Abstract
Background
Variations in the prevalence and systemic inflammatory (SI) status between non-alcoholic fatty liver disease (NAFLD) and newly defined metabolic dysfunction-associated fatty liver disease (MAFLD) have only been reported by few studies. Hence, this study aimed to compile data on the prevalence and the systemic inflammation levels of MAFLD and NAFLD in a general population from Southeast China was summarized to explore the potential effect of the transformation of disease definition.
Methods
A total of 6718 general population participants aged 35–75 were enrolled. Logistic regression and restricted cubic spline (RCS) models were used to examine the relationship between 15 SI indicators and NAFLD and MAFLD. The predicted values of MAFLD and NAFLD were analyzed using the receiver operating characteristic (ROC) curve.
Results
The prevalence of MAFLD and NAFLD was 34.7% and 32.4%, respectively. Their overlap** rate was 89.7%, while only 8.3% and 1.9% of participants were MAFLD-only and NAFLD-only. Among three FLD groups, the MAFLD-only group had the highest levels of 8 SI indicators, including CRP, WBC, LYMPH, NEUT, MONO, ALB, NLR, and SIRI. The non-FLD group had the lower levels of all 15 SI indicators compared with all FLD subgroups. The odds ratios (ORs) of 10 SI indicators were significant in both multivariable-adjusted logistic regression and RCS analyses of MAFLD or NAFLD, including CRP, WBC, LYMPH, NEUT, MONO, ALB, PLR, LMR, ALI and CA. ROC analysis showed that the AUC values of all SI were lower than 0.7 in both MAFLD and NAFLD.
Conclusions
MAFLD could cover more FLD than NAFLD, and the MAFLD-only group had a more severe inflammation status, whereas the NAFLD-only exhibited lower levels. Moreover, there was not a high AUC and a high sensitivity of SI indicators, suggesting that SI indicators are not good indicators to diagnose NAFLD/MAFLD.
Similar content being viewed by others
Background
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver disease globally, with an estimated prevalence of 25% [1, 2]. It is strongly associated with a range of metabolic disorders, including hyperglycemia, hypertension, abdominal obesity, and dyslipidemia [3]. In 2020, a recommendation was to introduce metabolic dysfunction-associated fatty liver disease (MAFLD) [4], This change in terminology aimed to shift the focus away from alcohol consumption as the defining factor in NAFLD, emphasizing instead the role of metabolic disorders in the progression of NAFLD-related pathologies [5]. In 2021, two separate meta-analyses highlighted a significant difference in the prevalence rates of MAFLD and NAFLD. Specifically, MAFLD identified a larger number of patients, though it is important to note that there was still a considerable overlap between the two conditions [6, 7]. It is crucial to recognize that the prevalence of subgroups such as MAFLD-only, NAFLD-only, and overlap-FLD can vary significantly based on the proportions of metabolic abnormalities and other concurrent conditions, as defined by their respective criteria. [6]. In China, the reported prevalence of MAFLD ranging from 21.0% to 46.7% [8,9,10,11,12,13,14] and prevalence of NAFLD ranging from 29.3% to 32.9% [15,16,17], respectively it is varied largely among geographic regions. Thus, more studies among general populations are warranted to understand the similarities and differences between the two conditions, before the transformation from NAFLD to MAFLD.
Inflammation is a physiological response to tissue injury or infection, leading to the release of various inflammatory mediators. When inflammation persists over time, it can lead to chronic systemic inflammatory changes, which can worsen tissue damage [18, 19]. The status of systemic inflammation (SI) is widely acknowledged as a primary pathogenic factor in the advancement of steatohepatitis, fibrosis, and adverse outcomes associated with chronic liver diseases [20,21,22,23]. In the meantime, studies have reported that MAFLD tends to be associated with advanced liver disease compared to NAFLD [6]. The potential disparity in systemic inflammation (SI) levels between MAFLD and NAFLD is still uncertain, yet it could bear significant implications for the shift in diagnostic criteria. This study seeks to examine the association between MAFLD and NAFLD from an inflammatory standpoint, and to determine whether the distinct definitions of MAFLD and NAFLD result in differences in the affected populations. Therefore, the current study sought to investigate and compare the prevalence of MAFLD and NAFLD in Southern China, and explore the chronic inflammatory status and indicators of MAFLD, NAFLD, and three subgroups, including MAFLD-only, NAFLD-only, and overlap-FLD. The results may provide insight into the clinical relevance of the novel MAFLD definition from the SI perspective.
Methods
Study design and population
This study was conducted in Fuqing, Fujian Province in Southern China from July 2020 to June 2021, which recruited residents aged 35–75 years. Of the 7662 participants, 7164 underwent liver ultrasound examination. Participants with missing anthropometric results or important clinical and laboratory data were excluded from the analysis. A total of 6718 individuals were included in the final analysis. A flowchart of the participant enrollment process is shown in Fig. 1.
The study protocol was approved by the ethical committee of the Fujian Medical University (approval number: 2020–58), and written informed consent was obtained from all participants.
Data collection
Data were gathered by well-trained interviewers and examiners, including demographic variables, anthropometric measures, laboratory measurements, and liver ultrasonography.
Demographic variables
A face-to-face interview was conducted using a structured electronic questionnaire by well-trained interviewers, to collect participants’ information, including socio-demographic characteristics, lifestyle variables (smoking, alcohol drinking, and physical activity), and history of disease and medication. The electronic questionnaire was independently developed by the research group (https://cohort.fjmu.edu.cn/cobl). The interview process was tape-recorded, and the degree of cooperation of the respondents and the reliability of the questionnaire were evaluated.
Anthropometric measures
Anthropometric measurements, including height, body weight, waist circumference (WC), hip circumference (HC), and systolic and diastolic blood pressure (SBP and DBP) were measured by trained staff. The body mass index (BMI) was also calculated as body weight in kilograms divided by height squared in meters (kg/m2). WC and HC were measured with a tape measure to horizontally circle the waist and hips of all subjects, after they took off their coats, loosened their belts, stood naturally on both legs, and maintained calm breathing. Blood pressure (BP) measurements were taken on the right upper arm by trained employees using an electronic BP monitor (OMRON, Kyoto, Japan) at the heart level. The BP was measured twice with an interval of 30 s. When the difference between two SBP/DBP measurements was greater than 5 mmHg, a third measurement was taken. The two closest of all measurements were taken to calculate the average SBP and DBP, which were used in the analysis.
Laboratory measurement
Fasting blood was collected from all participants after fasting for at least 8 h. All participants without self-reported diabetes mellitus were invited to perform a 75 g oral glucose tolerance test (OGTT), and 2-h post-load blood samples were collected. Fasting blood glucose (FBG), 2-h post-load blood glucose (2 h-PG), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), albumin (ALB), C-reactive protein (CRP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured using an automatic analyzer (Toshiba, Tokyo, Japan). Glycosylated hemoglobin (HbA1c) was measured using a high-performance liquid chromatography method (ARKRAY, Osaka, Japan). Fasting insulin (FINs) was measured by electrochemiluminescence immunoassay (Roche Diagnostics, Munich, Germany). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: FINs × FBG/22.5 [24]. White blood cell (WBC), lymphocyte (LYMPH), neutrophils (NEUT), monocyte (MONO), and platelet (PLT), and the mean platelet volume (MPV) were measured using an automated hematology analyzer (SYSMEX, Kyoto, Japan).
Liver ultrasonography
Abdominal ultrasound was performed after overnight fasting and was completed by well-trained technicians using a portable full-digital color Doppler ultrasound diagnostic instrument (Hitachi, Tokyo, Japan).
Diagnostic criteria of NAFLD, MAFLD, and metabolic disorders
NAFLD was defined as evidence of hepatic steatosis based on abdominal ultrasound and the exclusion of significant alcohol consumption (defined as ≥ 30 g/day for men and 20 g/day for women, respectively) [25].
MAFLD was defined as evidence of fatty liver based on abdominal ultrasound with at least one of the following three conditions [4]: (1) overweight or obesity (BMI ≥ 23.0 kg/m2 in Asians); (2) type 2 diabetes mellitus (T2DM); and (3) metabolic dysregulation among non-overweight individuals (BMI < 23.0 kg/m2 in Asians).
Hypertension was defined as an average SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, self-reported history of hypertension, and/or taking antihypertensive drugs [26].
T2DM was defined as an FBG ≥ 7.0 mmol/L, 2 h-PG ≥ 11.1 mmol/L, HbA1c ≥ 6.5%, self-reported history of DM, and/or use of antidiabetic drugs [27].
Prediabetes was defined as non-diabetic individuals with an FBG level of 5.6-6.9 mmol/L, 2h-PG of 7.8-11.1 mmol/L, and/or HbA1c of 5.7-6.4% [27].
Hyperlipidemia was defined as triacylglycerols ≥ 2.26 mmol/L and/or total cholesterol ≥ 6.22 mmol/L and/or HDL-c < 1.04 mmol/L and/or LDL-c ≥ 4.14 mmol/L and/or self-reported medication for hyperlipidemia.
Indicators of the SI level
A total of 15 indicators were applied to evaluate the SI level of the population. CRP, WBC, LYMPH, NEUT, MONO, MPV, and ALB were obtained from laboratory measurements, and eight indicators were calculated according to the following equations: neutrophil-to-lymphocyte ratio (NLR) = NEUT/lymphocyte (LYMPH) [28], derived NLR (d_NLR) = NEUT/(WBC-NEUT) [28], platelet-to-lymphocyte ratio (PLR) = PLT/LYMPH [28], lymphocyte-to-monocyte ratio (LMR) = LYMPH/MONO [28], systemic immune-inflammation index (SII) = PLT × NEUT/LYMPH [29], C-reactive protein-to-albumin ratio (CA) = CRP/ALB [30], advanced lung cancer inflammation index (ALI) = BMI × ALB/NLR [31], and systemic inflammation response index (SIRI) = NEUT × MONO/LYMPH [32].
Statistical analysis
Statistical analysis was performed using SAS (version 9.4, America), and a two-tailed P-value of < 0.05 was considered statistically significant. Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range (IQR)) based on data distribution and were compared using independent Student’s t-test or one-way analysis of variance (ANOVA). The Fisher’s least significant difference (LSD) method was used for pairwise comparison between groups. Categorical variables were expressed as numbers and percentages and compared using the Chi-squared test. Non-normally distributed data were analyzed using the nonparametric test and logarithmically transformed to normality when appropriate.
Multivariable logistic regression models were applied to calculate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for NAFLD and MAFLD with different inflammatory indicators. To prevent the bias caused by any possible leverage value, restricted cubic spline (RCS) models were used to fit the non-linear relationship between inflammatory status indicators and MAFLD and NAFLD. Additionally, to analyze the predictive power of 15 inflammatory indicators for MAFLD and NAFLD and determine the best threshold for each parameter, the receiver operating characteristic (ROC) curve was used to analyze each parameter and find the point at which the sum of sensitivity and specificity was maximized to determine the best threshold for each parameter.
Results
Prevalence and characteristics of MAFLD, NAFLD, and their subgroups
The demographics, anthropometrics, and laboratory test characteristics of 6718 subjects are presented in Table 1. The median age of the participants was 57 years (range, 50–65 years). Out of all the participants, 34.7% were male. A total of 2330 individuals were diagnosed with MAFLD, yielding a prevalence rate of 34.7%. Within the MAFLD group, the percentages of elderly individuals, unemployed individuals, farmers, as well as those with hypertension and T2DM were higher compared to the non-MAFLD group (all P < 0.05). Compared with the non-MAFLD group, the MAFLD group had dramatically higher levels of WC, BMI, SBP, DBP, ALT, AST, TG, FBG, and 2 h-PG and significantly lower HDL-c levels.
The prevalence of NAFLD was 32.4%. The proportions of elderly, women, unemployed, farmers, smokers, hypertensive and T2DM patients among NAFLD group were higher than those among non-NAFLD group (all P < 0.05). The NAFLD group had dramatically higher levels of WC, BMI, SBP, DBP, ALT, TG, FBG, and 2 h-PG and significantly lower HDL-c levels compared with the non-NAFLD group.
The entire population was regrouped into non-FLD, overlap-FLD, MAFLD-only, and NAFLD-only groups. Participants who met the criteria for both MAFLD and NAFLD were categorized in the overlap-FLD group. The overlap** population included 2132 subjects, with an overlap** rate of 89.7%. Participants who met the inclusion criteria for MAFLD but not NAFLD were classified as MAFLD-only, and those who met the criteria for NAFLD but not MAFLD were considered to be NAFLD-only. The prevalence of MAFLD-only and NAFLD-only was 8.3%, and 1.9%, respectively.
SI levels of MAFLD, NAFLD, and their subgroups
The 15 SI indicators are shown in Table 2. Except for MPV, d_NLR, and SII, other indicators dramatically differed between MAFLD and non-MAFLD groups, and the MAFLD group had higher levels of SI. Similarly, compared with the non-NAFLD group, NAFLD participants had higher levels of all the indicators, except for MPV, SII, and SIRI.
Compared among non-FLD, overlap-FLD, MAFLD-only, and NAFLD-only groups, the MAFLD-only group had the highest levels of CRP, WBC, LYMPH, NEUT, MONO, ALB, NLR, and SIRI, whereas the NAFLD-only group had the highest levels of PLR and the overlap-FLD group had the highest levels of LMR and ALI. The non-FLD group had the lowest levels of all 15 SI indicators (Table 3).
Inflammatory status of MAFLD with/without CRP
Considering that CRP was an item in the MAFLD definition, CRP was removed and re-defined MAFLD. Only 10 participants were excluded from the fully defined MAFLD group. No differences in SI indicators were observed after excluding the 10 participants. The Mann–Whitney U test was used to explore the relationship between SI indicators and MAFLD, and no significant differences in SI indicators were found between the re-defined and fully defined MAFLD (Table 4).
Relationship between SI indicators and MAFLD/NAFLD
Logistic regression analyses were used to explore the relationship between SI indicators and MAFLD, and the results are shown in Fig. 2 (a) and Supplementary Table 1. Except for MPV and SII, the ORs of other SI indicators were statistically significant in crude models. After adjusting for age, sex, BMI, smoking history, alcohol drinking history, education, and occupation, CRP, WBC, LYMPH, NEUT, MONO, ALB, PLR, SIRI, LMR, ALI, and CA were positively associated with MAFLD prevalence, and PLR was negatively associated. RCS analysis showed that a linear relationship existed between MPV, ALB, NLR, d_NLR, and PLR and MAFLD, whereas CRP, WBC, LYMPH, NEUT, MONO, SII, SIRI, LMR, ALI, and CA exhibited a non-linear relationship with MAFLD (Supplementary Fig. 1).
The results for the relationship between SI indicators and NAFLD are shown in Fig. 2 (b) and Supplementary table 2. The ORs of CRP, WBC, LYMPH, NEUT, MONO, ALB, PLR, LMR, ALI, and CA were greater than 1.0, and the OR of PLR was less than 1.0 in multivariable-adjusted logistic regression analysis. RCS analysis showed that there was a linear relationship between MPV, ALB, NLR, d_NLR, PLR, SIRI, and LMR and NAFLD, whereas CRP, WBC, LYMPH, NEUT, MONO, SII, ALI, and CA showed a non-linear relationship with NAFLD (Supplementary Fig. 2).
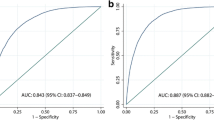
ROC analysis of SI indicators in MAFLD and NAFLD
The AUC, sensitivity, specificity, and positive predictive values of SI indicators for MAFLD and NAFLD are depicted in Fig. 3, Supplementary table 3 and Supplementary Figs. 3 and 4. The AUC values of all SI indicators were lower than 0.7 in both MAFLD and NAFLD. The AUC values of CRP, WBC, LYMPH, ALI and CA were all higher than 0.60 for both MAFLD (0.61, 0.62, 0.63, 0.63 and 0.60 respectively) and NAFLD (0.61, 0.61, 0.62, 0.62 and 0.60 respectively), and their ROCs are presented in Supplementary Fig. 5. The AUCs of MPV and SII were lower than those of other indicators for both MAFLD (0.51 and 0.51, respectively) and NAFLD (0.50 and 0.51, respectively). The sensitivity, specificity, and positive predictive values of LYMPH, ALI, and MPV in MAFLD were 0.69, 0.50, and 0.42, 0.65, 0.55, and 0.43, and 0.15, 0.82, and 0.31, respectively. The sensitivity, specificity, and positive predictive values of LYMPH, ALI, and MPV in NAFLD were 0.69, 0.49, and 0.38, 0.71, 0.47, and 0.38, and 0.86, 0.16, and 0.32, respectively.
Discussion
The current study compared the prevalence and SI levels of MAFLD and NAFLD in a general population. The prevalence of MAFLD was 34.7%, slightly higher than 32.4% of NAFLD. Their overlap** rate was 89.7%, while only 8.3% and 1.9% of participants were MAFLD-only and NAFLD-only, respectively. Of the 15 SI indicators, 12 indicators showed striking differences between MAFLD and non-MAFLD, and between NAFLD and non-NAFLD. Moreover, the MAFLD-only population showed slightly higher SI levels than the overlap-FLD group. Both MAFLD-only and overlap-FLD groups had a worse SI status than the NAFLD-only group. The results were similar after removing CRP from the definition of MAFLD. Among all the SI indicators, LYMPH and ALI were closely associated with MAFLD and NAFLD. However, they still showed poor discrimination ability between MAFLD and non-MAFLD as well as NAFLD and non-NAFLD.
NAFLD is closely associated with the presence and severity of multiple metabolic disorders [33]. With the develo** understanding of the mechanism of NAFLD, the nomenclature of NAFLD has changed to MAFLD, underscoring the underlying pathophysiology of NAFLD as a metabolically driven disease [4, 34]. A recent meta-analysis showed that the prevalence of MAFLD and NAFLD was 39.22% and 38.28%, respectively, and MAFLD identified more FLD than NAFLD [7]. In China, both higher and lower prevalence rates of MAFLD than NAFLD have been reported [ The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Nonalcoholic fatty liver disease Metabolic dysfunction-associated fatty liver disease Systolic blood pressure Diastolic blood pressure Body mass index Waist circumstance Oral glucose tolerance test Fasting blood glucose 2-H post-load blood glucose Triglyceride High-density lipoprotein cholesterol Albumin C-reactive protein Glycosylated hemoglobin Fasting insulin Homeostasis model assessment of insulin resistance White blood cell Lymphocyte Neutrophils Monocyte Platelet Mean platelet volume Type 2 diabetes mellitus Neutrophils-to-lymphocyte ratio Systemic immune inflammation index Systemic immune inflammation response index Li J, Zou B, Yeo YH, Feng Y, **e X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–98. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease: a review of epidemiology, risk factors, diagnosis and management. Intern Med J. 2020;50:1038–47. Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014 (e1991). Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–25. Ayada I, van Kleef LA, Alferink LJM, Li P, de Knegt RJ, Pan Q. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: focusing on the non-overlap groups. Liver Int. 2022;42:277–87. Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-Analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2021. Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, et al. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab (Lond). 2021;18:82. Fan J, Luo S, Ye Y, Ju J, Zhang Z, Liu L, et al. Prevalence and risk factors of metabolic associated fatty liver disease in the contemporary South China population. Nutr Metab. 2021;18:82. Li H, Guo M, An Z, Meng J, Jiang J, Song J, et al. Prevalence and risk factors of metabolic associated fatty liver disease in **nxiang, China. Int J Environ Res Public Health. 2020;17:188. Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107:88–97. Wang X, Wu S, Yuan X, Chen S, Fu Q, Sun Y, et al. Metabolic dysfunction-associated fatty liver disease and mortality among Chinese adults: a prospective cohort study. J Clin Endocrinol Metab. 2022;107:e745-755. Yu C, Wang M, Zheng S, **a M, Yang H, Zhang D, et al. Comparing the diagnostic criteria of MAFLD and NAFLD in the Chinese Population: a Population-based prospective cohort study. J Clin Transl Hepatol. 2022;10:6–16. Zeng J, Qin L, ** Q, Yang RX, Ning G, Su Q, et al. Prevalence and characteristics of MAFLD in Chinese adults aged 40 years or older: a community-based study. Hepatobiliary Pancreat Dis Int. 2022;21:154–61. Lee HW, Wong VW. Changing NAFLD epidemiology in China. Hepatology. 2019;70:1095–8. Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta-analysis. Hepatol Int. 2020;14:259–69. Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71:1851–64. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–41. Chen S, Guo H, **e M, Zhou C, Zheng M. Neutrophil: an emerging player in the occurrence and progression of metabolic associated fatty liver disease. Int Immunopharmacol. 2021;97:107609. Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 2016;150:1704–9. Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349–64. Torre P, Motta BM, Sciorio R, Masarone M, Persico M. Inflammation and fibrogenesis in MAFLD: role of the hepatic immune system. Front Med (Lausanne). 2021;8:781567. Lin SY, Li WC, Yang TA, Chen YC, Yu W, Huang HY, et al. Optimal threshold of homeostasis model assessment of insulin resistance to identify metabolic syndrome in a Chinese population aged 45 years or younger. Front Endocrinol (Lausanne). 2021;12:746747. Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283–98. Joint Committee for Guideline R. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese Guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16:182–241. American Diabetes Association Professional Practice C. 2. Classification and diagnosis of Diabetes: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:17–S38. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell Lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;22:803–10. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8:886–94. ** Z, Hao D, Song Y, Zhuang L, Wang Q, Yu X. Systemic inflammatory response index as an Independent risk factor for ischemic stroke in patients with rheumatoid arthritis: a retrospective study based on propensity score matching. Clin Rheumatol. 2021;40:3919–27. Feng RN, Du SS, Wang C, Li YC, Liu LY, Guo FC, et al. Lean-non-alcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J Gastroenterol. 2014;20:17932–40. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–9. Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol. 2021;19:2161-2171 e2165. Tsutsumi T, Eslam M, Kawaguchi T, Yamamura S, Kawaguchi A, Nakano D, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: generalized estimating equation approach. Hepatol Res. 2021;51:1115–28. Chun HS, Lee M, Lee JS, Lee HW, Kim BK, Park JY, et al. Metabolic dysfunction associated fatty liver disease identifies subjects with cardiovascular risk better than non-alcoholic fatty liver disease. Liver Int. 2023;43(3):608–25. Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease – novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–60. Peiseler M, Tacke F. Inflammatory mechanisms underlying nonalcoholic steatohepatitis and the transition to hepatocellular carcinoma. Cancers. 2021;13:730. Olveira A, Augustin S, Benlloch S, Ampuero J, Suárez-Pérez JA, Armesto S, et al. The essential role of IL-17 as the pathogenetic link between psoriasis and metabolic-associated fatty liver disease. Life. 2023;13(2):419. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46. Tsutsumi T, Nakano D, Kawaguchi M, Hashida R, Yoshinaga S, Takahashi H, et al. MAFLD associated with COPD via systemic inflammation Independent of aging and smoking in men. Diabetol Metab Syndr. 2022;14:14. Duan Y, Pan X, Luo J, **ao X, Li J, Bestman PL, et al. Association of inflammatory cytokines with non-alcoholic fatty liver disease. Front Immunol. 2022;13: 880298. Tong C, Li Q, Kong L, Ni X, Halengbieke A, Zhang S, et al. Sex-specific metabolic risk factors and their trajectories towards the non-alcoholic fatty Liver Disease incidence. J Endocrinol Invest. 2022;45:2233–45. Zhao D, Cui H, Shao Z, Cao L. Abdominal obesity, chronic inflammation and the risk of non-alcoholic fatty liver disease. Ann Hepatol. 2022;28:100726. Zhao Y, **a J, He H, Liang S, Zhang H, Gan W. Diagnostic performance of novel inflammatory biomarkers based on ratios of laboratory indicators for nonalcoholic fatty liver disease. Front Endocrinol (Lausanne). 2022;13:981196. Thanks to the participants, interviewers of the Fuqing cohort study and workers from local government officials, the Fuqing Hospital, and the Fifth Hospital of Fuqing City, who all have been providing generous support for the day-to-day research field work operation. This study was jointly supported by the National Natural Science Fund of the People's Republic of China (grant number: 82103923), General Program of the Natural Science Foundation of Fujian Province (grant number: 2022J01711), Government of Fuqing city (grant number: 2019B003), Department of Science and Technology of Fujian, China (grant number: 2019Y9021), and High-level Talents Research Start-up Project of Fujian Medical University (No. XRCZX2021026, No. XRCZX2017035 and No. XRCZX2020034). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Weimin Ye: Conceptualization (formulation or evolution of overarching research goals and aims). Shanshan Du: Project administration (Management and coordination responsibility for the research activity planning and execution). Qingdan Liu: wrote the manuscript. Meilan Han, Meilan Li, **aoyin Huang; Wanxin Li, Haiying He, Ruimei, Wenxin Zheng, Jun Chen, Zhijian Hu: Resources (Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools).All authors reviewed the manuscript. The study protocol was approved by the ethical committee of the Fujian Medical University (approval number: 2020–58), and written informed consent was obtained from all participants. Not applicable. The authors declare no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Supplementary Table 1. Logistic regression analysis of the relationship between systemic inflammatory indicators and MAFLD. Supplementary Table 2. Logistic regression analysis of the relationship between systemic inflammatory indicators and NAFLD. Supplementary Table 3. Diagnostic accuracy of systemic inflammatory indicators for MAFLD and NAFLD. Supplementary Figure 1. RCS analysis of systemic inflammatory indicators and MAFLD. Supplementary Figure 2. RCS analysis of systemic inflammatory indicators and NAFLD. Supplementary Figure 3. Diagnostic accuracy of systemic inflammatory indicators for MAFLD. Supplementary Figure 4. Diagnostic accuracy of systemic inflammatory indicators for NAFLD. Supplementary Figure 5. Comparison of multiple indicators ROC for MAFLD (a) and NAFLD (b). Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. Liu, Q., Han, M., Li, M. et al. Shift in prevalence and systemic inflammation levels from NAFLD to MAFLD: a population-based cross-sectional study.
Lipids Health Dis 22, 185 (2023). https://doi.org/10.1186/s12944-023-01947-4 Received: Accepted: Published: DOI: https://doi.org/10.1186/s12944-023-01947-4Availability of data and materials
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Additional information
Publisher’s Note
Supplementary Information
Additional file 1:
Rights and permissions
About this article
Cite this article
Keywords