Abstract
Rationale
About 50% of hospitalized coronavirus disease 2019 (COVID-19) patients with diabetes mellitus (DM) developed myocardial damage. The mechanisms of direct SARS-CoV-2 cardiomyocyte infection include viral invasion via ACE2-Spike glycoprotein-binding. In DM patients, the impact of glycation of ACE2 on cardiomyocyte invasion by SARS-CoV-2 can be of high importance.
Objective
To evaluate the presence of SARS-CoV-2 in cardiomyocytes from heart autopsy of DM cases compared to Non-DM; to investigate the role of DM in SARS-COV-2 entry in cardiomyocytes.
Methods and results
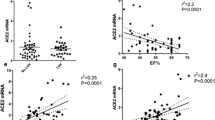
We evaluated consecutive autopsy cases, deceased for COVID-19, from Italy between Apr 30, 2020 and Jan 18, 2021. We evaluated SARS-CoV-2 in cardiomyocytes, expression of ACE2 (total and glycosylated form), and transmembrane protease serine protease-2 (TMPRSS2) protein. In order to study the role of diabetes on cardiomyocyte alterations, independently of COVID-19, we investigated ACE2, glycosylated ACE2, and TMPRSS2 proteins in cardiomyocytes from DM and Non-DM explanted-hearts. Finally, to investigate the effects of DM on ACE2 protein modification, an in vitro glycation study of recombinant human ACE2 (hACE2) was performed to evaluate the effects on binding to SARS-CoV-2 Spike protein. The authors included cardiac tissue from 97 autopsies. DM was diagnosed in 37 patients (38%). Fourth-seven out of 97 autopsies (48%) had SARS-CoV-2 RNA in cardiomyocytes. Thirty out of 37 DM autopsy cases (81%) and 17 out of 60 Non-DM autopsy cases (28%) had SARS-CoV-2 RNA in cardiomyocytes. Total ACE2, glycosylated ACE2, and TMPRSS2 protein expressions were higher in cardiomyocytes from autopsied and explanted hearts of DM than Non-DM. In vitro exposure of monomeric hACE2 to 120 mM glucose for 12 days led to non-enzymatic glycation of four lysine residues in the neck domain affecting the protein oligomerization.
Conclusions
The upregulation of ACE2 expression (total and glycosylated forms) in DM cardiomyocytes, along with non-enzymatic glycation, could increase the susceptibility to COVID-19 infection in DM patients by favouring the cellular entry of SARS-CoV2.
Similar content being viewed by others
The potential translational impact of the study results
In patients with diabetes mellitus, the upregulation of ACE2 expression in cardiomyocytes, together with non-enzymatic glycation favouring protein oligomerization, could increase the susceptibility to COVID-19 infection and worse prognosis. However, the control of the expression of ACE2 and its glycated form could represent a therapeutic target to prevent COVID-19 infection and worse prognosis in patients with diabetes.
Introduction
The coronavirus disease-19 (COVID-19), caused by the RNA single-stranded enveloped virus of severe acute respiratory syndrome (SARS)-CoV-2, has a significant impact on the cardiovascular (CV) system by direct myocardial damage [1]. Indeed, a considerable number of hospitalized COVID-19 patients could develop cardiac injury (24.4%), with a consequent higher rate of mortality (72.6%), [2]. Notably, among hospitalized COVID-19 patients with diabetes (DM), about half of them developed myocardial damage [3]. Indeed, DM is very common among hospitalized COVID-19 patients, has a significant impact on the treatment [4], and negatively influences clinical outcomes in affected patients [5,6,7]. However, specific therapies to prevent coagulopathies, over-inflammation, and hyperglycemia may represent a valid therapeutic option for treating asymptomatic and non-critically ill COVID-19 patients with DM as critically-ill DM patients [8,9,10,11,12].
In this context, the impact of hyperglycemia in the progression and deterioration of heart function in COVID-19 patients is currently of great importance. Indeed, in DM patients, the severity of SARS-CoV-2 infection has been attributed to impaired innate and adaptive immunity, upregulation of ACE2, and potential changes in the glycation of ACE2 [4, www.uniprot.org; entry: Q9BYF1, entry name: ACE2_HUMAN) showing in red the glycated lysine residues obtained after 12 days of incubation with 120 mM of glucose. b Position glycated lysine (K) after 12 days of incubation with 12 mM, 60 mM, and 120 mM of glucose and function of glycated sites. c Human ACE2 homodimer (PDB 1r42) showing the lysine 353 (K353), involved in the Spike-RBD binding to ACE2, lysine 470 (K470) (unknown function). ACE2 structure from PDB 6M17 showing the glycated lysine 619 (K619), 631 (K631), 659 (K659), and 689 (K689) in the polar neck region involved in the dimerization of ACE2
Results showed that among the seven glycated residues, only lysine 353 (Lys 353) (Fig. 4c) has previously been reported as important for binding Spike-RBD [31]. However, under our experimental conditions, minor glycation of Lys 353 (0.6%) was found. Also, in the hACE2 (Protein Data Bank, PDB 1r42), the Lys 470 with unknown function displayed a 4.98% of glycation at 120 mM of glucose (Fig. 4c). Notably, a higher number of glycated residues were detected in the polar region of ACE2 involved in the dimerization (neck domain), with Lys 619, Lys 631, Lys 659, and Lys 689 showing 1.47%, 5.28%, 7.62%, and 6.78% of glycation, respectively (Fig. 4c). The structures shown in Fig. 4c are from PDB 6M17. Next, we verified that the glycosylation-associated structural heterogeneity of hACE2 was quite difficult to eliminate. ACE2 glycosylation was removed by overnight incubation with PNGase F. SDS-PAGE results showed that the deglycosylated hACE2 has a lower apparent molecular weight due to the PNGase digestion. However, its band is still heterogeneous with a diffused aspect (Additional file 1: Figure SIX).
For SPR measurements of glycated ACE-SARS-CoV-2 Spike protein binding, an affinity coupling approach using a biosensor functionalized with an anti-human F.C. antibody immobilization kit (Cytiva, catalog no. BR-1008-39) was used. This system allowed to bind the Spike protein via its F.C. affinity tail with chip recycling for the whole study. The system showed to be functional, and an amount of 1 µg/assay of immobilized Spike protein was found to work optimally. In line with the low grade of glycation found at the Lys 353 located in the hACE2 domain of binding for SARS-CoV-2 Spike, results showed a minimal influence of glycation on the binding properties to SARS-CoV-2 Spike, as indicated by the Ka and Kd and the protein ligand-analyte affinity (K.D.) (9.69 nM in control and 11.07 nM in glycated hACE2 with 120 mM of glucose) (Additional file 1: Figure SX).
Glycation modifies ACE migration
The impact of the glycated residues detected in the neck domain of ACE2 on the protein structure was further investigated by SDS-PAGE under reducing and non-reducing conditions. To this end, the oligomerization state of hACE2 and SARS-CoV-2 Spike protein was first evaluated in samples before starting mild glycation experiments and binding measurement, confirming the purity of dimeric spike and the monomeric hACE2 protein (detected band at 100 kDa) used in this study (Fig. 5a). Notably, the SDS-PAGE of glycated hACE2 (Glyc-hACE) (120 mM glucose) showed important differences compared to non-glycated hACE2 (hACE) (Fig. 5b). In a non-reducing setting, hACE showed only one band at 100 kDa, whereas the Glyc-hACE showed both a predominant band over 100 kDa along with another band at the higher molecular weight (250 kDa). Under reducing conditions, both hACE and Glyc-hACE showed the predominant band of at 100 kDa. However, despite the experimental reducing conditions, a weak band at 250 kDa was still observed for Glyc-hACE. The evidence of glycation role on ACE2 dimer formation under a non-reducing setting (p < 0.05) was provided by the quantitative ratio of the dimeric to monomeric form (Fig. 5c).
Effect of glycation on hACE2 migration. a SDS-PAGE was conducted using hACE2 and SARS-CoCOVV-2 Spike protein aliquots collected before starting glycation and SPR measurements. b SDS-PAGE was conducted using 8% gels in reducing and non-reducing conditions with hACE2 incubated for 12 days with glucose 120 mM (Glyc hACE2). Molecular weight indicators are displayed at the center. c Ratio of the dimeric to monomeric form of ACE2 in reducing and non-reducing condition. *p < 0.05 vs. non glycated ACE2. Shown as mean ± SD. Statistical test: Student’s t-test
These results indicated the mild glycation modified hACE2 protein migration and oligomerization process, supporting non-enzymatic glycation at neck domain level on hACE2 oligomerization and dimer formation (Addition file 2).