Abstract
Exosomes are lipid bilayer membrane vesicles and are emerging as competent nanocarriers for drug delivery. The clinical translation of exosomes faces many challenges such as massive production, standard isolation, drug loading, stability and quality control. In recent years, artificial exosomes are emerging based on nanobiotechnology to overcome the limitations of natural exosomes. Major types of artificial exosomes include ‘nanovesicles (NVs)’, ‘exosome-mimetic (EM)’ and ‘hybrid exosomes (HEs)’, which are obtained by top-down, bottom-up and biohybrid strategies, respectively. Artificial exosomes are powerful alternatives to natural exosomes for drug delivery. Here, we outline recent advances in artificial exosomes through nanobiotechnology and discuss their strengths, limitations and future perspectives. The development of artificial exosomes holds great values for translational nanomedicine.

Similar content being viewed by others
Introduction
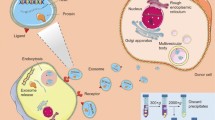
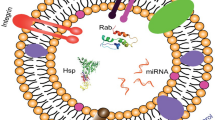
In recent decades, synthetic nanoparticles (NPs) including liposomes [1], micelles [2], dendrimers [3], nanocapsules [4], nanodiamonds [28], which are cell-derived proteolipid membrane vesicles, are emerging in nanomedicine-related fields [29]. Major types of EVs include exosomes, microvesicles, and apoptotic bodies [30]. Our understanding of the between-cell communication has been elevating in the last decade due to EVs, particularly exosomes, which are nano-sized (30–150 nm) subtype of EVs. Exosomes are enriched with various biological components, including proteins, nucleic acids and lipids from their parental cells [31]. Exosome-mediated cell-to-cell communication plays an important role in multiple physiological and pathological processes like tumor metastasis, drug resistance, immune responses and microenvironment homeostasis [32].
Exosomes are also competent candidates for targeted drug delivery [33, (Reprinted with permission from Ref. [3). Despite tropism of exosomes, the membrane engineering may be necessary for advanced drug delivery [96]. The hybrid NPs are slightly large in size but have similar morphology to exosomes and retained most protein markers of exosomes. Importantly, the hybrid NPs showed improved circulation and preferential accumulation in tumors and released drugs in response to temperatures. Genetically engineered thermosensitive liposome-exosome hybrid can escape mononuclear phagocytic system and target the tumor site, in which hyperthermia therapy stimulates the hybrid and induces combined immune-chemotherapy (Fig. 4). (Reprinted with permission from Ref [96]. Copyright WILEY–VCH 2020) A typical biohybrid strategy for generating hybrid exosomes by freeze-thawing induced fusion for combined tumor chemo-immunotherapy. Genetically engineered exosomes (gExos) were fused with thermosensitive liposomes (TLs) to form hybrid nanoparticles (gETL NPs), which can escape from clearance of mononuclear phagocytic system (MPS) and be activated by hyperthermic intraperitoneal chemotherapy (HIPEC) at the tumor site to release drugs for chemotherapy and polarize tumor-associated macrophages to M2 type to activate T cells for tumor immunotherapy. Incubation is a mild and commonly used method for various cellular processes and reactions. Fusion of liposomes and exosomes may occur during incubation as their membranes both have lipid bilayer structure. Incubation-induced spontaneous fusion may result in wide size distribution of hybrid particles with a high polydispersity index (PDI). The impact of incubation conditions on the fusion process requires further investigation. Exosomes are promising targeted delivery nanocarrier, but have limited efficiency, for their small size, in encapsulating exogenous large nucleic acids, such as CRISPR/Cas9 System. The fusion of exosomes and liposomes may achieve efficient loading of large plasmids into the hybrid. Lin et al. developed a hybrid NP by simple incubation of HEK293FT cell-derived exosomes with CRISPR/Cas9 expressing liposomes at 37℃ for 12 h [97]. The hybrid NPs delivered the CRISPR/Cas9 system to mesenchymal stem cells (MSCs) and achieved gene editing by expressing the encapsulated genes in the MSCs, which cannot be transfected by the liposome alone. Taken together, the exosome–liposome hybrid NPs can deliver CRISPR–Cas9 system in MSCs, providing a promising tool for targeted gene editing. The incubation-induced fusion of exosomes and liposomes can be enhanced by mediators on the surface, such as PEG. Piffoux et al. modified the EVs by incubation with liposomes via PEG-mediated fusion [98]. PEG-mediated fusion was proved to be an efficient approach to engineer EVs with exogenous compounds while preserving their inherent contents. Moreover, this fusion method enabled bioengineering of liposomal particles with biogenic molecules; importantly, the PEG-mediated fusion strategy allows efficient loading of cargoes and the feasible engineering of EV membranes with adaptable functions. Another reported method for generating biohybrid of exosomes and liposomes is co-extrusion. Under physical stress, membranes of exosomes and liposomes would break and re-assemble to form hybrid vesicles when passing through the membrane pore with controlled size. Exosomes have been explored as a drug delivery candidate owing to their natural functionalities. However, it has been reported that exosomes obtained by different method may differ in yield and purity [101]. The HEs efficiently accumulated in the fibrotic lesion and exhibited significant penetration of pulmonary fibrotic tissue for the improved affinity for fibroblasts by homologous exosome. Along with the rapid growth in nanobiotechnology, the research of exosomes has been advancing from biology [32, 106] to biomarkers [107, 108] and nanomedicines [109] over the past decade. For drug delivery, exosomes have shown various advantages compared with conventional synthetic materials such as liposomes [110]. The therapeutic potential of exosomes-mediated drug delivery are still in tests of preliminary clinical trials (pancreatic cancer: NCT03608631; acute ischemic stroke: NCT03384433; colon cancer: NCT01294072), while the efficacy of cell-derived exosome-like vesicles has been evidenced in several pilot trials [111,112,113]. Nevertheless, clinical translation of natural exosomes has been challenging [114]. Major hurdles including large-scale production, standard purification protocols, characterization of complex composition, cargo loading, quality control and storage stability are in their way to products for therapeutic applications [115]. Mass production of exosomes may be achieved through the development of bioreactors [38]; however, as biological components, their standardized and reproducible production requires comprehensive control of genetic stability and culturing condition of producing cells; purification requires subtype identification and quantification of contaminants [116]; efficacy is dependent on drug loading efficiency and capacity [117]; storage of therapeutic exosomes is supposed to have high recovery without damage to exosome particles as well as their biological contents [118, 119]. From a current perspective, the development of exosomes for therapeutic drug delivery is still in infancy and the cost for translational research and clinical application would be very high. In recent years, artificial exosomes have been developed with higher pharmaceutical acceptability to overcome the drawbacks of natural exosomes as new theranostic biomaterials for potential clinical applications [45]. However, there are some challenges for different strategies develo** artificial exosomes for the following aspects: yield, procedures, time–cost, manpower, sustainability, characterization and efficacy, which are summarized and compared to natural exosomes (Table 4). Top-down strategy is the most widely reported method for obtaining artificial exosomes. One major strength of the top-down strategy is the applicability because NVs are fully biological and have similar physiochemical and biological features to natural exosomes. Similar to natural exosomes, the yield of artificial exosomes by top-down strategies may vary from cell to cell. For the serial extruding method, most studies reported a nearly 100-fold higher yield than natural exosomes, but higher yields have also been reported and the maximum was 500-fold (Table 1). Compared to natural exosomes, preparation of artificial exosomes by top-down strategies could be cumbersome as UC-based purification procedures are still required following serial extruding or nitrogen cavitation. The development of specific devices may simplify the procedures and increase production efficiency and reduce time–cost and manpower [63]. However, for generating NVs, cells are sacrificed and broken into fragments, leading to limited production sustainability. Also, it has not been raised whether natural exosomes in cells should be considered as contaminants that may influence the characterization of NVs. Bottom-up strategies are able to produce “clean” artificial exosomes with determined formulations and have the highest scalability because synthetic materials could be feasibly obtained and used for massive production. Besides, the cost of time and manpower could be remarkably reduced by using synthetic materials. Multiple modifications of liposomes are still challenging for complex procedures, uncertain conditions and instability. Another major drawback of bottom-up approaches for generating liposome-based artificial exosomes is that synthetic materials can still hardly mimic the complex composition of natural exosomes. Therefore, the functions of natural exosomes can hardly be fully reproduced by artificial exosomes based on bottom-up strategies. Publications that have compared artificial exosomes with natural exosomes are scarce. Most studies only evaluated physicochemical and biological properties of artificial exosomes (Table 2). A previous study reported a preliminary comparison concerning drug delivery efficiency [87]. Currently, preparing artificial exosomes that fully assembles components of natural exosomes may be pharmaceutically impossible [64], modification of liposomes with key proteins is dependent on the purpose and may not be consistent with natural exosomes. Therefore, the translational applicability of bottom-up strategies is limited. Biohybrid approaches can only produce semi-artificial exosomes. The yield of artificial exosomes by biohybrid strategies would not be very high as natural exosomes are still required. In addition, the involvement of natural vesicles may face methodological challenges similar to natural exosomes. Preparation of biohybrid exosomes could be laborious as preparation of synthetic liposomes and isolation of natural vesicles are both required. Characterization of hybrid particles may be influenced by additional liposomes and exosomes and the purification would be rather difficult as liposomes, exosomes and the hybrid are very similar in multiple aspects. However, one strength of the biohybrid approach is that the hybrid possesses natural components from exosomes, despite dilution, those hybrid nanocarriers may have improved delivery efficiency than liposomes and higher stability than exosomes, leading to high applicability. Besides, the fusion method that is widely used in biohybrid strategies provided a feasible option for drug loading such as loading biological cargoes into liposomes and loading exogenous therapeutic agents into exosomes [98]. Biomimetic nanocarriers are the next generation of drug delivery systems in nanomedicine for improving health. Advancements in nanobiotechnology provide avenues for the development of artificial exosomes that may accelerate clinical translation for nanomedicine application. Currently, natural exosomes are just in their preliminary clinical trials and artificial exosomes are not yet ready for translation. Major challenges include the preparation protocols, characterization and biocompatibility concerns. Artificial exosomes have commercial advantages for their up-scale productivity. In the future, novel and multifunctional artificial exosomes will be developed, with contributions from multidiscipline efforts of biotechnology, nanotechnology, chemical engineering and pharmaceutical industry, to improve healthcare. We hold confidence for artificial exosomes’ potentials for personalized nanomedicine.
Biohybrid by freeze-thawing

Biohybrid by incubation
Biohybrid by co-extruding
Comparison of natural exosomes and artificial exosomes
Concluding remarks
References
Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–63.
Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–92.
Yamamoto K, Imaoka T, Tanabe M, Kambe T. New horizon of nanoparticle and cluster catalysis with dendrimers. Chem Rev. 2020;120:1397–437.
Deng S, Gigliobianco MR, Censi R, Di Martino P. Polymeric nanocapsules as nanotechnological alternative for drug delivery system: current status, Challenges and Opportunities. Nanomaterials (Basel). 2020;10:847.
Lai H, Stenzel MH, **ao P. Surface engineering and applications of nanodiamonds in cancer treatment and imaging. Int Mater Rev. 2020;65:189–225.
Allahyari S, Trotta F, Valizadeh H, Jelvehgari M, Zakeri-Milani P. Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opin Drug Deliv. 2019;16:467–79.
Elzayat A, Adam-Cervera I, Alvarez-Bermudez O, Munoz-Espi R. Nanoemulsions for synthesis of biomedical nanocarriers. Colloids Surf B Biointerfaces. 2021;203:111764.
Wang TT, **a YY, Gao JQ, Xu DH, Han M. Recent progress in the design and medical application of in situ self-assembled polypeptide materials. Pharmaceutics. 2021;13:753.
Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6:1–20.
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9.
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du Mond C, Mamelok RD, Henry DH. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445–51.
Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22.
Meazza C, Asaftei SD. State-of-the-art, approved therapeutics for the pharmacological management of osteosarcoma. Expert Opin Pharmacother. 2021. https://doi.org/10.1080/14656566.2021.1936499.
Koudelka S, Turanek J. Liposomal paclitaxel formulations. J Control Release. 2012;163:322–34.
Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastr J, Scheithauer W, Siena S, Tabernero J, Teixeira L, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015. https://doi.org/10.1093/jnci/dju413.
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803.
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P, Zhang H, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–62.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–51.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:1–12.
Price LSL, Stern ST, Deal AM, Kabanov AV, Zamboni WC. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci Adv. 2020;6:eaay9249.
Ben-David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R, Golub TR. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet. 2017;49:1567–75.
Pasut G, Paolino D, Celia C, Mero A, Joseph AS, Wolfram J, Cosco D, Schiavon O, Shen H, Fresta M. Polyethylene glycol (PEG)-dendron phospholipids as innovative constructs for the preparation of super stealth liposomes for anticancer therapy. J Control Release. 2015;199:106–13.
Nakamura K, Yamashita K, Itoh Y, Yoshino K, Nozawa S, Kasukawa H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim Biophys Acta. 2012;1818:2801–7.
Zhang P, Wang Y, Lian J, Shen Q, Wang C, Ma B, Zhang Y, Xu T, Li J, Shao Y, et al. Engineering the surface of smart nanocarriers using a pH-/Thermal-/GSH-responsive polymer zipper for precise tumor targeting therapy in vivo. Adv Mater. 2017;29:1702311.
Newman MR, Russell SG, Schmitt CS, Marozas IA, Sheu TJ, Puzas JE, Benoit DSW. Multivalent presentation of peptide targeting groups alters polymer biodistribution to target tissues. Biomacromol. 2018;19:71–84.
Gomes-da-Silva LC, Fonseca NA, Moura V, Pedroso de Lima MC, Simoes S, Moreira JN. Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res. 2012;45:1163–71.
Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: why and how? Adv Drug Deliv Rev. 2020;159:332–43.
Margolis L, Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol. 2019;17:e3000363.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. https://doi.org/10.1126/science.aau6977.
Patil SM, Sawant SS, Kunda NK. Exosomes as drug delivery systems: a brief overview and progress update. Eur J Pharm Biopharm. 2020;154:259–69.
Li YJ, Wu JY, Hu XB, Wang JM, **ang DX. Autologous cancer cell-derived extracellular vesicles as drug-delivery systems: a systematic review of preclinical and clinical findings and translational implications. Nanomedicine (Lond). 2019;14:493–509.
Mehryab F, Rabbani S, Shahhosseini S, Shekari F, Fatahi Y, Baharvand H, Haeri A. Exosomes as a next-generation drug delivery system: an update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020;113:42–62.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503.
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPalpha signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145–64.
Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24:242–56.
Ayala-Mar S, Donoso-Quezada J, Gallo-Villanueva RC, Perez-Gonzalez VH, Gonzalez-Valdez J. Recent advances and challenges in the recovery and purification of cellular exosomes. Electrophoresis. 2019;40:3036–49.
Hood JL. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine (Lond). 2016;11:1745–56.
Rankin-Turner S, Vader P, O’Driscoll L, Giebel B, Heaney LM, Davies OG. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv Drug Deliv Rev. 2021;173:479–91.
Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017;20:1.
Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular vesicle heterogeneity: subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 2018;9:738.
Huang G, Lin G, Zhu Y, Duan W, ** D. Emerging technologies for profiling extracellular vesicle heterogeneity. Lab Chip. 2020;20:2423–37.
Garcia-Manrique P, Gutierrez G, Blanco-Lopez MC. Fully artificial exosomes: towards new theranostic biomaterials. Trends Biotechnol. 2018;36:10–4.
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–710.
Wang JM, Li YJ, Wu JY, Cai JX, Wen J, **ang DX, Hu XB, Li WQ. Comparative evaluation of methods for isolating small extracellular vesicles derived from pancreatic cancer cells. Cell Biosci. 2021;11:37.
Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J. RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–8.
Jeong D, Jo W, Yoon J, Kim J, Gianchandani S, Gho YS, Park J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials. 2014;35:9302–10.
Oh K, Kim SR, Kim DK, Seo MW, Lee C, Lee HM, Oh JE, Choi EY, Lee DS, Gho YS, Park KS. In vivo differentiation of therapeutic insulin-producing cells from bone marrow cells via extracellular vesicle-mimetic nanovesicles. ACS Nano. 2015;9:11718–27.
Kim YS, Kim JY, Cho R, Shin DM, Lee SW, Oh YM. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med. 2017;49:e284.
Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, Gopal A, Baek SH, Jeong SY, Lee SW, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116.
Kim HY, Kumar H, Jo MJ, Kim J, Yoon JK, Lee JR, Kang M, Choo YW, Song SY, Kwon SP, et al. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18:4965–75.
Yang Z, **e J, Zhu J, Kang C, Chiang C, Wang X, Wang X, Kuang T, Chen F, Chen Z, et al. Functional exosome-mimic for delivery of siRNA to cancer: in vitro and in vivo evaluation. J Control Release. 2016;243:160–71.
Nasiri Kenari A, Kastaniegaard K, Greening DW, Shambrook M, Stensballe A, Cheng L, Hill AF. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteomics. 2019;19:e1800161.
Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25:241–55.
Wu JY, Ji AL, Wang ZX, Qiang GH, Qu Z, Wu JH, Jiang CP. Exosome-Mimetic Nanovesicles from Hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci Rep. 2018;8:2471.
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, Jeong GJ, Kwon SP, Song SY, Go S, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–93.
Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. Novel alternatives to extracellular vesicle-based immunotherapy—exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 2018;46:S166–79.
Wu JY, Li YJ, Hu XB, Huang S, Luo S, Tang T, **ang DX. Exosomes and biomimetic nanovesicles-mediated anti-glioblastoma therapy: a head-to-head comparison. J Control Release. 2021;336:510–21.
Fan Z, **ao K, Lin J, Liao Y, Huang X. Functionalized DNA enables programming exosomes/vesicles for tumor imaging and therapy. Small. 2019;15:e190361.
Guo P, Busatto S, Huang J, Morad G, Moses MA. A facile magnetic extrusion method for preparing endosome-derived vesicles for cancer drug delivery. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202008326.
Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, Yoon YJ, Kim SC, Gho YS, Park J. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6:12056–64.
Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, Storm G, Wang JW, Czarny B, Pastorin G. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7:14322.
Jo W, Jeong D, Kim J, Cho S, Jang SC, Han C, Kang JY, Gho YS, Park J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14:1261–9.
Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials. 2015;59:12–20.
Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016;224:208–16.
Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73.
Go G, Lee J, Choi DS, Kim SS, Gho YS. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019;8:e1801082.
Ingato D, Edson JA, Zakharian M, Kwon YJ. Cancer Cell-derived, drug-loaded nanovesicles induced by sulfhydryl-blocking for effective and safe cancer therapy. ACS Nano. 2018;12:9568–77.
Patty PJ, Frisken BJ. The pressure-dependence of the size of extruded vesicles. Biophys J. 2003;85:996–1004.
Olson F, Hunt C, Szoka F, Vail W, Papahadjopoulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochimica et Biophysica Acta BBA Biomembranes. 1979;557:9–23.
Liu D, Zhang H, Fontana F, Hirvonen JT, Santos HA. Current developments and applications of microfluidic technology toward clinical translation of nanomedicines. Adv Drug Deliv Rev. 2018;128:54–83.
Elvira KS. Microfluidic technologies for drug discovery and development: friend or foe? Trends Pharmacol Sci. 2021;42:518–26.
Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22.
Zhao Z, McGill J, Gamero-Kubota P, He M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip. 2019;19:1877–86.
Sezgin E, Kaiser HJ, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 2012;7:1042–51.
Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47:8572–610.
Krause MR, Regen SL. The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Acc Chem Res. 2014;47:3512–21.
Lu M, Huang Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials. 2020;242:119925.
Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015–28.
De La Pena H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GW, Selman A, Rees RC, Travers PJ, Dodi IA. Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods. 2009;344:121–32.
Martinez-Lostao L, Garcia-Alvarez F, Basanez G, Alegre-Aguaron E, Desportes P, Larrad L, Naval J, Martinez-Lorenzo MJ, Anel A. Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum. 2010;62:2272–82.
De Miguel D, Basanez G, Sanchez D, Malo PG, Marzo I, Larrad L, Naval J, Pardo J, Anel A, Martinez-Lostao L. Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol Pharm. 2013;10:893–904.
Li K, Chang S, Wang Z, Zhao X, Chen D. A novel micro-emulsion and micelle assembling method to prepare DEC205 monoclonal antibody coupled cationic nanoliposomes for simulating exosomes to target dendritic cells. Int J Pharm. 2015;491:105–12.
Lu M, Zhao X, **ng H, Liu H, Lang L, Yang T, Xun Z, Wang D, Ding P. Cell-free synthesis of connexin 43-integrated exosome-mimetic nanoparticles for siRNA delivery. Acta Biomater. 2019;96:517–36.
Vazquez-Rios AJ, Molina-Crespo A, Bouzo BL, Lopez-Lopez R, Moreno-Bueno G, de la Fuente M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J Nanobiotechnol. 2019;17:85.
Molinaro R, Martinez JO, Zinger A, De Vita A, Storci G, Arrighetti N, De Rosa E, Hartman KA, Basu N, Taghipour N, et al. Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma. Biomater Sci. 2020;8:333–41.
Li YJ, Wu JY, Hu XB, Ding T, Tang T, **ang DX. Biomimetic liposome with surface-bound elastase for enhanced tumor penetration and chemo-immumotherapy. Adv Healthc Mater. 2021. https://doi.org/10.1002/adhm.202100794.
Zhang KL, Wang YJ, Sun J, Zhou J, **ng C, Huang G, Li J, Yang H. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem Sci. 2019;10:1555–61.
Li J, Peng K, Li Y, Wang J, Huang J, Yan Y, Wang D, Tang BZ. Exosome-mimetic supramolecular vesicles with reversible and controllable fusion and fission*. Angew Chem Int Ed Engl. 2020;59:21510–4.
Kameritsch P, Renkawitz J. Principles of leukocyte migration strategies. Trends Cell Biol. 2020;30:818–32.
Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, et al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037–46.
Li J, Shi K, Drechsler M, Tang BZ, Huang J, Yan Y. A supramolecular fluorescent vesicle based on a coordinating aggregation induced emission amphiphile: insight into the role of electrical charge in cancer cell division. Chem Commun (Camb). 2016;52:12466–9.
Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, Shiku H, Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep. 2016;6:21933.
Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci (Weinh). 2020;7:2000515.
Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv Sci (Weinh). 2018;5:1700611.
Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830–42.
Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94.
Jhan YY, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, Bishop CJ. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharm. 2020;573:118802.
Sun L, Fan M, Huang D, Li B, Xu R, Gao F, Chen Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271:120761.
Liang Y, Duan L, Lu J, **a J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95.
Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015;22:231–42.
Kumar Pramanik S, Losada-Pe Rez P, Reekmans G, Carleer R, D’Olieslaeger M, Vanderzande D, Adriaensens P, Ethirajan A. Physicochemical characterizations of functional hybrid liposomal nanocarriers formed using photo-sensitive lipids. Sci Rep. 2017;7:46257.
Abri Aghdam M, Bagheri R, Mosafer J, Baradaran B, Hashemzaei M, Baghbanzadeh A, de la Guardia M, Mokhtarzadeh A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J Control Release. 2019;315:1–22.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
LeBleu VS, Kalluri R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer. 2020;6:767–74.
Hornung S, Dutta S, Bitan G. CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front Mol Neurosci. 2020;13:38.
Wei W, Ao Q, Wang X, Cao Y, Liu Y, Zheng SG, Tian X. Mesenchymal stem cell-derived exosomes: a promising biological tool in nanomedicine. Front Pharmacol. 2020;11:590470.
Escude Martinez de Castilla P, Tong L, Huang C, Marios Sofias A, Pastorin G, Chen X, Storm G, Schiffelers RM, Wang JW. Extracellular vesicles as a drug delivery system: a systematic review of preclinical studies. Adv Drug Deliv Rev. 2021;175:113801.
Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, ** X, Liang X, Lv J, Zhang Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743–53.
Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, Xu P, Sun Y, Ma R, Ji T, et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–27.
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282.
Perocheau D, Touramanidou L, Gurung S, Gissen P, Baruteau J. Clinical applications for exosomes: are we there yet? Br J Pharmacol. 2021;178:2375–92.
Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894–906.
Yang XX, Sun C, Wang L, Guo XL. New insight into isolation, identification techniques and medical applications of exosomes. J Control Release. 2019;308:119–29.
Donoso-Quezada J, Ayala-Mar S, Gonzalez-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit Rev Biotechnol. 2020;40:804–20.
Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol. 2018;9:1199.
Bari E, Perteghella S, Catenacci L, Sorlini M, Croce S, Mantelli M, Avanzini MA, Sorrenti M, Torre ML. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine (Lond). 2019;14:753–65.
Acknowledgements
Not applicable.
Funding
The work was supported by the Hunan Provincial Science and Technology Plan (No. 2016TP2002).
Author information
Authors and Affiliations
Contributions
YJL and DXX defined the focus of the review. YJL and JYW summarized studies. YJL drafted the manuscript. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, YJ., Wu, JY., Liu, J. et al. Artificial exosomes for translational nanomedicine. J Nanobiotechnol 19, 242 (2021). https://doi.org/10.1186/s12951-021-00986-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-021-00986-2