS coating, and the PLA nanofiber membrane contact angle was decreased from 110° to 39° (Fig. 4e). No significant differences were observed between PLA/SCS and PLA/SCS/PDA nanofiber membranes, which indicates that PDA modifications had not altered the hydrophobicity of PLA/SCS nanofiber membranes. However, the contact angle of PLA/SCS/PDA-GS nanofiber membranes was increased due to GS hydrophobicity properties (Fig. 4f). The swell behaviors of PLA, PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS nanofiber membranes were examined in PBS (Fig. 4g). All the nanofibers membranes reached equilibrium after 4 h, and the swelling ratios were increased from PLA (ca. 200%) to PLA/SCS (ca. 440%), PLA/SCS/PDA (ca. 1180%), and PLA/SCS/PDA-GS (ca. 1380%). GS release profiles from PLA/SCS/PDA-GS nanofiber membranes are shown in Fig. 4h. An initial burst release was observed in the first 12 h, which was followed by a sustained release over 7 days. Thus, the efficient initial GS release from PLA/SCS/PDA-GS nanofiber membranes was beneficial to prevent early-stage wound healing infection.
SEM images showing a porous PLA, b PLA/SCS, c PLA/SCS/PDA, and d PLA/SCS/PDA-GS nanofiber membranes, e Contact angle images of PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS membranes; f Quantitative contact angle data of PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS membranes; g swelling behaviors of PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS membranes immersed in PBS solution for 18 h; h GS release profiles from PLA/SCS/PDA-GS membranes in PBS solution. Data are expressed as mean ± SD (n = 3)
In vitro cell viability and pro-angiogenic marker gene expression
The Live/dead Raw 264.7 cell staining of all nanofiber membranes is shown in Fiugre 5a1-a8. Raw 264.7 cells attached to and spread well on the porous nanofiber membranes, with cells proliferating well on day 3 when compared with day 1. Nanofiber membrane cytotoxicity levels are shown in Fig. 5b. Consistent with live/dead staining data, no obvious cell toxicities were detected on all four nanofiber membranes after 1 and 3 days of culture. Interestingly, after both 1 and 3 days of culture, the PLA/SCS group exhibited higher cell viability when compared with the PLA group, whereas no significant differences were obseverd among PLA, PLA/SCS/PDA and PLA/SCS/PDA-GS groups. Thus, all porous PLA-based nanofibers were suitable for cell growth, and SCS may be beneficial for cell proliferation.
Fluorescence images of Raw 264.7 cells cultured on (a1, a5) PLA, (a2, a6) PLA/SCS, (a3, a7) PLA/SCS/PDA and (a4, a8) PLA/SCS/PDA-GS nanofiber membranes after 1 and 3 days of culture, respectively; b cell viabilities on PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS nanofiber membranes after 1 and 3 days of culture; c HIF-1α and d VEGF gene expressions in Raw.264.7 cells after cultured in PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS nanofiber memebranes for 24 h
We previously data showed that SCS alone induced the pro-angiogenic factor (HIF-1α and VEGF) expression in Raw 264.7 cells (Fig. 1c, d). Our in vitro gene expression data demonstrated that the PLA/SCS group expressed higher HIF-1α and VEGF levels when compared with the PLA group after 1 day of culture. Thus, after decorating the porous nanofiber membranes with SCS, the pro-angiogenic function of SCS was maintained. However, both PLA/SCS/PDA and PLA/SCS/PDA-GS groups did not increase HIF-1α and VEGF expression. These might have be due to a PDA layer forming on the PLA/SCS nanofiber membrane surface, and also more time might have been required for SCS to release and induce pro-angiogenic gene expression. Hyperglycemia in diabetes mellitus will cause endothelia dysfunction and damage the activity of VEGF, ultimately lead to impairment of neovascularization [42, 43]. In this work, SCS-containing PLA nanofiber membranes showed ability to increase endogenous VEGF expression and promote angiogenesis in HUVECs, and these will promote diabetic wound healing.
Macrophage immune responses to porous PLA-based nanofiber membranes
To investigate the influence of porous PLA-based nanofiber membranes on the polarization of Raw 264.7 cells, RT-PCR was carried out. Lipopolysaccharide (LPS, 500 ng/mL), a potent activator of the inflammatory response, was used to stimulate macrophage polarization toward the proinflammatory M1 phenotype. After 24 h coculture (Fig. 6), LPS markedly increased pro-inflammatory gene expression (TNF-α, IL-1β and IL-6) (***p < 0.001), with PLA/SCS, PLA/SCS/PDA, and PLA/SCS/PDA-GS groups showing significantly reduced the TNF-α, IL-1β and IL-6 expression, especially PDA-containing groups. Notably, PLA/SCS and PLA/SCS/PDA groups showed elevated anti-inflammatory gene expression (IL-4, IL-10 and Arg-1). Forty-eight hours after the LPS stimulation (Fig. 7), similar results were observed for pro-inflammatory gene expression (TNF-α, IL-1β and IL-6) in all nanofiber groups. PLA/SCS and PLA/SCS/PDA groups showed increased anti-inflammatory gene expression, whereas GS-containing groups dramatically promoted anti-inflammatory gene expression.
Long-term inflammatory environment in diabetic wounds impaired macrophage phenotype transition from inflammatory (M1) to anti-inflammatory (M2) status, which will also impede the trans-differentiation of macrophages into fibroblasts. Moreover, M2 macrophages also play a pivotal role in revascularization owing to their ability to release angiogenic factors, such as VEGF and PDGF. Our cell tests data demonstrated that PLA/SCS/PDA-GS nanofiber membranes were able to regulate the macrophage inflammatory responses, forming new vessels, thus promoting diabetic wound healing.
Antibacterial activity of porous PLA-based nanofiber membranes
Bacterial infection is a critical barrier to diabetic wound healing; therefore, antimicrobial activity is essential in diabetic wound dressings to prevent bacterial infection. Here, the S. aureus was used to investigate the in vitro antibacterial properties of the PLA-based nanofiber membrane. The ZOI assays showed no antibactericidal effects against S. aureus in PLA, PLA/SCS, and PLA/SCS/PDA groups, but for the PLA/SCS/PDA-GS nanofiber membranes, an 8 mm ZOI against S. aureus was observed (Fig. 8a, b). Additionally, the quantitative bactericidal effects from membranes were using through shake-flask culture method (Fig. 8c, d). Consistent with ZOI data, S. aureus had high survival rates when incubated with PLA, PLA/SCS, and PLA/SCS/PDA nanofiber membranes, but few S. aureus were observed in the PLA/SCS/PDA-GS treated group after co-cultivation for 15 min. It is worth noting that dopamine deposition had no obvious effects on S. aureus killing. Live/dead staining assays showed that bacteria grew well in PLA, PLA/SCS, and PLA/SCS/PDA groups, whereas GS-loaded nanofiber membranes (PLA/SCS/PDA-GS) showed efficient bacteria killing functions (99.23 ± 0.6%) (Fig. 8e1-e12). All the above results indicated that PLA, PLA/SCS and PLA/SCS/PDA nanofiber membranes had no significant anti-bactericidal abilities against S. aureus, whereas GS loading onto nanofiber membranes contributed to effective antibacterial activity.
a Bacteriostatic circles and b radius of bacteriostatic circle of S. aureus after treatment with PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS nanofiber membranes; c Photographs and d relative bacterial survival rates of S. aureus after coculture with PLA, PLA/SCS, PLA/SCS/PDA and PLA/SCS/PDA-GS nanofiber membranes for 0 min and 15 min respectively; Live/dead staining of S. aureus incubated with (e1, e5, e9) PLA, (e2, e6, e10) PLA/SCS, (e3, e7, e11) PLA/SCS/PDA and (e4, e8, e12) PLA/SCS/PDA-GS nanofiber membranes for 15 min (SYTO9 and PI stained live bacteria and dead bacteria, respectively)
Conclusions
In summary, in the present work, a multifunctional nanofiber membrane was developed via electrospinning combined with surface modification methods. Importantly, the coating of SCS, PDA and GS changed the porous structure of PLA nanofiber, increased the PLA fiber diameter and improved the hydrophilicity property of PLA nanofiber membrane. Additionally, in vitro data confirmed anti-inflammatory properties of PLA/SCS/PDA-GS nanofiber membranes toward macrophages (Raw 264.7 cells) and increased endogenous VEGF secretion to induce vascularization. Moreover, our in vitro antibacterial data demonstrated the efficient antibacterial ability of the GS-loaded nanofiber membrane toward S. aureus. Therefore, our novel nanofiber membranes with antibacterial, anti-inflammatory, and angiogenic properties have promising potential in diabetic wound regeneration applications.
Availability of data and materials
All the data and materials concerned with the manuscript are available with the corresponding author and can thereby asked.
References
Li WC, Jiang LB, Wu SJ, Yang SW, Ren L, Cheng B, **a J. A shape-programmable hierarchical fibrous membrane composite system to promote wound healing in diabetic patients. Small. 2022;18: e2107544.
Liu P, **ong Y, Chen L, Lin CC, Yang YY, Lin Z, Yu YS, Mi BB, Liu GH, **ao XF, Feng Q. Angiogenesis-based diabetic skin reconstruction through multifunctional hydrogel with sustained releasing of M2 Macrophage-derived exosome. Chem Eng J. 2022;431: 132413.
Li G, Wang QX, Feng JW, Wang JL, Wang YQ, Huang XT, Shao TT, Deng XF, Cao YM, Zhou MM, Zhao C. Recent insights into the role of defensins in diabetic wound healing. Biomed Pharmacother. 2022;155: 113694.
Augustine R, Rehman SRU, Ahmed R, Zahid AA, Sharifi M, Falahati M, Hasan A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int J Biol Macromol. 2020;156:153–70.
Dong RN, Guo BL. Smart wound dressings for wound healing. Nano Today. 2021;41: 101290.
Tu ZL, Chen M, Wang M, Shao ZX, Jiang XQ, Wang KY, Yao Z, Yang SW, Zhang XX, Gao WY. Engineering bioactive M2 macrophage-polarized anti-inflammatory, antioxidant, and antibacterial scaffolds for rapid angiogenesis and diabetic wound repair. Adv Funct Mater. 2021;31:2100924.
Wang W, Wang CG, Chen M, ** YW, Cheng W, Mao C, Xu TZ, Zhang XX, Lin C, Gao WY, Guo Y, Lei B. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultra-violet shielding nanodressing with exosome release. ACS Nano. 2019;13:10279–93.
Li YZ, Yang L, Hou Y, Zhang ZZ, Chen M, Wang MX, Liu J, Wang J, Zhao ZH, **e CM, Lu X. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–27.
Xu Z, Deng B, Wang XW, Yu J, Xu ZB, Liu PG, Liu CH, Cai Y, Wang F, Zong RL, Chen ZL, **ng H, Chen G. Nanofiber-mediated sequential photothermal antibacteria and macrophage polarization for healing MRSA-infected diabetic wounds. J Nanobiotechnol. 2021;19:404.
Yuan Y, Fan DD, Shen SH, Ma XX. An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds. Chem Eng J. 2022;433: 133589.
Zheng DT, Huang CX, Zhu XH, Huang HH, Xu CL. Performance of polydopamine complex and mechanisms in wound healing. Int J Mol Sci. 2021;22:10563.
Li T, Ma HS, Ma HZ, Ma ZJ, Qiang L, Yang ZZ, Yang XX, Zhou XJ, Dai KR, Wang JW. Mussel-inspired nanostructures potentiate the immunomodulatory properties and angiogenesis of mesenchymal stem cells. ACS Appl Mater Inter. 2019;11:17134–46.
Bao XF, Zhao JH, Sun J, Hu M, Yang XR. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12:8882–92.
Ma WJ, Zhang XX, Liu YX, Fan L, Gan JJ, Liu WL, Zhao YJ, Sun LY. Polydopamine decorated microneedles with Fe-MSC-derived nanovesicles encapsulation for wound healing. Adv Sci. 2022;9: e2103317.
Zhang H, Wu L, Wang GC, Liu PZ, Qin S, Xu KH, Tong D, Ding H, Tang H, Ji F. Macrophage polarization, inflammatory signaling, and NF-kappaB activation in response to chemically modified titanium surfaces. Colloids Surf B Biointerfaces. 2018;166:269–76.
Guan Y, Niu H, Liu ZT, Dang Y, Shen J, Zayed M, Ma L, Guan JJ. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7:ahj0153.
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Noyakavic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–88.
Wang YQ, Yao DY, Li LH, Qian ZY, He W, Ding R, Liu HF, Fan YB. Effect of electrospun silk fibroin-silk sericin films on macrophage polarization and vascularization. ACS Biomate Sci Eng. 2020;6:3502–12.
Yu YM, Chen R, Sun Y, Pan YZ, Tang W, Zhang S, Cao LY, Yuan Y, Wang J, Liu CS. Manipulation of VEGF-induced angiogenesis by 2-N, 6-O-sulfated chitosan. Acta Biomater. 2018;71:510–21.
Yu YM, Dai K, Gao ZH, Tang W, Shen T, Yuan Y, Wang J, Liu CS. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci Adv. 2021;7:8217.
Shen T, Dai K, Yu YM, Wang J, Liu CS. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020;117:192–203.
Chen H, Cheng RY, Zhao X, Zhang YH, Tam A, Yan YF, Shen HK, Zhang YS, Qi J, Feng YH, Liu L, Pan GQ, Cui WG, Deng LF. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019;11:3.
Chen J, Liu YJ, Cheng GP, Guo JH, Du S, Qiu JM, Wang C, Li CC, Yang XF, Chen TK, Chen ZB. Tailored hydrogel delivering niobium carbide boosts ROS-scavenging and antimicrobial activities for diabetic wound healing. Small. 2022;18:2201300.
Shi MQ, Du Z, Qi YC, Li WL, Hu HQ, Lin XH, Wang SJ, Tang Z, Zhou M. Wound microenvironment-responsive glucose consumption and hydrogen peroxide generation synergistic with azithromycin for diabetic wounds healing. Theranostics. 2022;12:2658–73.
Anand S, Ra**ikanth PS, Arya DK, Pandey P, Gupta RK, Sankhwar R, Chidambarm K. Multifunctional biomimetic nanofibrous scaffold loaded with asiaticoside for rapid diabetic wound healing. Pharmaceutics. 2022;14:273.
** YW, Ge J, Wang M, Chen M, Niu W, Cheng W, Xue YM, Lin C, Lei B. Bioactive antiinflammatory antibacterial antioxidative silicon-based nanofibrous dressing enables cutaneous tumor photothermo-chemo therapy and infection-induced wound healing. ACS Nano. 2020;14:2904–16.
Qian YN, Zheng YJ, ** J, Wu X, Xu KJ, Dai ML, Niu Q, Zheng H, He XJ, Shen JL. Immunoregulation in diabetic wound repair with a photoenhanced glycyrrhizic acid hydrogel scaffold. Adv Mater. 2022;34:2200521.
Asadi N, Mehdipour A, Ghorbani M, Mesgari-Abbasi M, Akbarzadeh A, Davaran S. A novel multifunctional bilayer scaffold based on chitosan nanofiber/alginate methacrylate hydrogel for full-thickness wound healing. Int J Biol Macromol. 2021;193:734–47.
Golipour H, Ezzatzadeh E, Sadeghianmaryan A. Investigation of co-electrospun gelatin: TiO2/polycaprolactone:silk fibroin scaffolds for wound healing applications. J Appl Polym Sci. 2022;139: e52505.
Li T, Sun MC, Wu SH. State-of-the-art review of electrospun gelatin-based nanofiber dressings for wound healing applications. Nanomaterials-basel. 2022;12:784.
Rad ZP, Mokhtari J, Abbasi M. Biopolymer based three-dimensional biomimetic micro/nanofibers scaffolds with porous structures via tailored charge repulsions for skin tissue regeneration. Polym Adv Technol. 2021;32:3535–48.
Yin J, Xu L, Ahmed A. Batch preparation and characterization of electrospun porous polylactic acid-based nanofiber membranes for antibacterial wound dressing. Adv Fiber Mater. 2022;4:832–44.
Chandika P, Oh GW, Heo SY, Kim SC, Kim TH, Kim MS, Jung WK. Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Mater Sci Eng C. 2021;121: 111871.
Yao QQ, Cosme JGL, Xu T, Miszuk JM, Picciani PHS, Fong H, Sun HL. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–27.
Yao QQ, Liu Y, Pan YN, Li YJ, Xu LM, Zhong YM, Wang W, Zuo JY, Yu H, Lv ZR, Chen H, Zhang LS, Wang BL, Yong HY, Meng YC. Long-term induction of endogenous BMPs growth factor from antibacterial dual network hydrogels for fast large bone defect repair. J Colloid Interf Sci. 2022;607:1500–15.
Huang JH, Han QX, Cai M, Zhu J, Li L, Yu LF, Wang Z, Fan GT, Zhu Y, Lu JW, Zhou GX. Effect of angiogenesis in bone tissue engineering. Ann Biomed Eng. 2022;50:898–913.
Recoraro AR, Hosfield BD, Li HG, Shelley WC, Markel TA. Angiogenesis: a cellular response to traumatic injury. Shock. 2021;55:301–10.
Hu C, Long LY, Cao J, Zhang SM, Wang YB. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem Eng J. 2021;411: 128564.
Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015: 549412.
Zhao Y, Zhang M, Lu GL, Huang BX, Wang DW, Shao Y, Lu MJ. Hypoxic preconditioning enhances cellular viability and pro-angiogenic paracrine activity: the roles of VEGF-A and SDF-1α in rat adipose stem cells. Front Cell Dev Biol. 2020;8: 580131.
Long G, Liu DY, He X, Shen YY, Zhao YN, Hou XL, Chen B, OuYang W, Dai JW, Li X. A dual functional collagen scaffold coordinates angiogenesis and inflammation for diabetic wound healing. Biomater Sci. 2020;8:6337–49.
Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012: 918267.
Maruhashi T, Higashi Y. Pathophysiological association between diabetes mellitus and endothelia dysfunction. Antioxidants. 2021;10:1306.
Acknowledgements
Hao Yu, Yijia Li contributed equally to this work.
Funding
This research is supported by the Project of State Key Laboratory of Ophthalmology, Optometry and Visual Science, Wenzhou Medical University (K03-20220203), Wenzhou Bureau of Science and Technology (Y2020233), which are greatly acknowledged.
Author information
Authors and Affiliations
Contributions
HY and YL contribute to the Methodology; YP, HW, WW and XR contribute to the Project administration; ZL contributes to Writing-review & editing; HY, ZL and YZ contribute to the Data curation; WL is responsible for paper editing; QY is responsible for the ideas of the paper, designing the experimental route and writing the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, H., Li, Y., Pan, Y. et al. Multifunctional porous poly (L-lactic acid) nanofiber membranes with enhanced anti-inflammation, angiogenesis and antibacterial properties for diabetic wound healing. J Nanobiotechnol 21, 110 (2023). https://doi.org/10.1186/s12951-023-01847-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-01847-w