Abstract
Background
Drug repurposing otherwise known as drug repositioning or drug re-profiling is a time-tested approach in drug discovery through which new medical uses are being established for already known drugs. Antibiotics are among the pharmacological agents being investigated for potential anti-SARS-COV-2 activities. The antibiotics are used either to resolve bacterial infections co-existing with COVID-19 infections or exploitation of their potential antiviral activities. Herein, we aimed to review the various antibiotics that have been repositioned for the management of COVID-19.
Methods
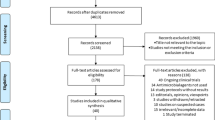
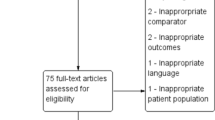
This literature review was conducted from a methodical search on PubMed and Web of Science regarding antibiotics used in patients with COVID-19 up to July 5, 2020.
Results
Macrolide and specifically azithromycin is the most common antibiotic used in the clinical management of COVID-19. The other antibiotics used in COVID-19 includes teicoplanin, clarithromycin, doxycycline, tetracyclines, levofloxacin, moxifloxacin, ciprofloxacin, and cefuroxime. In patients with COVID-19, antibiotics are used for their immune-modulating, anti-inflammatory, and antiviral properties. The precise antiviral mechanism of most of these antibiotics has not been determined. Moreover, the use of some of these antibiotics against SARS-CoV-2 infection remains highly controversial and not widely accepted.
Conclusion
The heavy use of antibiotics during the COVID-19 pandemic would likely worsen antibiotic resistance crisis. Consequently, antibiotic stewardship should be strengthened in order to prevent the impacts of COVID-19 on the antibiotic resistance crisis.
Similar content being viewed by others
Introduction
In December 2019, a pneumonia like disease of unknown cause emerged in Wuhan, an emerging business hub located in the Hubei province of China [1]. The disease was caused by a highly transmissible, hitherto undescribed beta-coronavirus, the SARS-coronavirus-2 (SARS-CoV-2) [2, 3]. The disease rapidly spread globally prompting the World Health Organisation (WHO) to declare it a global pandemic in March, 2020 [4]. As of 24th November 2020, 59,175,309 laboratory-confirmed COVID-19 cases were reported worldwide, with 1,396,403 deaths [5].
The rising biological, clinical, and socio-economic impacts of this COVID-19 diseases underscore the urgent need for effective resolution of this crisis [6, 7]. Currently, there is no specific vaccine or an approved antiviral for its effective treatment, several strategies are however being explored [3]. Drug repurposing offers a quick and cost-effective strategy to achieve this [8]. Drug repurposing otherwise known as drug repositioning or drug re-profiling is a time-tested approach in drug discovery through which new medical uses are being established for already known drugs, including approved, discontinued, shelved and experimental drugs [8]. This approach offers considerable advantage over the search for novel molecules. The advantages of drug repurposing have been summarised in a published review article on drug repurposing [8]. This approach has been successful used to brought back several drugs to the market [9]. Zidovudine for example, a well-known antiviral drug active against human immunodeficiency virus (HIV) has been shown to demonstrate in-vitro activity against colistin-resistant and carbapenem-resistant isolates [10]. Similarly, some anti-cancer drugs have been successfully repurposed for treatment of resistant bacterial infections [11]. Other successful examples abound in the literature.
Currently, various pharmacological agents are being investigated for potential use in the clinical management of coronavirus diseases [12,13,14,81]. However, no in-vitro or human clinical trial has been conducted to establish the proprieties of this finding.
Conclusion
Antibiotic repurposing is one of the therapeutic strategies being employed in the clinical management of COVID-19. This is aimed at either the resolution of any bacterial infections co-existing with the COVID-19 infections or exploitation of its potential antiviral properties. Though some of these antibiotics have shown promising results, their use remains highly controversial and not widely accepted. Moreover, the precise antiviral mechanism of most of these antibiotics has not yet been determined. Considering the positive association between heavy antibiotic use and worsening of antibiotic resistance crisis, efforts should be made to strengthen antibiotic stewardship at both national and sub-national levels so as to reduce the long and short impact of antibiotic use in COVID-19 on the antibiotic resistance crisis. Also, data are needed to increase the body of evidence and the clinicians’ confidence in the use of antibiotics for COVID-19 diseases.
Availability of data and materials
All relevant data are available within the paper. https://doi.org/10.6084/m9.figshare.12690077.v1.
References
Zhu N, Zhang D, Wang W, Li X, Bo Yang JS, Ma X, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33.
Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–63.
Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87:281–6.
World Health Organization (WHO). WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020. WHO. 2020. https://www.who.int/director-general/speeches/detail/. Accessed 9 Sept 2020.
University JH. COVID-19 Map. Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html. Accessed 9 Sept 2020.
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-jabir A. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020;78:185–93.
Amzat J, Aminu K, Kolo VI, Akinyele AA, Ogundairo JA, Danjibo CM. Coronavirus outbreak in Nigeria: burden and socio-medical response during the first 100 days. Int J Infect Dis. 2020;98:1–16.
Talevi A, Bellera CL. Expert opinion on drug discovery challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov. 2020;15:397–401.
Jourdan J. Drug repositioning: a brief overview. J Pharm Pharmacol. 2020. https://doi.org/10.1111/jphp.13273.
Peyclit L, Baron SA, Yousfi H, Rolain J, Peyclit L, Baron SA, et al. Zidovudine: a salvage therapy for mcr-1 plasmid-mediated colistin-resistant bacterial infections? To cite this version: HAL Id: hal-01858892. Int J Antimicrob Agents. 2019;52:11–3.
Soo V, Quezada H, Perez B, Martinez-vazquez M. Repurposing of anticancer drugs for the treatment of bacterial infections. Curr Top Med Chem. 2017;17:1157–76.
Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4539–47.
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949.
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2007016.
Zhou Q, Chen V, Shannon CP, Wei X-S, **ang X, Wang X, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11:1061. https://doi.org/10.3389/fimmu.2020.01061.
Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–75.
Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005.
Wu Q, **ng Y, Shi L, Li W, Gao Y, Pan S, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020;146:e20200961.
Bahal N, Nahata MC. The new macrolide antibiotics: azithromycin, clarithromycin, dirithromycin, and roxithromycin. Ann Pharmacother. 1992;26:46–55.
Madrid PB, Panchal RG, Warren TK, Shurtleff AC, Endsley AN, Green CE, et al. Evaluation of ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1:317–26.
Li C, Zu S, Deng Y-Q, Li D, Parvatiyar K, Quanquin N, et al. Azithromycin protects against Zika virus Infection by upregulating virus-induced type I and III interferon responses. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/AAC.00394-19.
Iannetta M, Ippolito G, Nicastri E. Azithromycin shows anti-Zika virus activity in human glial cells. Antimicrob Agents Chemother. 2017;61(9):e01152. https://doi.org/10.1128/AAC.01152-17.
Arikata M, Itoh Y, Shichinohe S, Nakayama M, Ishigaki H, Kinoshita T, et al. Efficacy of clarithromycin against H5N1 and H7N9 avian influenza a virus infection in cynomolgus monkeys. Antiviral Res. 2019;171:104591.
Takahashi E, Indalao IL, Sawabuchi T, Mizuno K, Sakai S, Kimoto T, et al. Clarithromycin suppresses induction of monocyte chemoattractant protein-1 and matrix metalloproteinase-9 and improves pathological changes in the lungs and heart of mice infected with influenza A virus. Comp Immunol Microbiol Infect Dis. 2018;56:6–13.
Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, et al. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54.
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663.
Javelot H, El-Hage W, Meyer G, Becker G, Michel B, Hingray C. COVID-19 and (hydroxy)chloroquine-azithromycin combination: should we take the risk for our patients? Br J Clin Pharmacol. 2020;86:1176–7.
Gbinigie K, Frie K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020. https://doi.org/10.3399/bjgpopen20X101094.
Ohe M, Shida H, Jodo S, Kusunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: will this be the way forward? Biosci Trends. 2020;14:159–60.
Million M, Lagier J-C, Gautret P, Colson P, Fournier P-E, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738.
Schwartz RA, Suskind RM. Azithromycin and COVID-19 prompt early use at first signs of this infection in adults and children an approach worthy of consideration. Dermatol Ther. 2020;33:e13785.
Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, et al. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228.
Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq M-P, et al. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281:86–100.
Pani A, Lauriola M, Romandini A, Scaglione F. Macrolides and viral infections: focus on azithromycin in COVID-19 pathology. Int J Antimicrob Agents. 2020;56(2):106053
Lin S-J, Kuo M-L, Hsiao H-S, Lee P-T. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int Immunopharmacol. 2016;40:318–26.
Amsden GW. Erythromycin, clarithromycin, and azithromycin: are the differences real? Clin Ther. 1996;18:56–72.
Millán-Oñate J, Millan W, Mendoza LA, Sánchez CG, Fernandez-Suarez H, Bonilla-Aldana DK, et al. Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann Clin Microbiol Antimicrob. 2020;19:16.
Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev Am Chem Soc. 2005;105:425–48.
Zhou N, Pan T, Zhang J, Li Q, Zhang X, Bai C, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV). J Biol Chem. 2016;291:9218–32.
Wang Y, Cui R, Li G, Gao Q, Yuan S, Altmeyer R, et al. Teicoplanin inhibits ebola pseudovirus infection in cell culture. Antiviral Res. 2016;125:1–7.
Baron SA, Devaux C, Colson P, Raoult D, Rolain J-M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents. 2020;55:105944.
Ceccarelli G, Alessandri F, d’Ettorre G, Borrazzo C, Spagnolello O, Oliva A, et al. Is teicoplanin a complementary treatment option for COVID-19? The question remains. Int J Antimicrob Agents. 2020;56:106029.
Zhang J, Ma X, Yu F, Liu J, Zou F, Pan T, et al. Teicoplanin potently blocks the cell entry of 2019-nCoV. bioRxiv. 2020. https://doi.org/10.1101/2020.02.05.935387.
Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev Am Soc Microbiol. 2001;65:232–60.
Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch Virol. 2014;159:711–8.
Rothan HA, Buckle MJ, Ammar YA, Mohammadjavad P, Shatrah O, Noorsaadah AR, et al. Study the antiviral activity of some derivatives of tetracycline and non-steroid anti inflammatory drugs towards dengue virus. Trop Biomed. 2013;30:681–90.
Sturtz FG. Antimurine retroviral effect of doxycycline. Methods Find Exp Clin Pharmacol. 1998;20:643.
Skulason S, Holbrook WP, Thormar H, Gunnarsson GB, Kristmundsdottir T. A study of the clinical activity of a gel combining monocaprin and doxycycline: a novel treatment for herpes labialis. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2012;41:61–7.
Wu Z, Wang X, Wei J, Li B, Shao D, Li Y, et al. Antiviral activity of doxycycline against vesicular stomatitis virus in vitro. FEMS Microbiol Lett. 2015;362(22):fnv195.
Bick MJ, Carroll J-WN, Gao G, Goff SP, Rice CM, MacDonald MR. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J Virol Am Soc Microbiol J. 2003;77:11555–62.
Tang Q, Wang X, Gao G. The short form of the zinc finger antiviral protein inhibits influenza a virus protein expression and is antagonized by the virus-encoded NS1. J Virol. 2017;91(2):e01909–16.
Sargiacomo C, Sotgia F, Lisanti MP. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12:6511–7.
Fredeking TM, Zavala-Castro JE, González-Martínez P, Moguel-Rodríguez W, Sanchez EC, Foster MJ, et al. Dengue patients treated with doxycycline showed lower mortality associated to a reduction in IL-6 and TNF levels. Recent Patents Anti-Infect Drug Disc. 2015;10:51–8.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395:1033–4.
Quartuccio L, Semerano L, Benucci M, Boissier M-C, De Vita S. Urgent avenues in the treatment of COVID-19: targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87:191–3.
Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–88.
Clercq ED. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti Infect Ther (Taylor and Francis). 2006;4:291–302.
Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J Chem Inf Model. 2020;60:3277–86.
Ezelarab HAA, Abbas SH, Hassan HA, Abuo-Rahma GE-DA. Recent updates of fluoroquinolones as antibacterial agents. Arch Pharm (Weinheim). 2018;351:e1800141.
Dalhoff A. Antiviral, antifungal, and antiparasitic activities of fluoroquinolones optimized for treatment of bacterial infections: a puzzling paradox or a logical consequence of their mode of action? Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:661–8.
Ikeda S, Yazawa M, Nishimura C. Antiviral activity and inhibition of topoisomerase by ofloxacin, a new quinolone derivative. Antiviral Res. 1987;8:103–13.
Richter S, Parolin C, Palumbo M, Palù G. Antiviral properties of quinolone-based drugs. Curr Drug Targets Infect Disord. 2004;4:111–6.
Witvrouw M, Daelemans D, Pannecouque C, Neyts J, Andrei G, Snoeck R, et al. Broad-spectrum antiviral activity and mechanism of antiviral action of the fluoroquinolone derivative K-12. Antivir Chem Chemother. 1998;9:403–11.
Karampela I, Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and moxifloxacin, prove to be beneficial as an adjunct treatment in COVID-19? Arch Med Res. 2020;51(7):741–2.
Marciniec K, Beberok A, Boryczka S, Wrześniok D. Ciprofloxacin and moxifloxacin could interact with SARS-CoV-2 protease: preliminary in silico analysis. Pharmacol Rep. 2020;72(6):1553–61.
Metlay JP, Waterer GW. Treatment of community-acquired pneumonia during the coronavirus disease (COVID-19) pandemic. Ann Intern Med. 2020. https://doi.org/10.7326/M20-2189.
Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–71.
Riesbeck K. Immunomodulating activity of quinolones: review. J Chemother Florence Italy. 2002;14:3–12.
Serio AW, Magalhaes ML, Blanchard JS, Connolly LE. Aminoglycosides: mechanisms of action and resistance. In: Mayers D, Sobel J, Ouellette M, Kaye K, Marchaim D, editors. Antimicrobial drug resistance. Cham: Springer; 2017. https://doi.org/10.1007/978-3-319-46718-4_14.
Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):a027029.
Woods Acevedo MA, Erickson AK, Pfeiffer JK. The antibiotic neomycin enhances coxsackievirus plaque formation. mSphere. 2019;4(1):e00632-18.
Gopinath S, Kim MV, Rakib T, Wong PW, van Zandt M, Barry NA, et al. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat Microbiol. 2018;3:611–21.
Chalichem NSS, Bethapudi B, Mundkinajeddu D. Aminoglycosides can be a better choice over macrolides in COVID-19 regimen: plausible mechanism for repurposing strategy. Med Hypotheses. 2020;144:109984.
Khurshid Z, Zafar MS, Naseem M, Khan RS, Najeeb S. Human oral defensins antimicrobial peptides: a future promising antimicrobial drug. Curr Pharm Des. 2018;24:1130–7.
Holly MK, Diaz K, Smith JG. Defensins in viral infection and pathogenesis. Annu Rev Virol. 2017;4:369–91.
Park MS, Kim JI, Lee I, Park S, Bae J-Y, Park M-S. Towards the application of human defensins as antivirals. Biomol Ther. 2018;26:242–54.
Venkataraman N, Cole AL, Ruchala P, Waring AJ, Lehrer RI, Stuchlik O, et al. Reawakening retrocyclins: ancestral human defensins active against HIV-1. PLoS Biol. 2009;7:e95.
Wilson SS, Wiens ME, Smith JG. Antiviral mechanisms of human defensins. J Mol Biol. 2013;425:4965–80.
Izquierdo-Dominguez A, Rojas-Lechuga MJ, Mullol J, Alobid I. Olfactory dysfunction in the COVID-19 outbreak. J Investig Allergol Clin Immunol. 2020;30(5):317–26.
Thiem U, Heppner H-J, Pientka L. Elderly patients with community-acquired pneumonia: optimal treatment strategies. Drugs Aging. 2011;28:519–37.
Durojaiye AB, Clarke J-RD, Stamatiades GA, Wang C. Repurposing cefuroxime for treatment of COVID-19: a sco** review of in silico studies. J Biomol Struct Dyn. 2020. https://doi.org/10.1080/07391102.2020.1777904.
Pani A, Lauriola M, Romandini A, Scaglione F. Macrolides and viral infections: focus on azithromycin in COVID-19 pathology. Int J Antimicrob Agents. 2020;56:106053.
Choudhary R, Sharma AK, Choudhary R. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. N Microbes N Infect. 2020;35:100684.
Touret F, Gilles M, Barral K, Nougairède A, Decroly E, de Lamballerie X, et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. bioRxiv (Cold Spring Harbor Laboratory). 2020;48:92.
Sathyamoorthy N, Chintamaneni PK, Chinni S. Plausible role of combination of chlorpromazine hydrochloride and teicoplanin against COVID-19. Med Hypotheses. 2020;144:110011.
He B, Garmire L. Prediction of repurposed drugs for treating lung injury in COVID-19. Ar**v. 2020;9:609.
Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy (Hoboken: Wiley). 2020;40:487–8.
Conforti C, Giuffrida R, Zalaudek I, Di Meo N. Doxycycline, a widely used antibiotic in dermatology with a possible anti-inflammatory action against IL-6 in COVID-19 outbreak. Dermatol Ther. 2020;33:e13437.
Farouk A, Salman S. Dapsone and doxycycline could be potential treatment modalities for COVID-19. Med Hypotheses. 2020;140:109768.
Malek AE, Granwehr BP, Kontoyiannis DP. Doxycycline as a potential partner of COVID-19 therapies. IDCases. 2020;21:e00864.
Szolnoky G. Further aspects of doxycycline therapy in COVID-19. Dermatol Ther. 2020;. https://doi.org/10.1111/dth.13810.
Bonzano C, Borroni D, Lancia A, Bonzano E. Doxycycline: from ocular rosacea to COVID-19 anosmia. New insight into the coronavirus outbreak. Front Med. 2020;7:200.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AY and AO conceived and designed the study. AY and AO drafted the manuscript. The manuscript was critically reviewed by IY. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interests were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yacouba, A., Olowo-okere, A. & Yunusa, I. Repurposing of antibiotics for clinical management of COVID-19: a narrative review. Ann Clin Microbiol Antimicrob 20, 37 (2021). https://doi.org/10.1186/s12941-021-00444-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-021-00444-9