Abstract
Diabetes is a metabolic disorder that affects millions of people worldwide. Diabetic heart disease (DHD) comprises coronary artery disease, heart failure, cardiac autonomic neuropathy, peripheral arterial disease, and diabetic cardiomyopathy. The onset and progression of DHD have been attributed to molecular alterations in response to hyperglycemia in diabetes. In this context, microRNAs (miRNAs) have been demonstrated to have a significant role in the development and progression of DHD. In addition to their effects on the host cells, miRNAs can be released into circulation after encapsulation within the exosomes. Exosomes are extracellular nanovesicles ranging from 30 to 180 nm in diameter secreted by all cell types. They carry diverse cargos that are altered in response to various conditions in their parent cells. Exosomal miRNAs have been extensively studied in recent years due to their role and therapeutic potential in DHD. This review will first provide an overview of exosomes, their biogenesis and function, followed by the role of exosomes in cardiovascular disease and then focuses on the known role of exosomes and associated miRNAs in DHD.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a chronic metabolic disorder that has a strong association with the development of cardiovascular diseases, referred to as diabetic heart disease (DHD) [1]. DHD is a broad definition encapsulating a cluster of conditions, including coronary artery disease, heart failure, cardiac autonomic neuropathy, peripheral arterial disease and diabetic cardiomyopathy [2,3,4,5,6]. A cohort study conducted by Shah et al. demonstrated that heart failure and peripheral arterial disease are the most common initial manifestations of cardiovascular disease in type 2 diabetic individuals [7]. Additionally, the Framingham study has shown that diabetic women had a higher risk of develo** cardiovascular disease and congestive heart failure than diabetic males [4]. The Framingham study also implied the direct association between diabetes and the development of cardiomyopathy independent of any associated comorbidities [2].
Multiple studies have identified several molecular alterations in response to diabetes that underlie the development of diabetes-induced comorbidities [8,9,10,11,12,13,14,15,16]. Among these mechanisms, microRNAs (miRNAs) have been extensively studied. Results have shown a clear link between miRNA dysregulation and DHD [9, 10, 12,13,14, 17,18,19,20,21]. Of note, circulating miRNAs under DHD conditions have been demonstrated to have the potential to act as biomarkers [22,23,24]. Additionally, these circulating miRNAs can also affect the progression of disease under diabetic conditions[25,26,27,28]. Further, in addition to being circulated in naïve form, miRNAs can also be encapsulated into exosomes. Exosomes are a common nanovesicle that can encapsulate miRNAs and transport them in circulation [29, 30]. miRNAs isolated from plasma exosomes showed higher stability than miRNAs isolated directly from plasma [29]. Exosomes are efficiently engulfed by recipient cells, allowing miRNAs to exert their effects [31,32,33].This review will first provide an overview of the biogenesis and function of exosomes and miRNAs. Next it will focus on the role of exosomes in cardiovascular disease and the known role of exosomes and the miRNAs encapsulated within them in DHD.
Exosomes
Exosome biogenesis
Exosome biogenesis. The invagination of the cell membrane results in the formation of the early endosome (A). Various budding into the endosomal lumen, initiates the process of forming exosomes/intraluminal vesicles in the late sorting endosome (B). Cargo is shuttled between the trans-golgi network (C), endoplasmic reticulum (D)and the late sorting endosome (B). Cumulatively, this leads to the formation of a multivesicular body (E) with fully formed intraluminal vesicles/exosomes in it. The multivesicular body can be further processed within the cell by the lysosome (F) or autophagosome (G) to breakdown the components of the multivesicular body into the cell. Otherwise, the multivesicular body can be docked (H) and fused to the cell membrane to release the exosomes (I) into the extracellular space. Exosomes can be characterized using different surface markers (J) that are consistently expressed by them. Exosomal functionality (K) is based on the varying cargo within the exosomes. Image Created with Biorender.com with valid license
Biogenesis of exosomes begins in the endosomal system, where the early endosomes mature into late endosomes [34]. During the maturation process, the endosomal membrane undergoes further invagination [34], resulting in the formation of intraluminal vesicles (ILVs) or exosomes within the organelles [34]. The accumulation of exosomes in the endosome results in the formation of multivesicular bodies (MVBs) [35]. The MVBs then fuse with the plasma membrane to release exosomes into the extracellular space [36]. The Ras associated binding protein (Rab) family of small GTPases is involved in intracellular vesicle transportation through cytoskeletal interactions and docking of the vesicles to the target compartments [36, 37]. The soluble N-ethylmaleimide-sensitive fusion attachment protein (SNAP) receptors (SNAREs) play a role in the docking and fusion of the MVB to the plasma membrane [38]. The exosomes released will have surface markers from their origin cells [39] (Summarized in Fig. 1).
Exosome cargo sorting
The endosomal sorting complex required for transport (ESCRT) plays an essential role in forming ILVs in the endosome [40, 41]. Colombo et al. demonstrated that ESCRTs were involved in forming and secreting a heterogeneous subpopulation of exosomal vesicles [41]. They further showed that specific ESCRT components were involved in cargo loading in exosome biogenesis [41]. Other studies demonstrated an ESCRT-independent pathway of exosome biogenesis, as silencing of ESCRTs still resulted in the production of exosomes [37]. Tetraspanins such as CD63, CD9 and CD81 play an essential role in ESCRT-independent cargo loading into exosomes [42]. Tetraspanins organize the exosomal membrane into tetraspanin enriched domains [42, 43]. This formation is through the interaction between the exosome tetraspanins and other cellular tetraspanins, cytosolic proteins and lipids [42, 43]. Therefore sorting exosome cargos into exosomes occurs in both ESCRT-dependent and independent pathways [37, 44].
Interestingly, despite being a nanoparticle, exosomes can contain a variety of cargo. Studies have demonstrated the presence of various DNA molecules [45], RNAs such as miRNAs, circular RNAs and messenger RNAs [46], and proteins [47]. Multiple studies have also shown the functional importance of these cargos [32, 48, 49]. Exosome cargos are altered depending on the physiological or pathological conditions of the parent cells [49, 50]. Many studies have demonstrated selective chaperoning of miRNAs into exosomes under pathological conditions [51, 52]. As exosomes prolong the miRNA stability in circulation, they can also be considered a potential biomarker or a therapeutic target for various diseases [49, 50] (Summarized in Fig. 1).
Exosome uptake
Exosome surface markers and cell surface markers determine the selectivity of exosome uptake by the recipient cells [48, 53, 54]. Exosomes can be taken up by nearby recipient cells (juxtracrine uptake), cells further away from donor cells (paracrine uptake), as well as by cells in distant tissues (endocrine) by travelling through the systemic circulation [23, 48, 55,56,57,58,59]. The uptake of exosomes by the selected host cells can occur through either the phagolysosomes, utilizing exosomal surface proteins, or phosphatidylserine receptors [48, 54, 55, 60]. Tian et al. demonstrated that exosomes were internalized through the endocytosis pathway, where the intracellular exosomes were trapped in vesicles and actively transported [53]. In contrast, Feng et al. demonstrated efficient exosome uptake by phagocytic cells, where the exosomes were targeted to large phagolysosomes [61]. They further concluded that the type of exosomal uptake was dependent on the type of cargos and surface markers expressed in the exosomes [61].
Once internalized, the exosomes release their cargo via the phagolysosome or by being localized in the late endosome [53, 61]. Tian et al. demonstrated that exosomal proteins separate from the exosomes approximately 3 h post-internalization, while exosome lipids were re-cycled into the plasma membrane [53]. Interestingly, Montecalvo et al. demonstrated that exosomes dock, bind and then fuse with the recipient cells, [62] thereby “injecting” the exosomal miRNAs into the recipient cells [62]. This injection allows the exosomal miRNAs to reach the recipient cell cytosol to initiate its functional processes [62]. Concerning the endocytosis exosomal uptake pathway, Tian et al. demonstrated that following endocytosis, exosomes were stored in lysosomes where the exosomal proteins were stored and utilized [55]. However, while they were able to determine successful miRNA transfer, they could not determine the exact pathway or mechanism of content release for exosomal RNAs [55] (Summarized in Fig. 1).
miRNAs
miRNAs are short non-coding RNAs (~ 22 nucleotides) which post-transcriptionally alters protein expression by inhibiting the translation of messenger RNA. Under conditions such as diabetes, the alterations that occur at the gene coding regions lead to changes in miRNA expression [63, 64]. These changes induce pathophysiological alterations such as accelerated cardiomyocyte death, vascular remodelling, fibrosis, arrhythmias and stem cells dysfunction [8, 9, 12, 65, 66], all of which play a critical role in the progression of DHD [13, 67,68,69].
miRNA biogenesis
The biogenesis of of miRNAs is initiated in the nucleus where the introns of protein-coding genes or independent genes are initially transcribed by RNA polymerase II/III to form primary miRNA transcripts (pri-miRNA) [70, 71]. The RNase III protein Drosha then processes the pri-miRNA with its essential cofactor DiGeorge syndrome critical region 8 (DGCR8), a microprocessor complex subunit to generate ~ 70 nucleotides long precursor miRNAs (pre-miRNAs) [72].
In the presence of the Ran-GTP cofactor, Exportin-5 binds specifically to pre-miRNAs, facilitating nuclear export of the pre-miRNAs into the cytoplasm[73]. The final stages of miRNA processing occur in the cytoplasm, where the pre-miRNA was then cleaved by the enzyme Dicer to generate the ~ 22 nucleotide miRNA duplex [70]. In order to generate mature miRNA, the miRNA duplex needs to be unwound [74]. Once unwound, the mature miRNAs are bound to argonaute proteins to maintain their stability within the cytoplasm [75].
miRNA function
Mature miRNAs bind with RNA-induced silencing complexes (RISCs) to identify their messenger RNA (mRNA) targets[76, 77]. If the complementarity between the miRNA-RISC complex and the target mRNA is partial, the translation of the target mRNA leads to protein repression [78]. If there is exact complementarity between the complex and the target mRNA, the target mRNA undergoes cleavage and degradation [78, 79]. This feature of the miRNA allows it to target multiple genes and hence regulating multiple different pathological processes in its host cells [78,79,80,81,82].
Physiological and pathological roles of miRNA
As miRNAs have a diverse range of targets, they control both physiological and pathological processes [83]. Physiologically, miRNAs regulate processes such as development, cardiac hypertrophy and neuronal function[83,84,85]. We along with others have demonstrated the crucial role of miRNAs in various physiological and pathological processes (reviewed elsewhere)[86,87,88,89,90,91,92]. For instance, studies have demonstrated that multiple different miRNAs, including miRNA-26b, -27a, -143 and -150, were upregulated in exercise-induced cardiac hypertrophy [93]. While another study showed significant modulation of miRNA-195, -125b, -199a, and -124 in response to pathological cardiac hypertrophy and heart failure [81], suggesting that different miRNAs respond to physiological or pathological hypertrophy. Furthermore, the cardiac-specific loss of miRNAs by deletion of DGCR8 results in the development of dilated cardiomyopathy and heart failure [85].
Studies have demonstrated alterations in plasma miRNA expression in response to type 2 diabetes [10, 12, 23]. In addition to their effects on host cells, miRNAs are also released into the circulation, affecting remote cells/tissues [94]. Once released into circulation, miRNAs are either bound to proteins, high-density lipoproteins or encapsulated in micro- or nanovesicles such as exosomes [94]. This encapsulation prevents miRNA degradation, increasing their stability in the circulation, allowing miRNAs to have various paracrine effects [29, 94]. Further, this stability also allows miRNAs to function as biomarkers for various conditions [50, 95, 96].
Exosomes in CVD
Pathophysiological role of exosomes in CVD
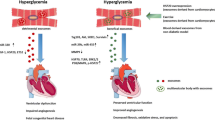
Function of exosomes under DHD conditions. Studies have been conducted on exosomes isolated from DHD biological fluids as well as from culture media under DHD conditions. Exosomes released from patients with DHD has been demonstrated to increase calcification, plaque rupture, adverse remodeling and blood pressure in the vasculature. This was mimicked in the in vitro experiments where diabetic exosomes increased endothelial cell apoptosis, while decreasing proliferation, angiogenesis and migration. Similarly, DHD exosomes in vivo resulted in increased apoptosis, fibrosis and adverse remodeling of the heart as well as reduced cell survival. Just as in in vitro experiments, DHD exosomes promoted cardiomyocyte apoptosis while reducing cardiomyocyte survival
Multiple studies have demonstrated exosomes to have various roles in CVD [31, 49, 57, 97,98,99]. Emmanueli et al. [23] demonstrated the potential of plasma exosomes as a biomarker in for recovery of patients post coronary artery bypass graft (CABG). They demonstrated increased density of the plasma exosomes post-CABG[23]. Importantly, these exosomes had increased expression levels of cardiac-specific miR-1, miR-24, miR-133a and miR-133b, both at 24- and 48-h post-surgery [23]. They also showed that positive correlation between alteration in the miRNA cargos within the exosomes and the expression of cardiac troponin I, a known biomarker for myocardial damage [23]. Another study demonstrated increased brain/head glycogen phosphorylase (PYGB) in circulating exosomes as an early and sensitive biomarker for cardiac injury [100]. The alteration of PYGB expression in exosomes isolated from rodents under doxorubicin-induced cardiac injury was observed before any alterations in the circulating cTn-I expressions [100], suggesting that in addition to miRNAs, exosomes can host protein cargos with biomarker potential. miRNA profiling of plasma exosomes from hypertensive rats showed exosomes could have a crucial role in hypertension [101]. Liu et al. demonstrated differential regulation of 27 exosomal miRNAs in spontaneously hypertensive rats compared to the control normotensive rats [101]. They attributed the differential miRNA expression to discriminatory packaging of the exosomal cargos under pathological conditions [101]. Further, another study demonstrated the role of circulating exosomal miRNA-194 that was upregulated in obesity to play a role in the development of cardiac injury and mitochondrial dysfunction [25]. Interestingly, treatment with a miRNA-194 sponge to downregulate the circulating miRNA levels attenuated obesity-related cardiac dysfunction in vivo [25] (Summarized in Fig. 2).
Therapeutic potential of exosomes in CVD
Exosomes have demonstrated both pro-and anti-angiogenic potential depending on the microenvironment [102, 103]. Using cardiosphere-derived cells (CDC), Ahmed et al. demonstrated that the CDCs treated with GW4869, a known exosome production blocker, lost their beneficial effects [104]. Results showed that CDC derived exosomes promoted angiogenesis and cardiac regeneration in scarred and infarcted hearts through activation of miRNA-146a [104]. This was supported by impaired protection against oxidative stress after injection of exosomes with miRNA-146a knockdown [104]. These data suggest that exosomes have therapeutic potential through multiple pro-angiogenic cargos.
Similarly, Gray et al. demonstrated an improved angiogenic potential of exosomes secreted by cardiac progenitor cells (CPC), extensively tested in CVD [105]. The exosomes produced by CPC under hypoxic conditions had increased angiogenic potential[105]. When added to the cultured endothelial cells, they increased the formation of networks compared to the exosomes produced from normoxic conditions[172]. However, the role of exosomal miRNA-1 and − 133a in circulation would need further study to determine the biomarker potential of exosomal miRNAs in diabetic cardiomyopathy.
Like other diseases, exosomes modified for their gene contents either through knockdown or overexpression have been used to treat of DCM. Induction of type 1 diabetes in transgenic mice overexpressed with HSP20 resulted in the production of beneficial exosomes compared to wild-type controls [99]. Notably, the study also showed that these exosomes, when transferred to the adjacent cells, induced beneficial effects, including angiogenesis, reduced oxidative stress, amelioration of fibrosis and apoptosis in the mouse diabetic heart [99]. Another study showed that cardiomyocyte-derived exosomes exhibited systemic effects and local effects. This study showed that both in vitro cellular stretch and in vivo pressure overload promote cardiomyocytes to produce exosomes enriched with angiotensin II type 1 receptor (AT1R [173]. These enriched exosomes were transferred to mesenteric vasculature and skeletal muscle to modulate peripheral vascular resistance and blood pressure [173].
The presented evidence shows that diabetes induces the release of exosomes from various cells, which plays a crucial role in the pathogenesis of the disease. On the other hand, treatments using exosomes derived from various types of stem cells or genetically modified exosomes with miRNA or genes showed therapeutic benefits in DHD. Under pathological conditions, exosomes released into circulation exhibit specific markers that can indicate the pathological state, making them a potential biomarker[23, 100]. In support of this, studies showed that exosomal miRNA expression was a better diagnostic marker for cardiac events in patients with coronary artery disease than freely circulating miRNAs [51]. This altered expression was primarily due to the selective sorting of miRNAs into exosomes in their host cells prior to release [51] (Pathophysiological role summarized in Fig. 2).
Conclusion and future direction
This review summarised the current knowledge regarding the role of exosomes in CVD and DHD. Exosomes originating from cardiovascular cells provide a means for cell to cell communication by transferring miRNA, protein and cytokine cargos. Alterations in the exosomal cargo sorting in the host cells determine the physiological or pathological responses in the recipient cells. There are still some challenges that need to be overcome before its translation into routine clinical practice. For instance, multiple studies have demonstrated the potential therapeutic effects and biomarker capabilities of exosomal miRNAs. However, only a few have looked into the pathophysiological role of miRNA cargos in the DHD [23, 110, 174, 175]. While these studies provided evidence for the possible involvement of multiple miRNAs within the exosome cargos in the protective/destructive effects exhibited by the exosomes on their recipient cells [32, 175], they are only a fraction of the associated miRNAs with diabetes [8,9,10, 17, 176]. Additionally, most of these studies were either assessing individual disease conditions or had yet to delve into the complex molecular pathways affected by exosomal miRNA cargos [31, 33, 99, 110]. Therefore, further comprehensive analysis is essential in determining the full potential of exosomes as biomarkers and therapeutics for DHD.
Another caveat of the studies using exosomes is the lack of standardised isolation protocols for exosomes. This is primarily due to the requirement of a specific size range of exosomes. Due to the vast difference in the isolation procedures, different studies alternate between naming the vesicles as either exosome, extracellular vesicles or microvesicles. Although exosomes can be identified due to the expression of specific surface markers, studies have shown that these can be altered in response to diabetes or other associated comorbidities. Therefore, establishing a standardised protocol for isolating exosomes from tissues, cells and body fluids is crucial before translating these findings to the clinic.
Data availability
Not applicable.
Abbreviations
- AT1R:
-
Angiotensin II type 1 receptor
- CDC:
-
Cardiosphere-derived cells
- CPC:
-
Cardiac progenitor cells
- CVD:
-
Cardiovascular disease
- DCM:
-
Diabetic cardiomyopathy
- DHD:
-
Diabetic heart disease
- ECDE:
-
Endothelial cells derived exosomes
- EPC:
-
Endothelial progenitor cells
- ESCRT:
-
Endosomal sorting complex required for transport
- hESC-pg:
-
human embryonic stem cell-derived cardiovascular progenitors
- HSP:
-
Heat shock protein
- IHD:
-
Ischemic heart disease
- mbPDGF-BB:
-
Membrane-bound platelet-derived growth factor-BB
- MCEC:
-
Mouse cardiac endothelial cell
- MI:
-
Myocardial infarction
- miRNA:
-
MicroRNA
- MSC:
-
Mesenchymal stem cells
- MVB:
-
Multivesicular bodies
- PAD:
-
Peripheral arterial disease
- PYGB:
-
Brain/head glycogen phosphorylase
- RNA:
-
Ribonucleic acid
- SNARES:
-
Soluble N-ethylmaleimide-sensitive fusion attachment protein receptors
- T2D:
-
Type 2 diabetes
- VEGF:
-
Vascular endothelial growth factor
- VSMC:
-
Vascular smoot muscle cells
References
Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med. 1998;15(7):539–53.
Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34(1):29–34.
Kannel WB. Diabetes and Cardiovascular Disease. 1979;241(19):2035.
Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2(2):120–6.
Van Hoeven KH, Factor SM. Diabetic heart disease-Part II: the clinical and pathological spectrum. Clin Cardiol. 1989;12(11):667–71.
Preis SR, Hwang S-J, Coady S, Pencina MJ, D’Agostino RB, Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham heart study, 1950 to 2005. Circulation. 2009;119(13):1728–35.
Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–13.
Munasinghe PE, Riu F, Dixit P, Edamatsu M, Saxena P, Hamer NSJ, Galvin IF, Bunton RW, Lequeux S, Jones G, et al. Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol. 2016;202:13–20.
Rawal S, Munasinghe PE, Shindikar A, Paulin J, Cameron V, Manning P, Williams MJA, Jones GT, Bunton R, Galvin I, et al. Down-regulation of proangiogenic microRNA-126 and microRNA-132 are early modulators of diabetic cardiac microangiopathy. Cardiovasc Res. 2017;113(1):90–101.
Rawal S, Munasinghe PE, Thevakar P, Lew JKS, Jones GT, Willams MJ, Davis P, Bunton RW, Galvin IF, Manning P, et al. Downregulation of miR-15a/b accelerates fibrotic remodelling in the type-2 diabetic human and mouse heart. Clin Sci. 2017;131:847.
Chen S, Puthanveetil P, Feng B, Matkovich SJ, Dorn GW, Chakrabarti S. Cardiac miR-133a overexpression prevents early cardiac fibrosis in diabetes. J Cell Mol Med. 2014;18(3):415–21.
Fomison-Nurse I, Saw EEL, Gandhi S, Munasinghe PE, Van Hout I, Williams MJA, Galvin I, Bunton R, Davis P, Cameron V, et al. Diabetes induces the activation of pro-ageing miR-34a in the heart, but has differential effects on cardiomyocytes and cardiac progenitor cells. Cell Death Differ. 2018;25(7):1336–49.
Feng B, Chen S, George B, Feng Q, Chakrabarti S. miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes Metab Res Rev. 2010;26(1):40–9.
Chandrasekera DN, Neale JP, van Hout I, Rawal S, Coffey S, Jones GT, Bunton R, Sugunesegran R, Parry D, Davis P. Upregulation of microRNA-532 enhances cardiomyocyte apoptosis in the diabetic heart. Apoptosis. 2020;25(5):388–99.
Reily-Bell M, Bahn A, Katare R. Reactive oxygen species-mediated diabetic heart disease: mechanisms and therapies. Antioxid Redox Signal. 2021;36:608.
Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–71.
Tang Y, Zheng J, Sun Y, Wu Z, Liu Z, Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int Heart J. 2009;50(3):377–87.
Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovas Res. 2011;92(1):75–84.
Raut SK, Singh GB, Rastogi B, Saikia UN, Mittal A, Dogra N, Singh S, Prasad R, Khullar M. miR-30c and miR-181a synergistically modulate p53–p21 pathway in diabetes induced cardiac hypertrophy. Mol Cell Biochem. 2016;417(1):191–203.
Shan Z-X, Lin Q-X, Deng C-Y, Zhu J-N, Mai L-P, Liu J-L, Fu Y-H, Liu X-Y, Li Y-X, Zhang Y-Y, et al. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584(16):3592–600.
Yu X-Y, Song Y-H, Geng Y-J, Lin Q-X, Shan Z-X, Lin S-G, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376(3):548–52.
Constanso-Conde I, Hermida-Prieto M, Barge-Caballero E, Núñez L, Pombo-Otero J, Suárez-Fuentetaja N, Paniagua-Martín MJ, Barge-Caballero G, Couto-Mallón D, Pan-Lizcano R. Circulating miR-181a-5p as a new biomarker for acute cellular rejection in heart transplantation. J Heart Lung Transplant. 2020;39(10):1100–8.
Emanueli C, Shearn AIU, Laftah A, Fiorentino F, Reeves BC, Beltrami C, Mumford A, Clayton A, Gurney M, Shantikumar S, et al. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS ONE. 2016;11(4): e0154274.
Wan S, Wang J, Wang J, Wu J, Song J, Zhang C-Y, Zhang C, Wang C, Wang J-J. Increased serum miR-7 is a promising biomarker for type 2 diabetes mellitus and its microvascular complications. Diabetes Res Clin Pract. 2017;130:171–9.
Nie H, Pan Y, Zhou Y. Exosomal microRNA-194 causes cardiac injury and mitochondrial dysfunction in obese mice. Biochem Biophys Res Commun. 2018;503(4):3174–9.
Venkat P, Cui C, Chopp M, Zacharek A, Wang F, Landschoot-Ward J, Shen Y, Chen J. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in Type 2 diabetes mellitus Mice. Stroke. 2019;50(10):2865–74.
Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, et al. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372-384.e312.
Castaño C, Kalko S, Novials A, Párrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci. 2018;115(48):12158–63.
Ge Q, Zhou Y, Lu J, Bai Y, **e X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19(2):1568–75.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013. https://doi.org/10.1038/ncomms3980.
Davidson SM, Riquelme JA, Takov K, Vicencio JM, Boi-Doku C, Khoo V, Doreth C, Radenkovic D, Lavandero S, Yellon DM. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J Cell Mol Med. 2018;22(1):141–51.
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al. Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Investig. 2014;124(5):2136–46.
**ao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7(6):e2277.
Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–500.
Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–81.
Hsu C, Morohashi Y, Yoshimura S-I, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C. J Cell Biol. 2010;189(2):223–32.
Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–37.
Zylbersztejn K, Galli T. Vesicular traffic in cell navigation. FEBS J. 2011;278(23):4497–505.
Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P, Altevogt P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72(9):1095–102.
Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5(9):a016766–a016766.
Colombo M, Moita C, Van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(24):5553–65.
Escola J-M, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–7.
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–91.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, **ang J, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–9.
Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Beckler MD, Weaver AM, Vickers K, Prasad N, Levy S. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4: e07197.
Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1(1):18397.
Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, **ang J. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28(2):126–31.
Beltrami C, Besnier M, Shantikumar S, Shearn AIU, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther. 2017;25(3):679–93.
Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21.
Kruger S, Elmageed ZYA, Hawke DH, Wörner PM, Jansen DA, Abdel-Mageed AB, Alt EU, Izadpanah R. Molecular characterization of exosome-like vesicles from breast cancer cells. BMC Cancer. 2014;14(1):44.
Tian C, Gao L, Zimmerman MC, Zucker IH. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol-Heart Circulat Physiol. 2018;314(5):H928–39.
Tian T, Wang Y, Wang H, Zhu Z, **ao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96.
Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11(1):108.
Tian T, Zhu Y-L, Zhou Y-Y, Liang G-F, Wang Y-Y, Hu F-H, **ao Z-D. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J Biol Chem. 2014;289(32):22258–67.
Sohel MMH, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, Rings F, Uddin MJ, Spencer TE, Schellander K, et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS ONE. 2013;8(11): e78505.
Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431(3):566–71.
Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, et al. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes. 2016;65(3):598–609.
Shi R, Zhao L, Cai W, Wei M, Zhou X, Yang G, Yuan L. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem Biophys Res Commun. 2017;483(1):602–8.
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–9.
Feng D, Zhao W-L, Ye Y-Y, Bai X-C, Liu R-Q, Chang L-F, Zhou Q, Sui S-F. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–87.
Montecalvo A, Larregina AT, Shufesky WJ, Beer Stolz D, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119(3):756–66.
Khalifa O, Pers Y-M, Ferreira R, Sénéchal A, Jorgensen C, Apparailly F, Duroux-Richard I. X-Linked miRNAs associated with gender differences in rheumatoid arthritis. Int J Mol Sci. 2016;17(11):1852.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci. 2004;101(9):2999–3004.
Fan F, Sun A, Zhao H, Liu X, Zhang W, ** X, Wang C, Ma X, Shen C, Zou Y. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr Pharm Des. 2013;19(27):4865–73.
Dixit P, Donnelly H, Edamatsu M, Galvin I, Bunton R, Katare R. Progenitor cells from atria, ventricle and peripheral blood of the same patients exhibit functional differences associated with cardiac repair. Int J Cardiol. 2017;228:412–21.
Lew JKS, Pearson JT, Schwenke DO, Katare R. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017. https://doi.org/10.1186/s12933-016-0484-4.
Chavali V, Tyagi SC, Mishra PK. Differential expression of dicer, miRNAs, and inflammatory markers in diabetic Ins2+/− Akita Hearts. Cell Biochem Biophys. 2014;68(1):25–35.
Yildirim SS, Akman D, Catalucci D, Turan B. Relationship between downregulation of miRNAS and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67(3):1397–408.
Lee Y. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21(17):4663–70.
Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13(12):1097–101.
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425(6956):415–9.
Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–6.
Bartel DP. MicroRNAs. Cell. 2004;116(2):281–97.
Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131(6):1097–108.
Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115(2):199–208.
Matkovich SJ, Van Booven DJ, Eschenbacher WH, Dorn GW. RISC RNA sequencing for context-specific identification of in vivo microRNA targets. Circ Res. 2011;108(1):18–26.
Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The microRNA world: small is mighty. Trends Biochem Sci. 2003;28(10):534–40.
Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17(4):438–42.
Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20.
Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci. 2006;103(48):18255–60.
Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH. MicroRNA expression in zebrafish embryonic development. Science. 2005;309(5732):310–1.
Thum T, Catalucci D, Bauersachs J. MicroRNAs: novel regulators in cardiac development and disease. Cardiovasc Res. 2008;79(4):562–70.
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, Van Der Made I, Herias V, Van Leeuwen RE, Schellings MW, Barenbrug P, et al. miR-133 and miR-30 regulate connective tissue growth factor. Circ Res. 2009;104(2):170–8.
Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, Reinhardt F, Liao R, Krieger M, Jaenisch R, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009;105(6):585–94.
Chandrasekera DNK, Neale JPH, van Hout I, Rawal S, Coffey S, Jones GT, Bunton R, Sugunesegran R, Parry D, Davis P, et al. Upregulation of microRNA-532 enhances cardiomyocyte apoptosis in the diabetic heart. Apoptosis. 2020;25(5–6):388–99.
Rawal S, Nagesh PT, Coffey S, Van Hout I, Galvin IF, Bunton RW, Davis P, Williams MJA, Katare R. Early dysregulation of cardiac-specific microRNA-208a is linked to maladaptive cardiac remodelling in diabetic myocardium. Cardiovasc Diabetol. 2019;18(1):13.
Rawal S, Munasinghe PE, Nagesh PT, Lew JKS, Jones GT, Williams MJA, Davis P, Bunton D, Galvin IF, Manning P, et al. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the Type 2 diabetic human and mouse heart. Clin Sci (Lond). 2017;131(9):847–63.
Rawal S, Munasinghe PE, Shindikar A, Paulin J, Cameron V, Manning P, Williams MJ, Jones GT, Bunton R, Galvin I, et al. Down-regulation of proangiogenic microRNA-126 and microRNA-132 are early modulators of diabetic cardiac microangiopathy. Cardiovasc Res. 2017;113(1):90–101.
Rawal S, Ram TP, Coffey S, Williams MJ, Saxena P, Bunton RW, Galvin IF, Katare R. Differential expression pattern of cardiovascular microRNAs in the human type-2 diabetic heart with normal ejection fraction. Int J Cardiol. 2016;202:40–3.
Rawal S, Manning P, Katare R. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc Diabetol. 2014;13:44.
Lew JK, Pearson JT, Schwenke DO, Katare R. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol. 2017;16(1):10.
Martinelli NC, Cohen CR, Santos KG, Castro MA, Biolo A, Frick L, Silvello D, Lopes A, Schneider S, Andrades ME, et al. An analysis of the global expression of microRNAs in an experimental model of physiological left ventricular hypertrophy. PLoS ONE. 2014;9(4): e93271.
Nassar W, El-Ansary M, Fayyad T, Aziz MA. Extracellular Micro-RNAs in health and disease: basic science, biogenesis and release. Am J Mol Biol. 2016;06(01):1–11.
Obradovic D, Rommel KP, Blazek S, Klingel K, Gutberlet M, Lücke C, Büttner P, Thiele H, Adams V, Lurz P. The potential role of plasma miR-155 and miR-206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Heart Failure. 2021;8(3):1850–60.
Zhu J, Yao K, Wang Q, Guo J, Shi H, Ma L, Liu H, Gao W, Zou Y, Ge J. Circulating miR-181a as a potential novel biomarker for diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;40(6):1591–602.
Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int. 2015;2015:1–12.
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell Mol Med. 2016;20(12):2318–27.
Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, et al. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes. 2016;65(10):3111–28.
Yarana C, Carroll D, Chen J, Chaiswing L, Zhao Y, Noel T, Alstott M, Bae Y, Dressler EV, Moscow JA. Extracellular vesicles released by cardiomyocytes in a doxorubicin-induced cardiac injury mouse model contain protein biomarkers of early cardiac injury. Clin Cancer Res. 2018;24(7):1644–53.
Liu X, Yuan W, Yang L, Li J, Cai J. miRNA profiling of exosomes from spontaneous hypertensive rats using next-generation sequencing. J Cardiovasc Transl Res. 2019;12(1):75–83.
Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–68.
Alcayaga-Miranda F, González PL, Lopez-Verrilli A, Varas-Godoy M, Aguila-Díaz C, Contreras L, Khoury M. Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget. 2016;7(28):44462–77.
Ahmed CK, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2(5):606–19.
Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2):255–63.
Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A, Song Y, Shen Z, Gardner G, Kuffel G, et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125–37.
Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Périer M-C, Toeg HD, Richart A. Cardiovascular progenitor–derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant. 2016;35(6):795–807.
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from AKt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2017;6(1):51–9.
Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med. 2015;19(9):2153–61.
Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan G-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50.
Huang G, Garikipati VNS, Zhou Y, Benedict C, Houser SR, Koch WJ, Kishore R. Identification and comparison of hyperglycemia-induced extracellular vesicle transcriptome in different mouse stem cells. Cells. 2020;9(9):2098.
Kim H, Bae Y-U, Lee H, Kim H, Jeon JS, Noh H, Han DC, Byun DW, Kim SH, Park HK, et al. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res Care. 2020;8(1): e001403.
Xu H, Du X, Xu J, Zhang Y, Tian Y, Liu G, Wang X, Ma M, Du W, Liu Y, et al. Pancreatic beta cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving beta cell function. PLoS Biol. 2020;18(2): e3000603.
Li D, Song H, Shuo L, Wang L, **e P, Li W, Liu J, Tong Y, Zhang CY, Jiang X, et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY). 2020;12(22):22719–43.
Villanueva M, Michie C, Parent S, Kanaan GN, Rafatian G, Kanda P, Ye B, Liang W, Harper M-E, Davis DR. Glyoxalase 1 prevents chronic hyperglycemia induced heart-explant derived cell dysfunction. Theranostics. 2019;9(19):5720.
Haffner SM. The importance of hyperglycemia in the nonfasting state to the development of cardiovascular disease. Endocr Rev. 1998;19(5):583–92.
Bäcklund T, Palojoki E, Saraste A, Eriksson A, Finckenberg P, Kytö V, Lakkisto P, Mervaala E, Voipio-Pulkki L-M, Laine M. Sustained cardiomyocyte apoptosis and left ventricular remodelling after myocardial infarction in experimental diabetes. Diabetologia. 2004;47(2):325–30.
Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26(8):2433–41.
Liu Y, Xu J, Gu R, Li Z, Wang K, Qi Y, Sun X, **e J, Wang L, Xu B, et al. Circulating exosomal miR-144-3p inhibits the mobilization of endothelial progenitor cells post myocardial infarction via regulating the MMP9 pathway. Aging (Albany NY). 2020;12(16):16294–303.
Yuan Lin FZ, Lian XF, Peng WQ, Yin Chao-Yao. Mesenchymal stem cell-derived exosomes improve diabetes mellitus-induced myocardial injury and fibrosis via inhibition of TGF-β1/Smad2 signaling pathway. Cell Mol Biol. 2019;65:4.
Association AD. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–41.
Sheth J, Shah A, Sheth F, Trivedi S, Nabar N, Shah N, Thakor P, Vaidya R. The association of dyslipidemia and obesity with glycated hemoglobin. Clin Diabetes Endocrinol. 2015. https://doi.org/10.1186/s40842-015-0004-6.
Ibrahim AA, Wahby AA, Ashmawy I, Saleh RM, Soliman H. Association of exosomal miR-34a with markers of dyslipidemia and endothelial dysfunction in children and adolescents with T1DM. J Clin Res Pediatr Endocrinol. 2020;12(4):401–9.
Santovito D, De Nardis V, Marcantonio P, Mandolini C, Paganelli C, Vitale E, Buttitta F, Bucci M, Mezzetti A, Consoli A, et al. Plasma exosome MicroRNA profiling unravels a new potential modulator of adiponectin pathway in diabetes: effect of glycemic control. J Clin Endocrinol Metab. 2014;99(9):E1681–5.
Izadi V, Farabad E, Azadbakht L. Epidemiologic evidence on serum adiponectin level and lipid profile. Int J Prev Med. 2013;4(2):133–40.
Collaboration ERF. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22.
Mendis S, Lindholm LH, Anderson SG, Alwan A, Koju R, Onwubere BJ, Kayani AM, Abeysinghe N, Duneas A, Tabagari S. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol. 2011;64(12):1451–62.
Coleman KJ, Haneuse S, Johnson E, Bogart A, Fisher D, O’Connor PJ, Sherwood NE, Sidney S, Theis MK, Anau J, et al. Long-term microvascular disease outcomes in patients with type 2 diabetes after bariatric surgery: evidence for the legacy effect of surgery. Diabetes Care. 2016;39(8):1400–7.
Stamler J, Vaccaro O, Neaton JD, Wentworth D, Group MRFITR. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16(2):434–44.
Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RTM, Alvarez-Hernandez D. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116(8):1312–23.
Tölle M, Reshetnik A, Schuchardt M, Höhne M, Van Der Giet M. Arteriosclerosis and vascular calcification: causes, clinical assessment and therapy. Eur J Clin Invest. 2015;45(9):976–85.
Krohn JB, Hutcheson JD, Martínez-Martínez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol. 2016;594(11):2895–903.
Togliatto G, Dentelli P, Rosso A, Lombardo G, Gili M, Gallo S, Gai C, Solini A, Camussi G, Brizzi MF. PDGF-BB carried by endothelial cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes. 2018;67(4):704–16.
Wang F, Chen F-F, Shang Y-Y, Li Y, Wang Z-h, Han L, Li Y-h, Zhang L, Ti Y, Zhang W. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE−/− mice. Int J Cardiol. 2018;265:181–7.
Li S, Zhan J-K, Wang Y-J, Lin X, Zhong J-Y, Wang Y, Tan P, He J-Y, Cui X-J, Chen Y-Y, et al. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci. 2019. https://doi.org/10.1186/s13578-018-0263-x.
Shyu K-G, Wang B-W, Fang W-J, Pan C-M, Lin C-M. Exosomal MALAT1 derived from high glucose-treated macrophages up-regulates resistin expression via miR-150-5p downregulation. Int J Mol Sci. 2022;23(3):1095.
Bouchareychas L, Duong P, Covarrubias S, Alsop E, Phu TA, Chung A, Gomes M, Wong D, Meechoovet B, Capili A, et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via microRNA cargo. Cell Rep. 2020;32(2): 107881.
Bai S, Yin Q, Dong T, Dai F, Qin Y, Ye L, Du J, Zhang Q, Chen H, Shen B. Endothelial progenitor cell–derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed Pharmacother. 2020;131: 110756.
Criqui MH. Peripheral arterial disease-epidemiological aspects. Vascular medicine. 2001;6(1_suppl):3–7.
Sorrentino TA, Duong P, Bouchareychas L, Chen M, Chung A, Schaller MS, Oskowitz A, Raffai RL, Conte MS. Circulating exosomes from patients with peripheral artery disease influence vascular cell migration and contain distinct microRNA cargo. JVS Vasc Sci. 2020;1:28–41.
Okonkwo U, Dipietro L. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18(7):1419.
He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021. https://doi.org/10.1002/ctm2.468.
**ong Y, Chen L, Yan C, Zhou W, Endo Y, Liu J, Hu L, Hu Y, Mi B, Liu G. Circulating Exosomal miR-20b-5p inhibition restores Wnt9b signaling and reverses diabetes-associated impaired wound healing. Small. 2020;16(3):1904044.
Chen K, Yu T, Wang X. Inhibition of circulating exosomal miRNA-20b-5p accelerates diabetic wound repair. Int J Nanomed. 2021;16:371–81.
Hu G-W, Li Q, Niu X, Hu B, Liu J, Zhou S-M, Guo S-C, Lang H-L, Zhang C-Q, Wang Y, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6(1):10.
Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, Lee SW, Lee J, Ahn BC. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release. 2017;264:112–26.
Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications. 2016;30(6):986–92.
Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Therapy. 2020. https://doi.org/10.1186/s13287-020-01824-2.
Kissela BM, Khoury J, Kleindorfer D, Woo D, Schneider A, Alwell K, Miller R, Ewing I, Moomaw CJ, Szaflarski JP. Epidemiology of ischemic stroke in patients with diabetes: the greater Cincinnati/Northern Kentucky Stroke Study. Diabetes Care. 2005;28(2):355–9.
Tuttolomondo A, Pinto A, Salemi G, Di Raimondo D, Di Sciacca R, Fernandez P, Ragonese P, Savettieri G, Licata G. Diabetic and non-diabetic subjects with ischemic stroke: differences, subtype distribution and outcome. Nutr Metab Cardiovasc Dis. 2008;18(2):152–7.
Lee S-H, Jang MU, Kim Y, Park SY, Kim C, Kim YJ, Sohn J-H. Effect of prestroke glycemic variability estimated glycated albumin on stroke severity and infarct volume in diabetic patients presenting with acute ischemic stroke. Front Endocrinol. 2020. https://doi.org/10.3389/fendo.2020.00230.
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS ONE. 2016;11(9): e0163645.
Chi NF, Chiou HY, Chou SY, Hu CJ, Chen KY, Chang CF, Hsieh YC. Hyperglycemia-related FAS gene and hsa-let-7b-5p as markers of poor outcomes for ischaemic stroke. Eur J Neurol. 2020;27(8):1647–55.
Venkat P, Zacharek A, Landschoot-Ward J, Wang F, Culmone L, Chen Z, Chopp M, Chen J. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp Neurol. 2020;334: 113456.
Jiang M, Wang H, ** M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X. Exosomes from MiR-30d-5p-ADSCs reverse acute ischemic stroke-induced, autophagy-mediated brain injury by promoting M2 microglial/macrophage polarization. Cell Physiol Biochem. 2018;47(2):864–78.
Wang J, Chen S, Zhang W, Chen Y, Bihl JC. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci Ther. 2020;26(12):1255–65.
Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y, Fujimiya M. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6(1):24805.
Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67(8):1639–49.
Gu S, Liu Y, Zou J, Wang W, Wei T, Wang X, Zhu L, Zhang M, Zhu J, **e T, et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp Eye Res. 2020;201: 108271.
Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, Chopp M, Zhang ZG, Liu XS. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63(2):431–43.
Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, Cepparulo P, Lu M, Li C, Zhang ZG. Exosomes derived from schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes. 2020;69(4):749–59.
Singh A, Shiekh PA, Qayoom I, Srivastava E, Kumar A. Evaluation of polymeric aligned NGCs and exosomes in nerve injury models in diabetic peripheral neuropathy condition. Eur Polymer J. 2021;146: 110256.
Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFbeta1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185–92.
Schrier RW, Estacio RO, Mehler PS, Hiatt WR. Appropriate blood pressure control in hypertensive and normotensive type 2 diabetes mellitus: a summary of the ABCD trial. Nat Clin Pract Nephrol. 2007;3(8):428–38.
Otani K, Yokoya M, Kodama T, Hori K, Matsumoto K, Okada M, Yamawaki H. Plasma exosomes regulate systemic blood pressure in rats. Biochem Biophys Res Commun. 2018;503(2):776–83.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602.
Kiencke S, Handschin R, Von Dahlen R, Muser J, Brunner-Larocca HP, Schumann J, Felix B, Berneis K, Rickenbacher P. Pre-clinical diabetic cardiomyopathy: prevalence, screening, and outcome. Eur J Heart Fail. 2010;12(9):951–7.
Salem ESB, Fan G-C. Pathological effects of exosomes in mediating diabetic cardiomyopathy. In: **ao J, Cretoiu S, editors. Exosomes in cardiovascular diseases. Singapore: Springer; 2017. p. 113–38.
Li H, Fan J, Zhao Y, Zhang X, Dai B, Zhan J, Yin Z, Nie X, Fu XD, Chen C, et al. Nuclear miR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ Res. 2019;125(12):1106–20.
Rijzewijk LJ, van der Meer RW, Smit JWA, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in Type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52(22):1793–9.
De Gonzalo-Calvo D, Van Der Meer RW, Rijzewijk LJ, Smit JWA, Revuelta-Lopez E, Nasarre L, Escola-Gil JC, Lamb HJ, Llorente-Cortes V. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-00070-6.
Pironti G, Strachan RT, Abraham D, Mon-Wei YuS, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA. Circulating exosomes induced by cardiac pressure overload contain functional Angiotensin II Type 1 receptors. Circulation. 2015;131(24):2120–30.
Emanueli C, Shearn AIU, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30.
Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2(5):606–19.
Rawal S, Ram TP, Coffey S, Williams MJ, Saxena P, Bunton RW, Galvin IF, Katare R. Differential expression pattern of cardiovascular microRNAs in the human type-2 diabetic heart with normal ejection fraction. Int J Cardiol. 2016;202:40–3.
Li Z, Zhao P, Zhang Y, Wang J, Wang C, Liu Y, Yang G, Yuan L. Exosome-based Ldlr gene therapy for familial hypercholesterolemia in a mouse model. Theranostics. 2021;11(6):2953–65.
Huang L, Yang L, Ding Y, Jiang X, **a Z, You Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle. 2020;19(3):339–53.
Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med. 2016;6:51.
Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, Gong P, Shen X, Ruan H, ** M, et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther—Nucleic Acids. 2018;11:103–15.
Acknowledgements
Not applicable.
Funding
Ms Chandrasekera is supported by the University of Otago Doctoral Scholarship.
Author information
Authors and Affiliations
Contributions
DC wrote the manuscript and prepared the figures; RK conceptualized the idea, designed the structure and made critical revisions of the manuscript. Both the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chandrasekera, D., Katare, R. Exosomal microRNAs in diabetic heart disease. Cardiovasc Diabetol 21, 122 (2022). https://doi.org/10.1186/s12933-022-01544-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01544-2