Abstract
Elevated low-density lipoprotein cholesterol (LDL-C) level is associated with an increased risk of atherosclerotic cardiovascular disease. Although high-intensity lipid-lowering therapies with statins and ezetimibe are highly effective for reducing LDL-C levels, over half of high-risk patients do not achieve guideline-recommended LDL-C goals. Thus, there is a significant gap between treatment guidelines and their implementation in daily clinical practice. The major causes are individual variability in the response to lipid-lowering therapies and variation in treatment adherence. Proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies combined with statins provide marked and consistent reduction in LDL-C levels; however, poor adherence due to the need for subcutaneous injections every 2 or 4 weeks and high cost are major obstacles to their use in real-world clinical settings. Inclisiran, a recently approved novel small interfering ribonucleic acid (siRNA) molecule that inhibits PCSK9 synthesis, provides robust and long-term reduction in LDL-C levels with a low inter-individual variability in the LDL-C-lowering response. Moreover, its administration by biannual injection is expected to greatly improve treatment adherence. Clinical trials of this drug lasting for up to 4 years showed acceptable safety profiles, and ongoing studies accumulate evidence of its longer-term safety. This narrative review summarizes the available evidence on the efficacy and safety of inclisiran and analyzes its potential to overcome the gap between guideline recommendations and real-world clinical practice in current LDL-C-lowering therapies, with a focus on reduced LDL-C level variability and improved treatment adherence.
Similar content being viewed by others
Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) level is a causal factor for develo** atherosclerotic cardiovascular disease (ASCVD), and evidences from randomized clinical trials, cohort studies, and Mendelian randomized studies have demonstrated a consistent dose-dependent log-linear association between absolute magnitude of exposure to LDL-C and the risk of ASCVD [1, 2]. Conversely, absolute reduction in LDL-C levels is associated with a proportional reduction in the risk of ASCVD, regardless of the presence of other risk factors. A meta-analysis of data from 26 randomized trials involving intensive statin therapy revealed that there was a 22% relative reduction in the rate of major cardiovascular events per 1 mmol/L (38.7 mg/dL) reduction in LDL-C levels [3]. Furthermore, achieving very low levels of LDL-C has beneficial effects on the risk for major cardiovascular events [4]. In a post-hoc analysis of the JUPITER trial, patients who achieved LDL-C levels below 50 mg/dL with high-intensity statin therapy experienced the fewest cardiovascular events without an increase in the rate of systemic adverse events [5]. Additionally, the IMPROVE-IT trial showed that adding ezetimibe to statin therapy provided incremental reduction in LDL-C levels by 53.2 mg/dL and improved cardiovascular outcomes for secondary prevention [6, 7]. In particular, high-risk patients with multiple commodities who are likely to have a high cardiovascular event rate despite statin therapy had an increased relative and absolute benefit from the addition of ezetimibe [8].
Therefore, the intensive LDL-C-lowering strategy was widely accepted and current guidelines for the management of dyslipidemia recommend more aggressive LDL-C reduction to achieve the target level for high-risk populations. For secondary prevention, the 2019 European Society of Cardiology/European Atherosclerosis Society Guideline for the Management of Dyslipidemias recommends ≥ 50% reduction from baseline values and an LDL-C target level of < 55 mg/dL, whereas the 2018 American College of Cardiology/American Heart Association Multisociety Guideline on the Management of Blood Cholesterol uses an LDL-C threshold of 70 mg/dL for initiation of non-statin therapy on top of statin therapy [9, 10]. However, many high-risk patients receiving statins fail to achieve the guideline-recommended reduction in LDL-C levels in real-world clinical setting. The DAVINCI study and EUROASPIRE V survey across European countries revealed that only half of the patients with established ASCVD received high-intensity statin therapy, and less than half achieved the target LDL-C level of < 70 mg/dL in secondary prevention [11, 12].
Inclisiran is a long-acting, small interfering ribonucleic acid (siRNA) that lowers consistently circulating LDL-C levels by approximately 50% with a twice-yearly dosing regimen [13]. Prior reviews of inclisiran therapy have not focused on its potential to reduce the gap between current guideline recommendations and their implementation. Therefore, this narrative review briefly summarizes the development of therapeutic agents with a potential to achieve this goal and focuses on the role of inclisiran in real-world clinical settings, with an overview of its pharmacological properties and the current evidence supporting its potential to provide robust and long-term reduction in LDL-C levels with low interindividual variability and improve treatment adherence for high-risk populations who have established ASCVD or ASCVD-risk equivalents with elevated LDL-C levels.
Novel strategies to target proprotein convertase subtilisin/kexin type 9
The LDL receptors (LDLRs) on the surface of hepatocytes control plasma LDL-C levels by binding to circulating LDL-C and subsequent internalization of the LDL-C/LDLR complex via clathrin-mediated endocytosis. In the acidic compartment of endosomes, LDL-C is released from the LDLR and degraded in lysosomes, while the LDLR is recycled to the surface of hepatocytes. LDLR expression is regulated at the transcriptional level by a negative feedback mechanism in the intracellular cholesterol pool [14, 15].
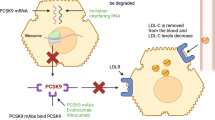
Proprotein convertase subtilisin/kexin type 9 (PCSK9), expressed predominantly in the liver and, to a lesser extent, in the small intestine, kidneys, pancreas, and the central nervous system, plays an important role in the regulation of LDL-C homeostasis via two pathways [16]. In the extracellular pathway, circulating PCSK9 binds to LDLRs on the hepatocyte surface and forms the PCSK9/LDLR complex, which is internalized via clathrin-mediated endocytosis and then directed to the lysosome for degradation of LDLRs. This degradation results in low levels of LDLRs at the cellular membrane of hepatocytes, leading to reduction in LDL-C uptake and increase in plasma LDL-C levels. In the intracellular pathway, PCSK9 enhances intracellular LDLR degradation by direct intracellular trafficking of the PCSK9/LDLR complex from the trans-Golgi network to the lysosomes without recycling LDLRs to the cellular surface [17,18,19] (Fig. 1).
The role of PCSK9 in lipid metabolism. LDLRs on the surface of the liver cells bind circulating LDL, and LDL/LDLR complexes are internalized via clathrin‐mediated endocytosis. LDL is released for degradation in the lysosome, while the LDLR is recycled to the cell surface. In extracellular pathway, PCSK9 binds to the LDLR on the surface of the liver cell and then internalizes with LDLR to intracellular degradation in the lysosome, which increase serum LDL levels by preventing LDLR recycling to the membrane. In intracellular pathway, the secreted PCSK9 from Golgi apparatus can be sorted directly to lysosomes as a PCSK9–LDLR complex, leading to intracellular degradation of the LDLR. LDLR LDL receptors, PCSK9 proprotein convertase subtilisin/kexin type 9
The PCSK9 gene was identified as a third causative gene for familial hypercholesterolemia [20]. Gain-of-function mutations in this gene promote degradation of LDLRs, resulting in higher LDL-C levels and increased ASCVD risk. In contrast, loss-of-function mutations in the PCSK9 gene were found to be associated with reduced LDLR degradation, leading to increased LDLR expression, lower LDL-C levels, and substantial reduction in the rate of cardiovascular events [21, 22]. In addition, individuals with severe loss-of-function mutations in the PCSK9 gene were apparently healthy without physical and neurological impairment despite the very low LDL-C levels (< 14 mg/dL) from birth [23]. These findings suggested that PCSK9 would be a promising therapeutic target for LDL-C level reduction for ASCVD prevention.
Anti-PCSK9 monoclonal antibodies
As a first approach to inhibit PCSK9, monoclonal antibodies (mAbs) were used to selectively prevent PCSK9 binding to LDLRs. Anti-PCSK9 mAbs bind to the catalytic domain of plasma extracellular PCSK9 with high affinity and specificity, blocking the interaction between LDLRs and PCSK9. Thus, PCSK9-mediated reduction in LDLR expression is attenuated, resulting in increased LDL-C uptake and subsequent reduction in plasma LDL-C levels [24]. Two fully human anti‐PCSK9 mAbs, alirocumab (SAR236553/REGN727) and evolocumab (AMG145), were eventually approved for clinical use by the United States Food and Drug Administration and the European Medicines Agency.
Subcutaneous injection of anti-PCSK9 mAbs every 2 or 4 weeks provides remarkable reduction in LDL-C levels by up to approximately 60% without serious adverse events in patients with maximally tolerated statin therapy. More importantly, two large randomized controlled trials, the FOURIER and ODYSSEY Outcomes trials, demonstrated not only a 50–60% reduction in LDL-C levels but also cardiovascular event risk reduction of approximately 15–20% in patients receiving statin therapy for secondary prevention [25,26,27]. The 2019 European Society of Cardiology/European Atherosclerosis Society guideline, 2018 American College of Cardiology/American Heart Association Multi-society guideline, and 2022 American College of Cardiology Expert Consensus for non-statin therapies acknowledge PCSK9 inhibitors as valuable options for combined LDL-C-lowering therapy in patients with very high cardiovascular risk [9, 10, 28].
Nevertheless, there are still several barriers to the wide implementation of anti-PCSK9 mAbs in daily clinical practice, such as the complex administration regimen, poor adherence, high cost, limited accessibility, reimbursement limitation, and lack of long-term clinical safety data.
Inclisiran: a small interfering ribonucleic acid-based inhibitor of PCSK9
Molecular structure and mechanism of action
Inclisiran (ALN-PCSSC; ALN-60212) is a first-in-class siRNA that inhibits the hepatic synthesis of PCSK9 by RNA interference, resulting in significant and long-term reduction in LDL-C levels. This duplex RNA contains one 2′-deoxy, 11 2′-fluoro, and 32 2′-O-methyl modified nucleotides. It consists of two complementary RNA strands, an antisense (guide) strand, and a sense (passenger) strand. The 3′ end of the sense strand is conjugated to the synthetic ligand triantennary N-acetyl galactosamine (GalNAc), which is a ligand of asialoglycoprotein receptors (ASGPRs), expressed mainly on the surface of hepatocytes. The siRNA enters the hepatocytes through the interaction between GalNAc and ASGPRs, which provides precise and rapid hepatic uptake. In addition, the formulated lipid nanoparticle encapsulation helps to increase endocytosis into target cells despite the high molecular weight and negative electrical charge of the siRNA [29].
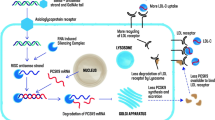
When the siRNA is loaded into the RNA induced-silencing complex (RISC) in hepatocytes, only the antisense strand is activated via selective removal of the sense strand by the Argonaute 2 [30]. This complex of the antisense strand and RISC binds to PCSK9 messenger RNA (mRNA) transcripts, and selectively and catalytically cleaves mRNA, inhibiting the translation of complementary mRNA transcript [31]. The consequent reduced synthesis of the PCSK9 protein results in significantly reduced plasma LDL-C levels (Fig. 2). As only approximately 100–200 loaded RISC complexes per cell are sufficient to eliminate the gene expression, each complex has a very long half-life from multiple mRNA copies, allowing dosing in patients to be months apart. In addition, the GalNAc-mediated liver specificity allows for use of lower cumulative doses, enabling subcutaneous administration [32].
The mechanism of PCSK9 synthesis inhibition by inclisiran. Inclisiran, GalNAc‐conjugated siRNA, binds to the asialoglycoprotein receptor on the surface of hepatocytes with high selectivity, which can be delivered into the cell via endocytosis. The antisense strand of the siRNA duplex is incorporated into the RNA-induced silencing complex intracellularly, which cleaves mRNA encoding PCSK9 specifically. The inhibition of PCSK9 synthesis reduces the degradation of LDL receptors. RISC ribonucleic acids‐induced silencing complex, ASGPRs asialoglycoprotein receptors, GalNAc N-acetyl galactosamine
These mechanisms give inclisiran the advantage of safety and less frequent administration. Subcutaneous administration at 0 and 3 months and every 6 months thereafter can provide significant reduction in LDL-C levels by approximately 50% in high-risk and very high-risk patients, as well as patients with statin intolerance.
Pharmacokinetics and pharmacodynamics
ALN-PCS, the early predecessor of inclisiran formulated in a lipid nanoparticle, reduced PCSK9 mRNA levels by 70% and LDL-C levels by 60% in animal models with a duration of 3 weeks [29]. In healthy volunteers who received ALN-PCS by intravenous infusion for 60 min, the highest dose of ALN-PCS reduced PCSK9 mRNA and protein levels by 70% and LDL-C levels by 40% after 3 days, and the effect sustained for 2 to 3 weeks after administration [33]. ALN-PCSsc (inclisiran), made by refinement of ALN-PCS, induced the optimal multivalent design of siRNA-GalNAc, enabling better stability and prolonged activity. ALN-PCSsc can be administered by subcutaneous injection. Tissue-specific delivery of ALN-PCSsc led to a potent and dose-dependent inhibition of PCSK9 gene expression. In healthy volunteers administered a single subcutaneous dose of inclisiran (100, 300, 500, or 800 mg), the least-square mean LDL-C reduction from baseline to day 84 was 36.7%, 50.0%, 50.6%, and 43.4%, respectively. Additionally, a dose of 300 mg or higher maintained PCSK9 and LDL-C level reduction over 6 months [34].
Following subcutaneous administration of a single dose in the range of 24–756 mg, exposure to inclisiran appears to increase in approximately dose-proportional manner. Peak plasma concentration was reached within 4 h after administration of the recommended dose (284 mg: equivalent 300 mg inclisiran sodium). It is rapidly cleared from the circulation through hepatic uptake and renal elimination, and is undetectable in the plasma by 48 h after administration. The major route of systemic clearance from the circulation is through hepatic uptake, with 16% being cleared through renal elimination [35]. Once in the tissue, it is mainly metabolized by non-specific nucleases to an inactive shorter nucleotide [36, 37]. The limited systemic exposure due to rapid clearance provides the benefit of lower theoretical risk for off-target effects.
Although individuals with mild or moderate hepatic and renal impairment had greater exposure to inclisiran, the pharmacodynamic effects remained relatively unchanged in terms of LDL-C level reduction, clearance, duration of effects, and safety [38, 39]. However, as the effect of inclisiran has not been evaluated in patients with severe hepatic impairment (Child–Pugh class C), its use in these patients should be cautioned. Inclisiran is not anticipated to have clinically significant interactions with other drugs, although limited data are available.
Overview of clinical trials
In a phase 1 trial (NCT02314442), healthy volunteers with LDL-C levels higher than 100 mg/dL were subcutaneously administered inclisiran in either a single-dose regimen (25–800 mg) or multiple-dose regimens of 125–500 mg with intervals of at least 1 week. At day 84, the maximal reduction by the single-dose regimen was 74.5% in PSCK9 levels after a 300 mg dose and 50.6% in LDL-C levels after a 500 mg dose. All multiple-dose regimens of inclisiran reduced LDL-C and PCSK9 levels by up to 59.7% and 83.8%, respectively. After administration of doses of 300 mg or higher, the reduction in LDL-C and PCSK9 levels was durable over at least 180 days. There were no serious adverse events versus placebo in this trial [34].
Based on these results, the ORION trials were initiated as a global clinical development program to evaluate the efficacy and safety of inclisiran [40] (Table 1). In phase 1 trials, the ORION-6 and ORION-7 (NCT03159416) trials demonstrated a favorable safety profile of inclisiran in patients with hepatic and renal impairment, respectively [38, 39], while the ORION-12 trial confirmed that there is no clinically significant effect on cardiac repolarization assessed by the QTc interval or other electrocardiographic parameters [41].
The ORION-1 trial (NCT02597127), the first phase 2 trial, was a multicenter, double-blind, placebo-controlled trial that enrolled 501 patients at high risk of ASCVD with elevated LDL-C levels [42]. Approximately 73% of the enrolled patients were receiving statin therapy. In this dose-finding study, patients received a single dose of inclisiran of 200, 300, or 500 mg, or placebo, or two doses of inclisiran of 100, 200, or 300 mg, or placebo (days 1 and 90). The primary endpoint was the change in LDL-C levels on day 180. At day 30 after the first injection, the PCSK9 level was reduced by 66.2–74.0% and the LDL-C level was reduced by 44.5–50.5%, depending on the received dose. At day 180, the least square mean reduction in LDL-C levels from baseline was 27.9–41.9% after a single dose and 35.5–52.6% after two doses. The greatest LDL-C level reduction was observed after two doses of inclisiran of 300 mg. Additionally, the mean reduction in LDL-C levels after 240 days was 26.7–47.2%, and the reduction in PCSK9 and LDL-C levels remained consistent across all dose regimens at day 240. These findings suggested that biannual dosing was appropriate as the most efficient administration regimen for inclisiran. Moreover, the prespecified analysis revealed that inclisiran provided sustained and concordant reduction in apolipoprotein B and non-HDL-C levels over 210 days, as well as modest reductions in VLDL-C and triglyceride levels [43]. The incidence of adverse events was similar in the inclisiran and placebo groups, with serious adverse event rates of 11% and 8%, respectively. Injection-site reactions occurred in 4% of patients who received a single dose and in 7% of those who received two doses of inclisiran.
The ORION-3 trial (NCT03060577), a phase 2 open-label extension of the ORION-1 trial, assessed the long-term efficacy and safety of inclisiran for up to 4 years in patients who previously received inclisiran in the ORION-1 trial and patients who received evolocumab for 1 year [44]. The median duration of exposure to inclisiran from baseline through ORION-3 was 4.5 years. The patients who received biannual injection of inclisiran achieved LDL-C level reduction of 47.5% from baseline to day 210 and LDL-C reductions were sustained over the window of 4 years without loss of efficacy, showing a significantly longer-lasting effect of inclisiran compared with that of the anti-PCSK9 mAb despite the similar relative reduction in LDL-C and PCSK9 levels. There were no new adverse event profiles during the 4-year study period. In addition, the ORION-14 (NCT04774003) and ORION-15 (NCT04666298) trials were conducted to assess the efficacy and safety of inclisiran in the Chinese and Japanese populations, respectively, and the ORION-18 trial (NCT04765657) is currently ongoing in Asian high-risk patients.
The ORION-10 (NCT03399370) and ORION-11 (NCT03400800) trials were double-blind, randomized, placebo-controlled, phase 3 trials to evaluate the percent change in LDL-C levels at the day 510 follow-up in 1561 patients with ASCVD in the United States and 1617 patients with ASCVD or risk equivalent in the European Union and South Africa, respectively [45]. The mean LDL-C levels at baseline in these two trials were 104.7 mg/dL and 105.5 mg/dL, respectively. At day 510, patients who received inclisiran achieved an LDL-C level reduction of 52.3% in the ORION-10 and 49.9% in the ORION-11 trial. Adverse events were not significantly different between the inclisiran and placebo groups in both trials.
The ORION-9 trial (NCT03397121) was conducted to assess the efficacy and safety of inclisiran in patients with heterozygous familial hypercholesterolemia (HeFH) [46]. The mean baseline LDL-C level was 153 mg/dL despite all patients receiving a maximally accepted dose of statin therapy with or without ezetimibe. At day 510, the mean percent change in LDL-C levels was a reduction of 39.7% in the inclisiran group vs. an increase of 8.2% in the placebo group. The substudy according to HeFH genotype revealed consistent reduction in LDL-C levels across all types of genetic defects in patients with HeFH. In the study summarizing the ORION-9, -10, and -11 trials for very high-risk patients, inclisiran reduced the levels of LDL-C, apolipoprotein B, and non-HDL-C by 51%, 42%, and 46%, respectively, which was associated with a 24% reduction in the rate of cardiovascular events in the subanalysis [47, 48]. In addition, a meta-analysis of five randomized controlled trials of inclisiran also demonstrated favorable effects on multiple lipid/lipoprotein parameters and an acceptable safety profile [49]. The ORION-8 trial (NCT03814187) is an ongoing open-label extension of the ORION-3, -9, -10, and -11 trials to assess the long-term effects and safety of inclisiran up to day 990. In the network meta-analysis of non-statin lipid-lowering therapies, PCSK9 mAbs reduced LDL-C by 64.7% (95% CI 67.4–62.0%), whereas inclisiran was found to reduce LDL-C by 50.2% (95% CI 55.0–45.4%). Inclisiran was expected to provide similar improvement in LDL-C levels though PCSK9 mAbs were more efficacious at reducing LDL-C than inclisiran [50]. The addition of inclisiran to maximally tolerated statins plus ezetimibe enables LDL-C reduction of more than 80% as well as PCSK9 mAbs [51, 52].
Based on the data from the phase 3 ORION-9, -10, and -11 trials that have confirmed the tolerability and efficacy of inclisiran in the long-term, inclisiran (Leqvio®, inclisiran 284 mg/1.5 mL solution for injection in a prefilled syringe) was approved by the European Medicines Agency in 2020 and by the United States Food and Drug Administration in 2021 for primary hypercholesterolemia or mixed dyslipidemia. It needs to be stored at room temperature of 20–25 °C (68–77°F) with allowable excursions between 15 and 30 °C (59–86°F) [35].
The ORION-4 trial (NCT03705234) is an ongoing double-blind, randomized, placebo-controlled phase 3 trial which has enrolled approximately 15,000 patients with established ASCVD in the United Kingdom and United States to assess the effect of inclisiran on the cardiovascular outcomes over a median follow-up duration of 5 years. The estimated primary completion date is December 2024. This result is expected worldwide, as it will provide valuable information on the clinical benefits of inclisiran in cardiovascular event prevention. Currently, the VICTORION program including a part of the ORION studies is underway to assess the benefit of inclisiran therapy on the life of high-risk patients (Table 1). The VICTORION-2PREVENT trial (NCT05030428) is a phase 3 cardiovascular outcome trial for patients with established ASCVD in multiple countries. This global study is designed to assess whether inclisiran reduces the risk of 3-point-major adverse cardiovascular events, defined as a composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal ischemic stroke. There are also many other ongoing trials performed to promote the different use of inclisiran in various clinical settings, such as VICTORION-INCEPTION (NCT04873934), -INICIATE (NCT04929249), -REAL (NCT05399992), -DIFFERENCE (NCT05192941), and -SPIRIT (NCT04807400).
The phase 2 ORION-2 trial (NCT02963311) in adult patients with homozygous familial hypercholesterolemia (HoFH) was a proof-of-concept pilot study conducted to confirm the dose and regimen for the subsequent phase 3 ORION-5 trial (NCT03851705) [53]. As reductions in LDL-C and PCSK9 levels by 300 mg of inclisiran sodium were observed in patients with HoFH without adjustment of dosing or regimen, the ORION-5 trial, a double-blind, placebo-controlled, open-label, multicenter trial, was initiated with the standard dose and regimen to assess the long-term effects, tolerability, and safety of inclisiran. The results have not yet been published. Moreover, the ORION-13 (NCT04659863) and -16 (NCT04652726) trials, which were designed to assess the efficacy and safety of inclisiran in adolescents aged 12 to 17 years with HoFH and HeFH, respectively, are ongoing [54]. These trials may provide promising therapeutic options for adults and children with severe familial hypercholesterolemia in the current settings of limited availability of treatment.
Adverse events
The available clinical trial data on the safety of inclisiran have shown that it is overall safe and well tolerated. In the ORION trials, adverse events occurred both in the placebo and treatment arms with a relatively similar frequency. There were no drug-related serious adverse events, and most reported adverse events were mild and moderate, including myalgia, cough, musculoskeletal pain, mild rash and hyperpigmentation, headache, musculoskeletal pain, nasopharyngitis, and dizziness. In the pooled safety analysis of the ORION-9, -10, and -11 trials that included 3655 patients, the safety findings were similar in the inclisiran and placebo groups for 18 months, but injection site reactions were more frequent in patients receiving inclisiran (5.0% vs. 0.7%) [48]. Additionally, the results of the ORION-3 trial showed well-tolerated safety profile consistent with other trials during 4 years of follow-up [44]. The network meta-analysis of PCSK9 inhibitors revealed that inclisiran was identified as the top ranked drug in association with less serious adverse events by the surface under the cumulative ranking curve, and there were no findings that LDL-C lowering therapies with PCSK9 mAbs and inclisiran were associated with new-onset diabetes and neurocognitive disorders [55].
In addition, abnormal hematological effects were not found in these studies, although, in a previous study, another gene-silencing approach of selective protein inhibition by targeting its mRNA was associated with thrombocytopenia [56]. Furthermore, in the prespecified analysis of the ORION-1 trial, there was no evidence of significant increase in the levels of inflammatory biomarkers, such as interleukin-6 or tumor necrosis factor-α, or relevant formation of antidrug antibodies leading to a possible loss of function as illustrated with the anti- PCSK9 mAb bococizumab [57]. However, these results were obtained through a relatively short-term follow-up; therefore, longer-term data are needed to fully evaluate the safety profile of inclisiran. Recent meta-analysis of genetic association studies demonstrated that the exposure to LDL-C lowering genetic variants was associated with a high risk of new-onset type 2 diabetes [58]. It may suggest the potential adverse metabolic effects of inclisiran.
Challenges in current lipid-lowering therapies and perspectives of inclisiran therapy
Variability in LDL-C levels
Statin therapy is the gold standard treatment worldwide for the management of dyslipidemia and prevention of ASCVD; however, there is an individual variation in LDL-C levels, even in clinical trials that enrolled patients with greater adherence to treatment than that encountered in routine clinical practice [59]. Contemporary registry data demonstrated that less than half of the patients with established ASCVD achieved the target LDL-C level of < 70 mg/dL with high-intensity statin therapy [11, 12]. The LDL-C-lowering response to statin therapy varies according to age, sex, race, baseline LDL-C levels, genetic diversity, and various metabolic factors. Although adherence is an important factor as a determinant, the variability in LDL-C levels has been reported to be an independent predictor of adverse cardiovascular events as well as blood pressure variations [60,61,62]. A meta-analysis of statin clinical trials demonstrated that the variability in LDL-C level percent reduction was wide, ranging from modest to significant reduction [59]. Therefore, we need to consider possible poor responses to statin treatment in individual patients.
Attainment of consistently lower LDL-C levels without major variation is essential to obtain the greatest benefit from LDL-C-lowering therapy. The addition of ezetimibe to statin therapy has been shown to be associated with a lesser variation in the LDL-C-lowering response compared to that with statin therapy alone [63]. Furthermore, anti-PCSK9 mAbs combined with statin therapy offered a more sustained LDL-C level reduction among a large majority of patients in a sub-analysis of the FOURIER trial [64]. The combination therapy with statin and non-statin LDL-C-lowering drugs is an effective way to overcome the heterogeneity in the response to statin therapy.
Poor treatment adherence
The major causes for the variation in LDL-C levels have been shown to be not only insufficient responses to drugs, but poor adherence by patients and low use of intensive LDL-C-lowering therapy by physicians. More importantly, these factors lead to increased ASCVD risk and mortality rates in daily clinical settings [65,66,67]. Despite the substantial benefits from statin therapy, the adherence to the guideline-recommended lipid-lowering therapy with high-intensity statins is suboptimal in both patients and physicians. The Patient and Provider Assessment of Lipid Management (PALM) registry, a study performed to assess the current practice of lipid management in 5693 patients at outpatient clinics in the United States, observed that 25% of guideline-eligible patients failed to receive statins and 15% of clinicians continued to have concerns about the safety of statins despite their belief of benefit [66, 68]. In addition, the EUROASPIRE V study revealed that statin therapy was interrupted or its intensity was decreased in approximately 20% of patients because of statin intolerance or based on physician’s advice, according to the medical records and interviews [12].
The most common causes of statin intolerance have been shown to be statin-associated muscle symptoms; however, a meta-analysis of the results from randomized trials of statins revealed that more than 90% of muscle symptoms in patients receiving statin therapy were not due to the statins [69]. Typically, the average incidence of myopathy is approximately one case per 10,000 patients treated per year, and that of rhabdomyolysis is 2–3 cases per 100,000 patients [70, 71]. Moreover, a series of n-of-1 trials among statin-intolerant patients found no difference in the frequency of muscle symptoms compared with placebo, suggesting a nocebo effect among users of statins [72]. These results imply that the fear of adverse effects caused by confusing information regarding the side effects of statins could be a contributing factor in poor adherence.
Other potential contributors to poor adherence to lipid-lowering therapy include inconvenience of administration, high cost, low health literacy, poor awareness, and clinical inertia. In particular, physicians should avoid complex treatment regimens, frequent dosing, and increased number of pills to improve treatment adherence in daily practice [73, 74]. Adherence to dosing regimens remains challenging because oral small molecules require daily dosing and anti-PCSK9 mAbs are administered by subcutaneous injection once or twice a month via self-injection or with the help of a caregiver. Additionally, anti-PCSK9 mAbs are more expensive than other lipid-lowering therapies, which hampers treatment adherence despite the bi-weekly administration [75, 76]. A pooled analysis of the results from anti-PCSK9 mAbs clinical trials that included 4197 patients demonstrated a high level of adherence for over 1 year, whereas a cohort study including 13,151 patients in real-world clinical settings revealed that the anti-PCSK9 mAb therapy was discontinued within 6 months after treatment initiation in approximately 40% of patients [77, 78]. Moreover, in a survey to evaluate the patients’ experience with anti-PCSK9 mAbs, 33.7% of 1216 patients who initiated treatment discontinued it because of its high cost and lack of insurance approval [79].
Expectations and challenges of inclisiran therapy
Inclisiran provided robust and sustained reduction in LDL-C levels in patients receiving the maximum tolerated dose of statins while achieving high consistency in individual LDL-C-lowering responses. In the ORION-1 trial, more than 95% of patients who received two doses of inclisiran of 300 mg experienced a persistent response that had not returned to within 20% of the change from the baseline LDL-C level at day 180 [80]. As a more than 50% reduction in LDL-C levels persists over 4 months after the second administration of inclisiran of 300 mg, the low inter-individual variability in LDL-C levels over time may provide a potential benefit to reduce the risk of cardiovascular events in a large number of high-risk patients. The degree of individual variation in the response to inclisiran is smaller than that for other LDL-C-lowering therapies, which could be due to the mechanism of direct inhibition of PCSK9 synthesis as opposed to the extracellular inhibition by anti-PCSK9 mAbs.
The greatest advantage of inclisiran is the infrequent, biannual administration schedule, which is expected to greatly improve treatment adherence despite its robust LDL-C-lowering effect being similar to that of anti-PCSK9 mAbs in combination with the maximal tolerated statin/ezetimibe therapy. Because the anti-PCSK9 mAbs bi-weekly dosing schedule by subcutaneous injection negatively impacts treatment adherence, the biannual dosing regimen of inclisiran may be considered in patients with poor adherence to anti-PCSK9 mAb therapy and those unable to self-inject anti-PCSK9 mAbs. Administration twice a year by a healthcare provider may completely solve the problem regarding treatment adherence. Additionally, as an annual injection of inclisiran results in a more than 30% reduction in LDL-C levels over a year, it would be possible to apply this regimen for primary prevention, for example, along with the flu vaccine (Fig. 3).
Potentials and challenges of inclisiran in current lipid lowing therapies. The individual variability in response to statins/ezetimibe and poor treatment adherence to daily administration remain challenging issues in primary care. PCSK9 monoclonal antibodies provide a consistent reduction in LDL-C levels, but poor adherence due to subcutaneous injection every 2- or 4-weeks and high cost are barrier. Robust and durable reduction in LDL-C with inclisiran has the potential to improve both large variability in LDL-C levels and poor treatment adherence, on the other hand, there are some challenges such as high cost, lack of cardiovascular outcome data, and longer-term safety over 5 years. CV outcomes cardiovascular outcomes, PCSK9 mAbs monoclonal antibodies
Nonetheless, a major barrier to the wide implementation of inclisiran is its cost-effectiveness and the lack of adequate data on cardiovascular outcomes and long-term safety. The cost of inclisiran will likely limit its widespread use in daily clinical practice. The Institute for Clinical and Economic Review (ICER) conducted a cost-effectiveness analysis and proposed a value-based price range of inclisiran reflecting the health economic outcome. This benchmark price range of inclisiran is USD 3600–6000 per year to be cost-effective, while the current average annual price for anti-PCSK9 mAbs is approximately USD 5400–5850 [81, 82]. However, the price of publicly available inclisiran was set to USD 3250 per dose, or USD 6500 annually based on the biannual two-dose regimen, which might result in high out-of-pocket costs for individual patients and increased financial burden to healthcare systems. To avoid limiting its use due to the high cost, as for anti-PCSK9 mAbs, the price needs to be lowered to a level that is affordable and acceptable for most high-risk patients.
In addition, there is no evidence on whether inclisiran provides greater cardiovascular benefits than those obtained with anti-PCSK9 mAbs. The results of the ORION-4 trial evaluating cardiovascular outcomes are eagerly awaited. However, meta-analyses of the results from the ORION-9, -10, and -11 trials showed that inclisiran was expected to reduce the rate of major adverse cardiovascular events by as much as 24–30% [47, 83]. In terms of the safety of inclisiran, the ORION trials reported a relatively favorable safety profile, but the duration of the follow-up period was relatively short. Therefore, the ongoing ORION-8 long-term extension study after the ORION-9, -10, and -11 trials will provide additional information on the safety of inclisiran for up to an additional 3 years. As the concern about statin safety based on widespread disinformation or confusion among both patients and physicians was associated with suboptimal statin use, the safety of inclisiran should be evaluated carefully and accurate information should be provided about its side effects. Furthermore, it is important that its long-term safety over decades is demonstrated, as it is the first-in-class siRNA therapeutic compound against PCSK9.
Conclusions
Inclisiran is a novel siRNA molecule that inhibits PCSK9 synthesis, providing robust and sustained LDL-C level reduction with low inter-individual variability in the LDL-C-lowering response. The convenience of a biannual administration schedule is expected to greatly improve treatment adherence, which might contribute to better clinical outcomes in a large number of high-risk patients. However, lowering its price should be considered to promote its use in a broader range of patient populations for ASCVD prevention. Moreover, further study is needed to evaluate the long-term safety of this promising siRNA therapy.
Availability of data and materials
Not applicable.
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- ASGPRs:
-
Asialoglycoprotein receptors
- GalNAc:
-
Triantennary N-acetylgalactosamine
- HeFH:
-
Heterozygous familial hypercholesterolemia
- HoFH:
-
Homozygous familial hypercholesterolemia
- LDL-C:
-
Low-density lipoprotein cholesterol
- LDLR:
-
Low-density lipoprotein receptor
- mAb:
-
Monoclonal antibody
- mRNA:
-
Messenger ribonucleic acid
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- siRNA:
-
Small interfering ribonucleic acid
References
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–72.
Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–30.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81.
Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–94.
Hsia J, MacFadyen JG, Monyak J, Ridker PM. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). J Am Coll Cardiol. 2011;57:1666–75.
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2:547–55.
Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, et al. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–21.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA /ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2018;139:e1082–143.
Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. 2020;28:1279–89.
De Backer G, Jankowski P, Kotseva K, Mirrakhimov E, Reiner Z, Ryden L, et al. Management of dyslipidaemia in patients with coronary heart disease: results from the ESC-EORP EUROASPIRE V survey in 27 countries. Atherosclerosis. 2019;285:135–46.
Samuel E, Watford M, Egolum UO, Ombengi DN, Ling H, Cates DW. Inclisiran: a first-in-class siRNA therapy for lowering low-density lipoprotein cholesterol. Ann Pharmacother. 2022. https://doi.org/10.1177/10600280221105169.
Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–54.
Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47.
Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431–8.
Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, ** W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–75.
Park SW, Moon YA, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279:50630–8.
Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–36.
Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol. 2010;55:2833–42.
Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23.
Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA. 2009;106:9820–5.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Guedeney P, Giustino G, Sorrentino S, Claessen BE, Camaj A, Kalkman DN, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehz430.
Writing C, Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Covington AM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80:1366–418.
Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105:11915–20.
Sheu-Gruttadauria J, MacRae IJ. Structural foundations of RNA silencing by argonaute. J Mol Biol. 2017;429:2619–39.
Khvorova A. Oligonucleotide therapeutics—a new class of cholesterol-lowering drugs. N Engl J Med. 2017;376:4–7.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–61.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8.
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51.
European Medicines Agency. Leqvio (inclisiran) 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/leqvio. Accessed 15 Nov 2022.
McDougall R, Ramsden D, Agarwal S, Agarwal S, Aluri K, Arciprete M, et al. The nonclinical disposition and pharmacokinetic/pharmacodynamic properties of N-acetylgalactosamine-conjugated small interfering RNA are highly predictable and build confidence in translation to human. Drug Metab Dispos. 2022;50:781–97.
Humphreys SC, Davis JA, Iqbal S, Kamel A, Kulmatycki K, Lao Y, et al. Considerations and recommendations for assessment of plasma protein binding and drug–drug interactions for siRNA therapeutics. Nucleic Acids Res. 2022;50:6020–37.
Kallend D, Stoekenbroek R, He Y, Smith PF, Wijngaard P. Pharmacokinetics and pharmacodynamics of inclisiran, a small interfering RNA therapy, in patients with hepatic impairment. J Clin Lipidol. 2022;16:208–19.
Wright RS, Collins MG, Stoekenbroek RM, Robson R, Wijngaard PLJ, Landmesser U, et al. Effects of renal impairment on the pharmacokinetics, efficacy, and safety of inclisiran: an analysis of the ORION-7 and ORION-1 studies. Mayo Clin Proc. 2020;95:77–89.
Stoekenbroek RM, Kallend D, Wijngaard PL, Kastelein JJ. Inclisiran for the treatment of cardiovascular disease: the ORION clinical development program. Future Cardiol. 2018;14:433–42.
Kallend D, Mason J, Smith PF, Koren MJ, Stoekenbroek R, He Y, et al. An evaluation of a supratherapeutic dose of inclisiran on cardiac repolarization in healthy volunteers: a phase I, randomized study. Clin Transl Sci. 2022;15:2663–72.
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430–40.
Ray KK, Stoekenbroek RM, Kallend D, Leiter LA, Landmesser U, Wright RS, et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: pre-specified secondary end points in ORION 1. Circulation. 2018;138:1304–16.
Ray KK, Troquay RP, Visseren FL, Leiter LA, Wright RS, Vikarunnessa S, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023. https://doi.org/10.1016/S2213-8587(22)00353-9.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–19.
Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382:1520–30.
Khan SA, Naz A, Qamar Masood M, Shah R. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69–73.
Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. 2021;77:1182–93.
Cicero A, Fogacci F, Zambon A, Toth P, Borghi C. Efficacy and safety of inclisiran a newly approved FDA drug: a systematic review and pooled analysis of available clinical studies. Am Heart J Plus. 2022. https://doi.org/10.1016/j.ahjo.2022.100127.
Toth PP, Bray S, Villa G, Palagashvili T, Sattar N, Stroes ESG, et al. Network meta-analysis of randomized trials evaluating the comparative efficacy of lipid-lowering therapies added to maximally tolerated statins for the reduction of low-density lipoprotein cholesterol. J Am Heart Assoc. 2022;11: e025551.
Banach M, Surma S, Reiner Z, Katsiki N, Penson PE, Fras Z, et al. Personalized management of dyslipidemias in patients with diabetes—it is time for a new approach (2022). Cardiovasc Diabetol. 2022;21:263.
Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337.
Hovingh GK, Lepor NE, Kallend D, Stoekenbroek RM, Wijngaard PLJ, Raal FJ. Inclisiran durably lowers low-density lipoprotein cholesterol and proprotein convertase subtilisin/kexin type 9 expression in homozygous familial hypercholesterolemia: the ORION-2 pilot study. Circulation. 2020;141:1829–31.
Reijman MD, Schweizer A, Peterson ALH, Bruckert E, Stratz C, Defesche JC, et al. Rationale and design of two trials assessing the efficacy, safety, and tolerability of inclisiran in adolescents with homozygous and heterozygous familial hypercholesterolaemia. Eur J Prev Cardiol. 2022;29:1361–8.
Wang X, Wen D, Chen Y, Ma L, You C. PCSK9 inhibitors for secondary prevention in patients with cardiovascular diseases: a Bayesian network meta-analysis. Cardiovasc Diabetol. 2022;21:107.
Crooke ST, Baker BF, Witztum JL, Kwoh TJ, Pham NC, Salgado N, et al. The effects of 2′-O-methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucleic Acid Ther. 2017;27:121–9.
Landmesser U, Haghikia A, Leiter LA, Wright RS, Kallend D, Wijngaard P, et al. Effect of inclisiran, the small-interfering RNA against proprotein convertase subtilisin/kexin type 9, on platelets, immune cells, and immunological biomarkers: a pre-specified analysis from ORION-1. Cardiovasc Res. 2021;117:284–91.
Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA. 2016;316:1383–91.
Ridker PM, Mora S, Rose L, the JUPITER Trial Study Group. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–9.
Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH, TNT Steering Committee and Investigators. Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015;65:1539–48.
Liu X, Wu S, Song Q, Wang X. Visit-to-visit variability of lipid measurements and the risk of myocardial infarction and all-cause mortality: a prospective cohort study. Atherosclerosis. 2020;312:110–6.
Bangalore S, Fayyad R, Messerli FH, Laskey R, DeMicco DA, Kastelein JJ, et al. Relation of variability of low-density lipoprotein cholesterol and blood pressure to events in patients with previous myocardial infarction from the IDEAL trial. Am J Cardiol. 2017;119:379–87.
Descamps O, Tomassini JE, Lin J, Polis AB, Shah A, Brudi P, et al. Variability of the LDL-C lowering response to ezetimibe and ezetimibe + statin therapy in hypercholesterolemic patients. Atherosclerosis. 2015;240:482–9.
Qamar A, Giugliano RP, Keech AC, Kuder JF, Murphy SA, Kurtz CE, et al. Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab: an analysis of FOURIER trial data. JAMA Cardiol. 2019;4:59–63.
Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. Am Heart Assoc. 2019;8: e011765.
Lowenstern A, Navar AM, Li S, Virani SS, Goldberg AC, Louie MJ, et al. Association of clinician knowledge and statin beliefs with statin therapy use and lipid levels (a survey of US practice in the PALM registry). Am J Cardiol. 2019;123:1011–8.
Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–95.
Navar AM, Wang TY, Li S, Robinson JG, Goldberg AC, Virani S, et al. Lipid management in contemporary community practice: results from the provider assessment of lipid management (PALM) registry. Am Heart J. 2017;193:84–92.
Cholesterol Treatment Trialists’ Collaborators. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet. 2022;400:832–45.
Cholesterol Treatment Trialists’ Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90.
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–61.
Herrett E, Williamson E, Brack K, Beaumont D, Perkins A, Thayne A, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ. 2021;372: n135.
Fung V, Graetz I, Reed M, Jaffe MG. Patient-reported adherence to statin therapy, barriers to adherence, and perceptions of cardiovascular risk. PLoS ONE. 2018;13: e0191817.
Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–14.
Bagepally BS, Sasidharan A. Incremental net benefit of lipid-lowering therapy with PCSK9 inhibitors: a systematic review and meta-analysis of cost-utility studies. Eur J Clin Pharmacol. 2022;78:351–63.
Hlatky MA, Kazi DS. PCSK9 inhibitors. Economics and policy. J Am Coll Cardiol. 2017;70:2677–87.
Farnier M, Colhoun HM, Sasiela WJ, Edelberg JM, Asset G, Robinson JG. Long-term treatment adherence to the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab in 6 ODYSSEY phase III clinical studies with treatment duration of 1 to 2 years. J Clin Lipidol. 2017;11:986–97.
Hines DM, Rane P, Patel J, Harrison DJ, Wade RL. Treatment patterns and patient characteristics among early initiators of PCSK9 inhibitors. Vasc Health Risk Manag. 2018;14:409–18.
Bradley CK, Shrader P, Sanchez RJ, Peterson ED, Navar AM. The patient journey with proprotein convertase subtilisin/kexin type 9 inhibitors in community practice. J Clin Lipidol. 2019;13:725–34.
Ray KK, Stoekenbroek RM, Kallend D, Nishikido T, Leiter LA, Landmesser U, et al. Effect of 1 or 2 doses of inclisiran on low-density lipoprotein cholesterol levels. JAMA Cardiol. 2019;4:1067.
Institute for Clinical and Economic Review. High cholesterol: an assessment of treatments for high cholesterol. 2022. https://icer.org/assessment/high-cholesterol-2021/. Accessed 15 Nov 2022.
Desai NR, Campbell C, Electricwala B, Petrou M, Trueman D, Woodcock F, et al. Cost effectiveness of inclisiran in atherosclerotic cardiovascular patients with elevated low-density lipoprotein cholesterol despite statin use: a threshold analysis. Am J Cardiovasc Drugs. 2022;22:545–56.
Cordero A, Santos-Gallego CG, Facila L, Rodriguez-Manero M, Bertomeu-Gonzalez V, Castellano JM, et al. Estimation of the major cardiovascular events prevention with Inclisiran. Atherosclerosis. 2020;313:76–80.
Acknowledgements
None to report.
Funding
None.
Author information
Authors and Affiliations
Contributions
TN conceived and designed the paper, performed the literature review, wrote the manuscript, produced the figures, and critically revised the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nishikido, T. Clinical potential of inclisiran for patients with a high risk of atherosclerotic cardiovascular disease. Cardiovasc Diabetol 22, 20 (2023). https://doi.org/10.1186/s12933-023-01752-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01752-4