Abstract
Background
Circulating thrombospondin-2 (TSP2) levels were associated with the development of heart failure (HF) in recent studies. However, these studies included only a minority of patients with type 2 diabetes, which is associated with an increased HF risk. As hyperglycemia induces TSP2 expression and its tissue expression increases in type 2 diabetes, we investigated the prospective association of circulating TSP2 with incident HF hospitalization (HHF), and its associations with longitudinal changes of echocardiographic parameters in type 2 diabetes.
Methods
Baseline serum TSP2 levels were measured in 4949 patients with type 2 diabetes to determine its association with incident HHF using multivariable Cox regression analysis. In the echocardiographic study, baseline serum TSP2 levels were measured in another 146 patients with type 2 diabetes but without cardiovascular diseases who underwent detailed transthoracic echocardiography at baseline and after 1 year.
Results
Over a median follow-up of 7.8 years, 330 of 4949 patients (6.7%) developed incident HHF. Baseline serum TSP2 levels were independently associated with the development of HHF (HR 1.31, 95%CI 1.06–1.62, p = 0.014) after adjustments for baseline conventional cardiovascular risk factors, atrial fibrillation, estimated glomerular filtration rate, albuminuria and high-sensitivity C-reactive protein level, use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, loop-diuretics, aspirin, insulin, metformin and sodium-glucose co-transporter 2 inhibitors. Moreover, baseline serum TSP2 levels were independently associated with increase in average E/e’ and left atrial volume index (p = 0.04 and < 0.01, respectively).
Conclusion
Serum TSP2 levels were independently associated with both incident HHF and deterioration in diastolic function in type 2 diabetes.
Trial registration
Not Applicable
Similar content being viewed by others
Introduction
Thrombospondin-2 (TSP2) is a matricellular protein that interacts with various ligands such as extracellular matrix (ECM) structural proteins and is implicated in the pathogenesis of cardiovascular diseases (CVD)[1, 2]. Circulating TSP2 levels have been associated with adverse cardiovascular outcomes in patients with heart failure (HF) with preserved and reduced ejection fraction (HFpEF and HFrEF, respectively) [3, 4]. Recently, plasma proteomic profiling had demonstrated that circulating TSP2 level was associated with incident HF in a population-based cohort, as well as the presence of acute HF among individuals attending the emergency department, independent of N-terminal pro-hormone BNP (NT-proBNP) and B-type natriuretic peptide (BNP), respectively [5, 6]. In patients with advanced HF, circulating TSP2 levels decreased significantly from baseline following heart transplantation [5, 6]. However, in all these cohorts, patients with type 2 diabetes only constituted a small proportion, and the prognostic significance of circulating TSP2 levels with regard to HF in type 2 diabetes remains undefined. This is a significant knowledge gap because while patients with type 2 diabetes are at high risk of HF and its adverse outcomes [7, 8], the use of several anti-diabetic agents such as glitazones and sodium glucose co-transporter 2 inhibitors (SGLT2i) has been associated with altered risk of hospitalization for heart failure (HHF) [9, 10]. Most importantly, hyperglycemia induces TSP2 expression and increased tissue expression has been observed in patients with type 2 diabetes [11,12, In Part 1, a total of 4949 patients with type 2 diabetes were included in the study. At baseline, serum TSP2 levels positively correlated with age, BMI, WC, systolic BP and serum hsCRP levels, and negatively correlated with eGFR levels of the study participants (all p < 0.001). (Supplemental Table S1) Moreover, serum TSP levels were significantly higher in men (p < 0.001), and among participants who had hypertension (p < 0.001), atrial fibrillation (p < 0.001), CVD (p = 0.004) and albuminuria (p < 0.001) than those who did not. (Supplemental Table S2) Over a median follow-up of 7.8 years, 330 (6.7%) of them developed incident HHF, with a rate of 8.7 per 1000 person-years. Those who developed HHF had significantly longer duration of diabetes (p < 0.001), higher BMI (p = 0.002), WC (p < 0.001), systolic BP (p < 0.001), prevalence of dyslipidemia (p = 0.007), atrial fibrillation (p < 0.001), CVD (p < 0.001) and albuminuria (p < 0.001) at baseline than those who did not. Participants with incident HHF also had significantly higher glycated hemoglobin (HbA1c) and hsCRP, but lower eGFR levels (all p < 0.001) at baseline than those without. Moreover, there were significantly more users of insulin, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta-blockers, loop-diuretics, statin and aspirin (all p < 0.001) but less users of metformin (p = 0.021) among participants who had incident HHF than those who did not. Importantly, baseline serum TSP2 levels were significantly higher in participants with incident HHF than those without (Men: 3.70 ng/ml vs. 3.32 ng/ml; women: 4.51 ng/ml vs. 3.71 ng/ml; p < 0.001) (Table 1). In multivariable Cox regression analysis, baseline serum TSP2 levels were independently associated with the development of HHF (HR 1.27, 95%CI 1.03–1.58, p = 0.028), together with age (HR 1.04, 95%CI 1.03–1.06, p < 0.001), duration of diabetes (HR 1.01, 95%CI 1.00–1.03, p = 0.034), BMI (HR 1.04, 95%CI 1.02–1.07, p = 0.003), systolic BP (HR 1.008, 95%CI 1.003–1.010, p = 0.009), HbA1c (HR 1.14, 95%CI 1.05–1.23, p < 0.001), eGFR (HR 0.98, 95%CI 0.98–0.99, p < 0.001), presence of atrial fibrillation (HR 1.96, 95%CI 1.44–2.67, p < 0.001) and albuminuria (HR 2.18, 95%CI 1.65–2.88, p < 0.001), in a model also consisting of sex, smoking status, dyslipidemia, CVD and serum hsCRP levels at baseline (Table 2). The association between baseline serum TSP2 levels and incident HHF remained significant after adjustments for the use of insulin, metformin, ACEI/ARB, beta-blockers, loop-diuretics and aspirin (HR 1.29, 95%CI 1.04–1.60, p = 0.02), as well as the time-dependent cDDD of SGLT2i (HR 1.31, 95%CI 1.06–1.62, p = 0.014) (Table 3). When study participants were stratified by the presence of CVD at baseline, serum TSP2 levels were significantly associated with incident HHF among those with baseline CVD (p = 0.029) but not in those without. Notably, 9.4% and 5.3% of participants with and without CVD at baseline developed HHF, respectively (Table 3). Since SGLT2i was not locally available until 2015, a sensitivity analysis was further conducted involving only participants who had survived and remained free of HHF in 2015. Among these 4812 participants, 296 developed HHF after 2015. In multivariable Cox regression analysis, baseline serum TSP2 levels remained significantly associated with incident HHF (HR 1.32, 95%CI 1.06–1.64, p = 0.014), together with the use of SGLT2i with cDDD ≥ 180 (HR 0.42, 95%CI 0.20–0.90, p = 0.025) (Supplementary Table S3). The addition of serum TSP2 levels into a clinical model, which consisted of age, sex, duration of diabetes, smoking status, BMI, hypertension, dyslipidemia, atrial fibrillation, CVD, HbA1c, CKD, albuminuria and serum hsCRP level at baseline, led to a significant improvement of c-statistics in predicting HHF from 0.79 (95%CI 0.78–0.81) to 0.81 (95%CI 0.79–0.83) (p = 0.003). This was accompanied by improvement in both NRI (0.33, 95%CI 0.26–0.41; p < 0.001) and IDI (0.02, 95%CI 0.01–0.03; p < 0.001) (Table 4), In Part 1, the association between baseline serum TSP2 levels and incident HHF seemed to be more readily observed among patients who had type 2 diabetes and CVD. To evaluate whether serum TSP2 levels were still associated with changes in ventricular function in patients with type 2 diabetes but without CVD, a longitudinal echocardiographic analysis was conducted as Part 2 of the study in 146 participants with type 2 diabetes who did not have CVD at baseline. Among them, participants who were in the highest serum TSP2 tertile had significantly higher baseline BMI than those in the lower tertiles (p < 0.01). Otherwise, their baseline clinical characteristics were comparable between the two groups (Supplemental Table S4). Table 5 summarizes the echocardiographic parameters of these 146 participants at baseline and follow-up after a median interval of 27 months (Range 12–46 months). Compared to participants in the lower baseline serum TSP2 tertiles, those in the highest serum TSP2 tertile had significantly higher LVPWd and LV mass at baseline (both p < 0.05), as well as lower LVEF (p < 0.05), higher average E/e’ (p < 0.01) and LAVi (p < 0.01) at follow-up. Importantly, the increase in average E/e’ and LAVi from baseline were both significantly greater among participants in the highest serum TSP2 tertile than those in the lower tertiles (Both p < 0.05). In multivariable linear regression analysis, baseline serum TSP2 levels were positively and independently associated with both changes in average E/e’ (beta 0.75, 95%CI 0.02–1.49, p = 0.04) and LAVi (beta 4.12, 95%CI 1.48–6.77, p < 0.01) (Table 6). To our knowledge, the current study is the first demonstration of a clinically significant association between circulating TSP2 levels and incident HHF in an exclusively diabetic population, known to be at increased HF risk, and was independent of the use of SGLT2i during the study period. Moreover, using an echocardiographic study, we found that baseline serum TSP2 levels were significantly associated with left ventricular remodeling and the subsequent deterioration of diastolic function in patients with type 2 diabetes but without CVD, highlighting the potential of circulating TSP2 level as a novel biomarker of HF in type 2 diabetes, regardless of the presence of CVD. Although the association between circulating TSP2 and HF has been demonstrated previously in studies involving community-dwelling individuals and patients across the various stages of HF, only a minority of the participants had type 2 diabetes in all these studies [3,4,5,6, 27]. Notably, type 2 diabetes significantly elevates HF risk and is also associated with increased TSP2 tissue expression [7, 11,12,27]. Similarly, another recent study demonstrated that circulating TSP2 levels were inversely correlated with LVEF measured at 4 months post-myocardial infarction [28]. Therefore, our findings that circulating TSP2 levels were also associated with indices of LV hypertrophy and subsequent development of diastolic dysfunction in patients without pre-existing CVD or HF at baseline provided further clinical support that TSP2 could possibly be implicated in adverse left ventricular remodeling earlier on, even before the development of clinical HF. TSP2 is a pro-fibrotic and anti-angiogenic protein that plays a major role in matrix assembly during tissue injury and remodelling processes [29, 30]. In preclinical studies, lack of TSP2 expression in mice after fetal murine cardiomyocyte grafting was shown to promote graft integration, vascularization leading to better graft survival [30]. On the other hand, mice with genetic ablation of TSP2 had difficulty to cope with increased cardiac loading, with augmented matrix metalloproteinase (MMP)-2 and MMP-9 activities which disrupted myocardial matrix integrity, resulting in cardiomyopathy and fatal cardiac rupture [31]. Subsequent studies also demonstrated that the absence of TSP2 predisposed mice to age-related dilated cardiomyopathy, doxorubicin-induced cardiomyopathy and heightened cardiac inflammation in viral myocarditis [24,25,26,27,28,29,30,31,32, 34]. Interestingly, TSP2 expression in the heart was paradoxically elevated during cardiac injury in both rodents and humans, especially among those that would further progress to HF with worse outcomes [31, 34]. Moreover, TSP2 expression was significantly higher in human hypertrophied hearts that were accompanied by LV systolic dysfunction than those with preserved LVEF [31]. Consistently, in our echocardiographic study, participants in the highest serum TSP2 tertile had significantly greater LV mass and LVPWd at baseline indicating LV hypertrophy. Furthermore, they also experienced significantly more deterioration in average E/e’ and LAVi which were two indices of diastolic dysfunction. Taken together, it is likely that TSP2 activation forms part of an important early-stage molecular program that precedes the development of HF. In other words, the resultant high cardiac TSP2 expression, and possibly its circulating levels in HF, may represent a futile adaptive response that was initially a bodily attempt to overcome HF development. Indeed, recent in-vitro studies demonstrated that pro-inflammatory and pro-fibrotic stimuli could increase THBS2 gene expression, which encodes TSP2, in cardiac fibroblasts [28]. Therefore, in patients with established CVD, this compensatory protective mechanism against cardiac dysfunction might even be more pronounced leading to their higher circulating TSP2 levels. Furthermore, this could also explain the significant reduction in circulating TSP2 levels from baseline following heart transplantation, as demonstrated in recent studies [5, 6], and the observation in the current study that a significant association of serum TSP2 levels with incident HHF was found only amongst those with CVD at baseline. Our study has a few limitations. First, the observational study design in both parts of our study precludes any inferred causal relationship between high circulating TSP2 levels and incident HF. Secondly, echocardiographic data is not available in Part 1 of our study, and hence rendering it difficult to evaluate the relationship between baseline serum TSP2 levels and longitudinal changes in ECHO parameters in patients who had type 2 diabetes and CVD. Thirdly, serum TSP2 levels were measured only once in all participants and it was therefore impossible to investigate if changes in serum TSP2 levels would also contribute to the development of HHF. Furthermore, external validation of our findings using an independent cohort was not performed. Lastly, neither BNP nor NT-proBNP level was measured in our study. Interestingly, in a preclinical study of hypertensive renin-overexpressing rats, although the cardiac expression of BNP and TSP2 were both up-regulated with pressure overload, it was found that TSP2, but not BNP, more reliably identified those that were prone to develop HF progression [31]. Nonetheless, as ELISA kits for measuring serum TSP2 levels are now commercially available from various sources, further studies, preferably together with the measurements of these established HF markers, are clearly required to validate our current findings, as well as to assess the cost-effectiveness of using this novel biomarker for early HF risk stratification in type 2 diabetes. Over the years, reductions in cardiovascular mortality due to coronary heart disease and stroke have been reported especially among older individuals with type 2 diabetes [35]. However, mortality related to HF did not change significantly and remained high. On the other hand, the advent of SGLT2i has introduced a paradigm shift in the management of type 2 diabetes and HF, with several landmark randomized controlled trials showing consistent benefits in reducing the rates of HHF regardless of the presence of CVD and the status of LV function [14,15,16,17,18, 36,37,38,39,40] While we recently demonstrated that circulating TSP2 had the potential to become a novel prognostic marker of liver fibrosis in type 2 diabetes [41], our current findings further proposed that it might also be usefully employed for HF risk stratification in patients with type 2 diabetes.Results
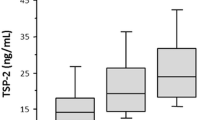
Baseline serum TSP2 levels were higher in patients with type 2 diabetes who developed HHF
Baseline serum TSP2 levels were independently associated with incident HHF in type 2 diabetes
Discrimination and reclassification performance of the addition of baseline serum TSP2 levels in predicting HHF in type 2 diabetes
Baseline serum TSP2 levels were associated with deterioration in average E/e’ and LAVi in patients with type 2 diabetes but without CVD
Discussion
Conclusion
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A wave:
-
Peak trans-mitral flow velocities in late diastole
- ACEI:
-
Angiotensin converting enzyme inhibitors
- ARB:
-
Angiotensin II receptor blockers
- BMI:
-
Body mass index
- BNP:
-
B-type natriuretic peptide
- BP:
-
Blood pressure
- BW:
-
Body weight
- cDDD:
-
Cumulative daily defined dose
- CDHS:
-
Chinese Diabetic Heart Study
- CI:
-
Confidence interval
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- CVD:
-
Cardiovascular diseases
- DDD:
-
Daily defined dose
- E’ Peak:
-
velocities of septal and lateral mitral annulus in early diastole
- ECHO:
-
Echocardiography
- ECM:
-
Extracellular matrix
- eGFR:
-
Estimated glomerular filtration rate
- E-wave:
-
Peak trans-mitral flow velocities in early diastole
- HbA1c:
-
Glycated haemoglobin
- HDL-C:
-
High-density lipoprotein cholesterol
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HHF:
-
Hospitalization for heart failure
- HKWDR:
-
Hong Kong West Diabetes Registry
- HR:
-
Hazard ratio
- hsCRP:
-
High sensitivity C-reactive protein
- IDI:
-
Integrated discrimination index
- IVSd:
-
Inter-ventricular septal dimension at end-diastole
- LAV:
-
Left atrial volume
- LAVi:
-
Left atrial volume index
- LDL-C:
-
Low-density lipoprotein cholesterol
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVPWd:
-
Left ventricular posterior wall thickness at end-diastole
- MMP:
-
Matrix metalloproteinase
- NRI:
-
Net reclassification index
- NTproBNP:
-
N-terminal pro-hormone BNP
- SD:
-
Standard deviation
- SGLT2i:
-
Sodium glucose co-transporter 2 inhibitors
- TG:
-
Triglyceride
- TSP2:
-
Thrombospondin-2
- UACR:
-
Urine to albumin creatinine ratio
- WC:
-
Waist circumference
References
Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36(6):1115–25.
Zhang K, Li M, Yin L, Fu G, Liu Z. Role of thrombospondin1 and thrombospondin2 in cardiovascular diseases (Review). Int J Mol Med. 2020;45(5):1275–93.
Hanatani S, Izumiya Y, Takashio S, Kimura Y, Araki S, Rokutanda T, Tsujita K, Yamamoto E, Tanaka T, Yamamuro M, et al. Circulating thrombospondin-2 reflects disease severity and predicts outcome of heart failure with reduced ejection fraction. Circ J. 2014;78(4):903–10.
Kimura Y, Izumiya Y, Hanatani S, Yamamoto E, Kusaka H, Tokitsu T, Takashio S, Sakamoto K, Tsujita K, Tanaka T, et al. High serum levels of thrombospondin-2 correlate with poor prognosis of patients with heart failure with preserved ejection fraction. Heart Vessels. 2016;31(1):52–9.
Egerstedt A, Berntsson J, Smith ML, Gidlof O, Nilsson R, Benson M, Wells QS, Celik S, Lejonberg C, Farrell L, et al. Profiling of the plasma proteome across different stages of human heart failure. Nat Commun. 2019;10(1):5830.
Wells QS, Gupta DK, Smith JG, Collins SP, Storrow AB, Ferguson J, Smith ML, Pulley JM, Collier S, Wang X, et al. Accelerating biomarker discovery through electronic health records, automated biobanking, and proteomics. J Am Coll Cardiol. 2019;73(17):2195–205.
Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, et al. Type 2 diabetes mellitus and heart failure, a scientific statement from the American heart association and heart failure society of America. J Card Fail. 2019;25(8):584–619.
Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI With ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 2016, 9(1).
Erdmann E, Charbonnel B, Wilcox RG, Skene AM, Massi-Benedetti M, Yates J, Tan M, Spanheimer R, Standl E, Dormandy JA, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care. 2007;30(11):2773–8.
Giugliano D, Longo M, Scappaticcio L, Bellastella G, Maiorino MI, Esposito K. SGLT-2 inhibitors and cardiorenal outcomes in patients with or without type 2 diabetes: a meta-analysis of 11 CVOTs. Cardiovasc Diabetol. 2021;20(1):236.
Raman P, Harry C, Weber M, Krukovets I, Stenina OI. A novel transcriptional mechanism of cell type-specific regulation of vascular gene expression by glucose. Arterioscler Thromb Vasc Biol. 2011;31(3):634–42.
Bae ON, Wang JM, Baek SH, Wang Q, Yuan H, Chen AF. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013;33(8):1920–7.
Kunkemoeller B, Bancroft T, **ng H, Morris AH, Luciano AK, Wu J, Fernandez-Hernando C, Kyriakides TR: Elevated Thrombospondin-2 contributes to delayed wound healing in diabetes. Diabetes 2019.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021.
Lee CH, Cheung CYY, Woo YC, Lui DTW, Yuen MMA, Fong CHY, Chow WS, Xu A, Lam KSL. Prospective associations of circulating adipocyte fatty acid-binding protein levels with risks of renal outcomes and mortality in type 2 diabetes. Diabetologia. 2019;62(1):169–77.
Chen Y, Zhao CT, Zhen Z, Wong A, Tse HF, Yiu KH. Association of myocardial dysfunction with vitamin D deficiency in patients with type 2 diabetes mellitus. J Diabetes Complicat. 2014;28(3):286–90.
Wu MZ, Lee CH, Chen Y, Yu SY, Yu YJ, Ren QW, Fong HC, Wong PF, Tse HF, Lam SK, et al. Association between adipocyte fatty acid-binding protein with left ventricular remodelling and diastolic function in type 2 diabetes: a prospective echocardiography study. Cardiovasc Diabetol. 2020;19(1):197.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Chapter 2. Definition, identification, and prediction of CKD progression. Kidney International Supplements 2013(3):63–72.
Methodology WCCfDS: Anatomical Therapeutic Chemical (ATC) / Defined Daily Dose (DDD) index on sodium-glucose co-transporter 2 inhibitors. 2019.
D. S: Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika 1980, 67(1):145–153.
Kresoja KP, Rommel KP, Wachter R, Henger S, Besler C, Kloting N, Schnelle M, Hoffmann A, Buttner P, Ceglarek U, et al. Proteomics to improve phenoty** in obese patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(10):1633–44.
Chan MY, Efthymios M, Tan SH, Pickering JW, Troughton R, Pemberton C, Ho HH, Prabath JF, Drum CL, Ling LH, et al. Prioritizing candidates of post-myocardial infarction heart failure using plasma proteomics and single-cell transcriptomics. Circulation. 2020;142(15):1408–21.
Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831–9.
Reinecke H, Robey TE, Mignone JL, Muskheli V, Bornstein P, Murry CE. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc Pathol. 2013;22(1):91–5.
Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95(5):515–22.
Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120(16):1585–97.
van Almen GC, Swinnen M, Carai P, Verhesen W, Cleutjens JP, D’Hooge J, Verheyen FK, Pinto YM, Schroen B, Carmeliet P, et al. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 2011;51(3):318–28.
Papageorgiou AP, Swinnen M, Vanhoutte D, VandenDriessche T, Chuah M, Lindner D, Verhesen W, de Vries B, D’Hooge J, Lutgens E, et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc Res. 2012;94(1):115–24.
Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, Imperatore G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391(10138):2430–40.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Lee CH, Seto WK, Lui DT, Fong CH, Wan HY, Cheung CY, Chow WS, Woo YC, Yuen MF, Xu A, et al. Circulating Thrombospondin-2 as a novel fibrosis biomarker of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Care 2021.
Acknowledgements
We thank Ms. Rachel Wong for her technical assistance in the measurements of serum TSP2 levels.
Funding
This work was supported by Hong Kong Research Grants Council/ Area of Excellence (AoE/M/707-18).
Author information
Authors and Affiliations
Contributions
C.H.L. and M.Z.W. researched the data and wrote the manuscript. D.T.W.L., Q.W.R., S.Y.Y., M.M.A.Y., W.S.C. and J.Y.H. researched the data. C.H.Y.F. performed statistical analyses. A.X., K.H.Y. and K.S.L.L. critically reviewed and edited the manuscript. K.S.L.L. and K.H.Y. initiated and supervised the study, had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocols were approved by the Institutional Review Board of the University of Hong Kong / Hospital Authority Hong Kong West Cluster (Ref: UW 07-378 and UW 11–121, respectively). Written informed consent was obtained from all recruited participants prior to any study related procedures.
Consent for publication
All authors have approved the manuscript and agreed with its publication.
Competing interests
K.S.L. is an advisory board member of Merck Sharp and Dohme. C.H.L. received speaker’s fees from AstraZeneca, Bayer and Sanofi Aventis. The remaining authors have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12933_2022_1646_MOESM1_ESM.pdf
Supplementary Material 1. Supplemental Table S1 Pearson correlation analysis of serum TSP2 level with clinical variables at baseline. Supplemental Table S2 Serum TSP2 level and baseline clinical characteristics at baseline. Supplementary Table S3 Sensitivity analysis showing the association between baseline circulating TSP2 levels and incident HF hospitalization in participants who survived and remained free of outcome events in 2015 (N = 4812). Supplementary Table S4 Baseline characteristics of the participants by serum TSP2 levels in Part 2 of the study (N = 146).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, C., Wu, M., Lui, D. et al. Prospective associations of circulating thrombospondin-2 level with heart failure hospitalization, left ventricular remodeling and diastolic function in type 2 diabetes. Cardiovasc Diabetol 21, 231 (2022). https://doi.org/10.1186/s12933-022-01646-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01646-x