Abstract
Purpose
Previous studies have shown that at a similar body mass index, Middle Eastern immigrants are more insulin resistant and at higher risk for type 2 diabetes (T2D) than native Europeans. Insulin resistance is strongly associated with disturbed fat metabolism and cardiovascular disease (CVD). However, fat metabolism is poorly investigated comparing Middle Eastern and European ethnicities.
Methods
This observational study included 26 Iraqi and 16 Swedish-born men without T2D or clinical risk factors for CVD. An oral fat tolerance test (OFTT) was performed, where plasma triglycerides (p-TG) were measured for 6 h. mRNA expression and adipocyte size were measured in subcutaneous adipose tissue biopsies collected prior to OFTT, and magnetic resonance imaging was conducted to assess body fat distribution.
Results
The median p-TG accumulation was higher and the clearance slower among Iraqis than Swedes. None of the groups reached their fasting p-TG (Iraqis 1.55 mmol/l; Swedes 0.95 mmol/l) after 6 h (Iraqis p-TG 3.10 mmol/l; Swedes p-TG 1.50 mmol/l). Adipocyte size, mRNA expression, and fat accumulation in the liver, muscle and abdomen were similar in both groups.
Conclusion
Postprandial p-TG levels rather than fat distribution may reflect early signs of disturbed fat metabolism in Iraqi immigrants without CVD risk factors.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) and insulin resistance are associated with an increased risk of cardiovascular disease (CVD) and mortality [1]. T2D is increasing in most areas of the world, with the Middle East (ME) having among the highest prevalence, varying between 7 and 12% [2]. Immigrants from ME constitute the largest non-European immigrant population in Sweden. Interestingly, the Iraqi immigrant population is more insulin resistant and displays relative insulin deficiency in the nondiabetic stages compared to native Swedes [3, 4]. However, the underlying mechanisms for these observations are poorly understood.
Metabolic abnormalities leading to T2D are thought to be secondary to overweight and insulin resistance [5]. Specifically, abnormal lipid metabolism in insulin resistance is characterized by elevated fasting levels of plasma triglycerides (p-TGs), a decrease in high-density lipoprotein cholesterol, and an increase in free fatty acid levels [6]. The daily impact of fasting (i.e., 10 hours without intake of food or drinks other than water) is fairly short in real life, and individuals are in the postprandial state for most of the day. Hence, postprandial, rather than fasting, p-TG may be a more informative marker for an individual’s capacity to metabolize lipids following a meal [7].
A decreased capacity to store excess energy in subcutaneous adipocytes leads to ectopic fat accumulation in the visceral fat depot, liver and peripheral muscle tissue, which in turn contributes to increased insulin resistance [8]. This notion is supported by observations that ectopic fat accumulation, rather than the amount of subcutaneous adipose tissue (SAT), predicts the future development of T2D [9]. Furthermore, an increased amount of visceral adipose tissue (VAT) serves as a marker of increased ectopic fat in other locations, including the heart [10]. Other ectopic fat depots are the intermuscular adipose tissue (IMAT), found beneath the fascia and within the muscles, and so-called intramyocellular lipids (IMCL). An increase in either IMAT or IMCL levels has been associated with insulin resistance [11, 12].
The release and storage of fatty acids, as well as the differentiation of adipocytes, is tightly regulated by insulin [13]. Adipose tissue expands by increasing both adipose cell size (hypertrophy) and cell number (hyperplasia). Previous studies have shown that enlarged adipocytes have an impaired insulin response [14, 15] and that increased adipose cell size is positively correlated with impaired systemic insulin sensitivity and glucose tolerance [16]. Furthermore, the expression and activity of adipose tissue lipoprotein lipase are affected in insulin-resistant individuals and have been proposed to contribute to a delayed clearance of postprandial and fasting p-TG levels [17].
Pronounced insulin resistance and relative insulin deficiency reflect metabolic abnormalities in the nondiabetic stages in ME immigrants. We hypothesized that healthy ME men would differ in fat tolerance and accumulation of ectopic and visceral fat compared with healthy, native Swedish men. To address these hypotheses, we compared 1) the p-TG response to a standardized fat meal, 2) adipocyte size, 3) mRNA expression of key regulators of lipid and glucose metabolism, and 4) fat distribution in nonobese, nonsmoking men born in Iraq or Sweden without a history of T2D or CVD.
Material and methods
Study population
The MEDIM cohort is a cross-sectional study conducted 2010–2012 including Iraqi and Swedish-born residents of Malmö, Sweden (30–75 years of age, residing in the same geographical area in Malmö, matched for age) as previously described [18]. A subset of men participating in the MEDIM study, where the health examination showed that they were never smokers, nonobese (body mass index (BMI) < 30 kg/m2), and without a history of hypertension, T2D, hyperlipidemia or CVD, were invited to participate in the study (either March to May 2017 or March to May 2018).
Physical activity was self-assessed using the international physical activity questionnaire (IPAC) capturing time (minutes) physically active over the last 7 days [19]. Those who were less active than 150 minutes were considered physically inactive.
Food habits were also self-assessed using National Board of Health and Welfare guidelines for methods of preventing disease [20], capturing frequency of intake of vegetables, fruit and berries, fish and seafood as well as intake of sweet food and drinks (such as bakery, chocolate, dessert, sweets, etc.). A diet index can capture the quality of food intake, and low points < 9 are considered unhealthy eating habits [20].
Participants with at least three of the following were considered to have metabolic syndrome: systolic blood pressure > 140 mmHg; diastolic blood pressure > 90 mmHg; fasting TG > 1.7 mmol/l; insulin resistance (HOMA-IR above the 4th quartile, i.e., > 2.6) or BMI > 30 kg/m2 [21].
All subjects completed a magnetic resonance imaging (MRI) exclusion form with standard MRI patient safety criteria. Only subjects without any contraindications for MRI (defined as noncompatible metallic implants or devices or claustrophobia) were eligible.
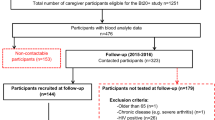
In total, 259 nonobese males (born in Iraq n = 140; born in Sweden n = 119) without CVD risk factors were invited to participate in the study (Fig. 1). A total of 42 men, 26 Iraqi born and 16 Swedish born, participated in the study, including fasting samples and oral fat tolerance tests (OFTTs). Biopsies were conducted in 25 Iraqi and 16 Swedish-born men. In 13 Iraqi-born and 11 Swedish-born men, mRNA was assessed, and adipocyte size was measured in 15 Iraqi-born and 8 Swedish-born men.
In total, 23 men born in Iraq and 15 men born in Sweden were examined by MRI. Due to technical errors, four thigh data sets (two Swedish-born and two Iraqi-born), eight abdominal data sets (four Swedish born and four Iraqi born), and eleven spectra (five Swedish born and six Iraqi born) could not be evaluated and were therefore excluded from the MRI results.
Laboratory assessments
Anthropometrics, body composition, and laboratory data were assessed by trained Swedish research nurses with the presence of an Arabic-, English- and Swedish-speaking interpreter. Body weight was measured using an electronic scale (Coline, 34–5062 RTC3010, China), while participants were requested to wear light clothing and remove shoes. A wall-mounted stadio-meter was used to measure height. Waist circumference was measured to the nearest cm in the standing position after gentle expiration. A tape measure was placed around the bare midriff of each participant, and the waist circumference was measured midway between the lower border of the rib cage and the superior border of the iliac crest. Hip circumference was measured at the maximum level, to the nearest cm, using a tape measure. Bioelectrical impedance analysis was used to assess fat mass and fat % (Tanita Pro, Tanita Europe BV, The Netherlands).
Fasting blood samples were collected and analyzed for blood glucose, total cholesterol, triglycerides (p-TG), high-density lipoprotein cholesterol (p-HDL) and low-density lipoprotein cholesterol (p-LDL). Homeostatic model assessment (HOMA) was used to estimate both insulin resistance (HOMA-IR) and beta cell function (HOMA-beta) [22].
Indices were calculated as follows:
HOMA - IR = fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5.
HOMA - β = [20 × fasting insulin (mU/L)/[fasting glucose (mmol/L) - 3.5].
Oral fat tolerance test (OFTT)
An OFTT was conducted after a 12-h overnight fast [7]. A standard fat meal of 4425 kJ (1057 kcal) was prepared from 100.0 g vanilla ice cream, 150.0 g cream, 70.0 g chocolate sauce, and 35.0 g natural cottage cheese, in total containing 5 energy percent (E%) protein (13.5 g), 66 E% fat (78.7 g) and 29 E% carbohydrates (74.0 g). For lacto-intolerant individuals, there was a corresponding lactose-free alternative of 4433 kJ (1059 kcal), containing 14.0 g protein, 78.5 g fat (of which 49.8 g was saturated fat) and 74.0 g carbohydrates. The participants were instructed to drink the meal within 15 minutes and were asked to keep physical activity to a minimum and not drink or eat during the following 6 h. Blood samples were drawn at baseline (fasting samples), 30 minutes, 1 h, 2 h, 3 h, 4 h and 6 h for determination of p-TG.
Fat distribution in the liver, thigh and abdomen
To examine fat accumulation in the liver and thigh as well as the abdominal adipose tissue distribution, subjects from both groups underwent magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS) examinations of the liver, abdomen, and thigh using a 3 T MRI scanner (TIM Trio, Siemens Healthineers, Erlangen, Germany). To estimate the IMCL/water concentration ratio in the thigh, two point resolved spectral selection techniques (PRESS) were acquired: one with water suppression for the estimation of the IMCL signal amplitude and one without water suppression for the estimation of the water signal amplitude.
Fat/water imaging using six echoes was performed to obtain proton density fat fraction (PDFF) maps of the liver and muscle (thigh) [23]. Semiautomated approaches were used to outline the liver, SAT of the thigh, and thigh muscle regions of interest (ROIs). Using the estimated PDFF maps of the left thigh, the IMAT and SAT-thigh volumes were calculated by summing the PDFFs within the respective ROIs and multiplying by the voxel size. IMAT was defined as fat within the muscle fascia [24]. Fat-only images from the volumetric interpolated breath-hold examination (VIBE) acquisition were used to estimate the volumes of abdominal SAT and VAT. A semiautomated region-grow method was used to outline the subcutaneous depot, while the visceral depot was manually outlined to avoid the spinal cord. To further separate the VAT from other structures and organs within the abdominal cavity, a threshold was used. Only voxels above the cutoff value were considered adipose tissue and included in the VAT ROI [25, 26].
The MRS analysis was conducted using JMRUI software [27, 28]. The amplitude of IMCL was estimated from the water-suppressed spectra, while the water amplitude was assessed from the spectra without water suppression. The ratio between the concentration of IMCL and water was then calculated as suggested by Boesch et al. [29]. All the MRI and MRS sequences and corresponding settings are shown in Table 2.
Adipocyte-size distribution and mRNA expression in subcutaneous fat
Subcutaneous adipose tissue samples were obtained through a needle biopsy following local anesthesia at the right side of the umbilicus at a distance of approximately 7–10 cm. Samples were analyzed for adipocyte-size distribution using a Beckman-Coulter counter after osmium fixation as described previously [30]. For mRNA analysis, samples were immediately snap-frozen in liquid nitrogen, lysed and homogenized in Qiazol™ lysis reagent (Qiagen). RNA was isolated using an RNeasy® Mini Kit (Qiagen) according to the manufacturer’s recommendations. RT-qPCRs were performed using the Quantifast SYBR Green RT–PCR kit (Qiagen) and Quantitect primer assays for 18S, SLC2A4, COL1A1, LPL, and CIDEA according to the manufacturer’s instructions. Primer sequences are considered proprietary information by Qiagen. mRNA expression levels were measured using a StepOnePlus real-time thermal cycler (Applied Biosystems, Waltham, USA) and quantitated using the ΔΔCT method as described by Livak and Schmittgen [31]. 18S mRNA expression levels were used for normalization.
Statistical analyses
Analyses were performed using SPSS version 24 (IBM). Comparisons of baseline characteristics between the Swedish and Iraqi populations were assessed using the independent-sample T test or the Mann–Whitney U test for normal and nonnormally distributed continuous variables, respectively (Table 1). Data on total area under the curve (tAUC), incremental AUC (iAUC) of postprandial p-TG concentration from Iraqis and Swedes were Log10 transformed (i.e., residuals tested for normality) and analyzed by general linear models (GLM) with age and BMI as covariates. Two-sided P values < 0.05 were considered statistically significant.
Wilcoxon rank sum tests were conducted to compare mRNA expression, MRI-estimated PDFFs, adipose tissue volumes, and IMCL/water concentrations of the Iraqi-born men to those of the Swedish-born men. The adipose cell size distribution is illustrated by plotting the average frequency within each bin measured from all subjects in each group (Iraqi versus Swedish). Maximum adipocyte size was collected at the peak value in the large adipose cell population for each individual.
Results
Baseline characteristics
There were no differences in lifestyle habits between the groups (Table 1). However, Swedish-born men presented with higher systolic blood pressure than Iraqi-born men. Furthermore, two Swedish but no Iraqi men fulfilled the criteria for metabolic syndrome. No differences in baseline characteristics were observed between the two groups, except for lower HDL and ApoA1 levels in Iraqi compared to native Swedish participants (Iraqis 1.00 mmol/l HDL, Swedes 1.35 mmol/l HDL, P = 0.001; Iraqis 1.20 g/l ApoA1, Swedes 1.45 g/l ApoA1, P = 0.003) (Table 1). No adjustments were made since the groups were well matched.
Oral fat tolerance test (OFTT)
The median fasting p-TG values were within the normal range at baseline (< 1.7 mmol/l) and peaked at 3 h post-ingestion in both groups (Fig. 2). However, during the 6-h load, the p-TG accumulation was generally higher in the Iraqi born group, with higher p-TG concentrations at 2 h, 3 h, 4 h and 6 h, higher tAUC and iAUC than in the Swedish born group (Table 1, Fig. 2). Six hours after ingestion, none of the groups had reached their fasting p-TG value (Iraqis 3.10 mmol/l versus Swedes 1.50 mmol/l, P = 0.049 (Table 1).
Plasma triglyceride concentrations during an oral fat tolerance test (OFTT). a Comparison of Iraqi immigrants and native Swedes assessed as estimated marginal means (95% confidence interval). b Same as in a, restricted to participants with BMI 25–30 kg/m2. a-b) Blue lines represent Iraqis, purple lines represent Swedes
Fat distribution in the liver, thigh and abdomen
MRI measurements revealed no difference in liver PDFF (3.84% vs. 3.27%, P = 0.2), VAT volume (2500 mL vs. 2652 mL, p = 0.7), or abdominal SAT volume (2378 mL vs. 2387 mL, P = 0.8) between Iraqis and Swedes (Fig. 3a-c). Examples of MRI and MRS data and corresponding ROIs are presented in Fig. 4. Furthermore, both IMAT volumes (28 mL vs. 26 mL, P = 0.8) and SAT volume in thighs (114 ml versus 145 ml, P = 0.1) were similar between Iraqis and Swedes (Fig. 3e-f). Likewise, no difference was found in the concentration of IMCL and water ratio estimated with MRS (0.8*10− 4 vs. 1.3*10− 4, P = 0.5) (Fig. 3h) or the ratio between VAT and abdominal SAT (0.91 versus 0.76, P = 0.3) and IMAT and thigh SAT (0.17 versus 0.21, P = 0.2) (Fig. 3d and g).
Comparison of fat accumulation in various fat depots between Iraqi immigrants and native Swedes, estimated with magnetic resonance imaging (MRI). Boxplots of the magnetic resonance imaging (MRI)-estimated a liver proton density fat fraction (PDFF), b visceral adipose tissue (VAT) volume, c abdominal subcutaneous adipose tissue (SAT) volume and d VAT/abdominal SAT ratio. Shown are the boxplots of the estimated e intermuscular adipose tissue (IMAT) volume, f thigh SAT volume, g IMAT/thigh SAT ratio, and h) ratio between the concentration of intramyocellular lipids (IMCL) and water of the semitendinosus muscle of the left thighs
Example of MRI imaging. Examples of a acquired liver VIBE (in-phase image), b calculated liver PDFF, c acquired abdominal VIBE (fat image), d visceral (yellow) and subcutaneous (green) adipose tissue ROIs, e acquired thigh VIBE (in-phase image), f subcutaneous adipose tissue (green) and IMAT (yellow) ROIs, g calculated thigh PDFF images, and h a water-suppressed PRESS spectrum of the semitendinosus muscle
Adipocyte-size distribution and mRNA expression in subcutaneous fat
Cell-size analysis revealed a bimodal adipocyte-size distribution in both the Swedish and Iraqi born groups (Fig. 5a), with a fraction of small cells, defined by the nadir [32], which is the lowest point between the two cell populations and a fraction of large cells (right of the nadir). The distribution curves were similar between Iraqi and Swedes (Fig. 5a), where the large cell fraction ranged between 75 and 200 μm, with a mean peak cell size of ~ 105 and 103 μm (Iraqis and Swedes, respectively) (Fig. 5b). Furthermore, we observed no differences in the mRNA expression of key regulators of lipid and glucose metabolism (SLC2A4, COL1A1, LPL, CIDEA), but there was a trend toward higher mRNA expression of LPL in Iraqi individuals (Fig. 5c).
Subcutaneous adipose tissue analyses. The adipocyte-size distribution of fixed, intact subcutaneous adipose tissue was measured using a coulter counter, ~ 6000 counts/sample, and each sample was run in duplicate. a The graph displays the average distribution curves from n = 15 Iraqi and n = 8 Swedish subjects. b The fraction of small cells is defined to the left of the nadir (arrow), and the fraction of large cells is defined to the right of the nadir. c mRNA expression of COL1A1, LPL, SLC2A4, and CIDEA, measured by qPCR, n = 11–13 Iraqi and n = 8–11 Swedish subjects. Data are normalized to 18S expression
Discussion
In the current study, we show that the Iraqi-born participants had higher postprandial TG levels than Swedish-born participants, even though participants in both groups were nonobese (BMI < 30 kg/m2) and nondiabetic. Information about postprandial TG levels between ethnic groups is scarce [33,34,35], and to the best of our knowledge, our study is the first to compare postprandial TG between men of Middle Eastern and European ancestry. Bower et al. found that lean African American women had lower fasting p-TG levels as well as low postprandial p-TG levels compared to lean Caucasians; however, no differences were seen between obese African American and obese Caucasians in p-TG levels [33]. When comparing young black and white males, the overall postprandial p-TG responses were similar between the groups, although white men showed a higher incremental response during two to 4 h following the fat load [35]. Furthermore, the lipid response to a high-fat meal did not differ between South Asians, Latin Americans and Northern Europeans in a small pilot study [34]. On the other hand, there are several studies focusing on the effects of OFTT without analyzing the influence of ethnicity. In these studies, postprandial hypertriglyceridemia was prolonged in obese adults with high insulin resistance [47]. Interestingly, the OFTT unmasked changes in lipid metabolism between Iraqis and Swedes only noticeable in the postprandial state. Therefore, we propose that the OFTT may be a way to identify metabolically healthy Iraqis at risk of develo** metabolic disturbances.
Limitations
The main strengths of our study are the well-defined populations from the same sociodemographic areas and that the groups are well balanced in anthropometrics. In addition, our study is based on an OFTT, which has been shown to better reflect postprandial p-TG than other methods [7]. Limitations involve the relatively small study population with fewer participants born in Sweden. Furthermore, our study was based on young and middle-aged men, and the results may therefore not be generalizable to other populations.
Conclusion
In conclusion, we found that despite having normal fasting p-TG levels and similar body fat distribution, healthy Iraqi male immigrants conducting an OFTT displayed a high p-TG peak and a delayed postprandial p-TG clearance compared with Swedes. Our findings pinpoint differences in fat metabolism across ethnicities, which needs to be further investigated in a larger study.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available upon request by the authors, with undue reservation.
Abbreviations
- BMI:
-
Body mass index
- HOMA:
-
Homeostasis model assessment
- iAUC:
-
Incremental area under the curve
- IMCL:
-
Intramyocellular lipids
- IMAT:
-
Intermuscular adipose tissue
- ME:
-
Middle eastern
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- OFTT:
-
Oral fat tolerance test
- PDFF:
-
Proton density fat fraction
- p-TG:
-
Plasma triglycerides
- p-HDL:
-
Plasma high-density protein
- p-LDL:
-
Plasma low-density protein
- ROI:
-
Region of interest
- SAT:
-
Subcutaneous adipose tissue
- VAT:
-
Visceral adipose tissue
- tAUC:
-
Total area under the curve
- T2D:
-
Type 2 diabetes
References
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34.
IDF Diabetes Atlas: International Diabetes Federation 2019:Available from: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf.
Bennet L, Groop L, Franks PW. Ethnic differences in the contribution of insulin action and secretion to type 2 diabetes in immigrants from the Middle East compared to native swedes. Diabetes Res Clin Pract. 2014;105(1):79–87.
Bennet L, Stenkula K, Cushman SW, Brismar K. BMI and waist circumference cut-offs for corresponding levels of insulin sensitivity in a middle eastern immigrant versus a native Swedish population - the MEDIM population based study. BMC Public Health. 2016;16(1):1242.
Khetan AK, Rajagopalan S. Prediabetes. Can J Cardiol. 2018;34(5):615–23.
Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–99.
Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258–70.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28.
Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–9.
Graner M, Siren R, Nyman K, Lundbom J, Hakkarainen A, Pentikainen MO, et al. Cardiac steatosis associates with visceral obesity in nondiabetic obese men. J Clin Endocrinol Metab. 2013;98(3):1189–97.
Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:309570.
Coen PM, Goodpaster BH. Role of intramyocelluar lipids in human health. Trends Endocrinol Metab. 2012;23(8):391–8.
Cignarelli A, Genchi VA, Perrini S, Natalicchio A, Laviola L, Giorgino F. Insulin and insulin receptors in adipose tissue development. Int J Mol Sci. 2019;20(3).
Franck N, Stenkula KG, Ost A, Lindstrom T, Stralfors P, Nystrom FH. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia. 2007;50(8):1716–22.
Laurencikiene J, Skurk T, Kulyte A, Heden P, Astrom G, Sjolin E, et al. Regulation of lipolysis in small and large fat cells of the same subject. J Clin Endocrinol Metab. 2011;96(12):E2045–9.
Acosta JR, Douagi I, Andersson DP, Backdahl J, Ryden M, Arner P, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–70.
Panarotto D, Remillard P, Bouffard L, Maheux P. Insulin resistance affects the regulation of lipoprotein lipase in the postprandial period and in an adipose tissue-specific manner. Eur J Clin Investig. 2002;32(2):84–92.
Bennet L, Groop L, Lindblad U, Agardh CD, Franks PW. Ethnicity is an independent risk indicator when estimating diabetes risk with FINDRISC scores: a cross sectional study comparing immigrants from the Middle East and native Swedes. Prim Care Diabetes. 2014;8(3):231-8.
International Physical Activity Questionnaire Available from: https://sites.google.com/site/theipaq/.
Disease NGfMoP. Available from: https://untobaccocontrol.org/impldb/wp-content/uploads/reports/sweden_annex5_national_guidelines_for_methods_of_preventing_disease_2011.pdf
Charity HU. Available from: https://www.heartuk.org.uk/genetic-conditions/metabolic-syndrome.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Yu H, Shimakawa A, Hines CD, McKenzie CA, Hamilton G, Sirlin CB, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66(1):199–206.
Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido-Gamio J, et al. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012;35(4):899–907.
Marzetti M, Brunton T, McCreight L, Pearson E, Docherty S, Gandy SJ. Quantitative MRI evaluation of whole abdomen adipose tissue volumes in healthy volunteers-validation of technique and implications for clinical studies. Br J Radiol. 2018;91(1087):20180025.
Storz C, Heber SD, Rospleszcz S, Machann J, Sellner S, Nikolaou K, et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br J Radiol. 2018;91(1089):20170808.
Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12(2–3):141–52.
Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997;129(1):35–43.
Boesch C, Machann J, Vermathen P, Schick F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006;19(7):968–88.
Li Y, Periwal V, Cushman SW, Stenkula KG. Adipose cell hypertrophy precedes the appearance of small adipocytes by 3 days in C57BL/6 mouse upon changing to a high fat diet. Adipocyte. 2016;5(1):81–7.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
McLaughlin T, Sherman A, Tsao P, Gonzalez O, Yee G, Lamendola C, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(8):1707–15.
Bower JF, Deshaies Y, Pfeifer M, Tanenberg RJ, Barakat HA. Ethnic differences in postprandial triglyceride response to a fatty meal and lipoprotein lipase in lean and obese African American and Caucasian women. Metabolism. 2002;51(2):211–7.
Cruz ML, Evans K, Frayn KN. Postprandial lipid metabolism and insulin sensitivity in young Northern Europeans, South Asians and Latin Americans in the UK. Atherosclerosis. 2001;159(2):441–9.
Friday KE, Srinivasan SR, Elkasabany A, Dong C, Wattigney WA, Dalferes E Jr, et al. Black-white differences in postprandial triglyceride response and postheparin lipoprotein lipase and hepatic triglyceride lipase among young men. Metabolism. 1999;48(6):749–54.
Wang F, Lu H, Liu F, Cai H, **a H, Guo F, et al. Consumption of a liquid high-fat meal increases triglycerides but decreases high-density lipoprotein cholesterol in abdominally obese subjects with high postprandial insulin resistance. Nutr Res. 2017;43:82–8.
Larsen MA, Goll R, Lekahl S, Moen OS, Florholmen J. Delayed clearance of triglyceride-rich lipoproteins in young, healthy obese subjects. Clin Obes. 2015;5(6):349–57.
Ai M, Tanaka A, Ogita K, Sekinc M, Numano F, Numano F, et al. Relationship between plasma insulin concentration and plasma remnant lipoprotein response to an oral fat load in patients with type 2 diabetes. J Am Coll Cardiol. 2001;38(6):1628–32.
Schneeman BO, Kotite L, Todd KM, Havel RJ. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc Natl Acad Sci U S A. 1993;90(5):2069–73.
Karpe F, Steiner G, Olivecrona T, Carlson LA, Hamsten A. Metabolism of triglyceride-rich lipoproteins during alimentary lipemia. J Clin Invest. 1993;91(3):748–58.
Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. 2012;55(10):2622–30.
Siddiqui F, Kurbasic A, Lindblad U, Nilsson PM, Bennet L. Effects of a culturally adapted lifestyle intervention on cardio-metabolic outcomes: a randomized controlled trial in Iraqi immigrants to Sweden at high risk for type 2 diabetes. Metabolism. 2017;66:1–13.
Bennet L, Nilsson C, Mansour-Aly D, Christensson A, Groop L, Ahlqvist E. Adult-onset diabetes in middle eastern immigrants to Sweden: novel subgroups and diabetic complications - the ANDIS cohort diabetic complications and ethnicity. Diabetes Metab Res Rev. 2021;37(6):e3419.
Lonn M, Mehlig K, Bengtsson C, Lissner L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010;24(1):326–31.
Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–506.
Domingo-Espin J, Lindahl M, Nilsson-Wolanin O, Cushman SW, Stenkula KG, Lagerstedt JO. Dual actions of apolipoprotein A-I on glucose-stimulated insulin secretion and insulin-independent peripheral tissue glucose uptake Lead to increased heart and skeletal muscle glucose disposal. Diabetes. 2016;65(7):1838–48.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35.
Acknowledgments
We are indebted to Margit Bergström, Sophie Örjansdottir, and Ulrika Björklund Brogren for their excellent work in examining the participants and collecting data.
Funding
Open access funding provided by Lund University. This study was funded by grants from Lund University (ALF grants 20101641, 20101837, and 162641) and the Swedish Research Council (Linné grant to LUDC 349–2006-237, Exodiab 2009- IRC15–0067). The Skåne University funds against cancer (Allmäna Sjukhusets Fonder för bekämpande av. Cancer).
Author information
Authors and Affiliations
Contributions
LB contributed to the study concept and design. LEK, LB, KS, WA, MLO, LT and SM contributed to the data acquisition. LEK, LT and LB performed the statistical analysis. LB, LT, KS, CF, NW, WA and MLO provided material support and technical support. LEK, LB, LT, KS, CF, NW, WA, MLO and SM drafted the manuscript. All authors contributed to the analysis and interpretation of the data and critically revised the manuscript. All authors have approved the final version of the manuscript. LB, KS and SM obtained the funding.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Lund University, Sweden (approval no 2015/507). All participants were provided both oral and written information about the study and signed a written consent form before participation. They were informed that participation was not mandatory and that they at any time could leave the study without explanation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stenkula, K.G., Klemendz, L.E., Fryklund, C. et al. Postprandial triglyceride levels rather than fat distribution may reflect early signs of disturbed fat metabolism in Iraqi immigrants. Lipids Health Dis 21, 68 (2022). https://doi.org/10.1186/s12944-022-01679-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01679-x