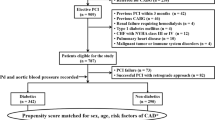
Abstract
Background
The extent of coronary collateral formation is a primary determinant of the severity of myocardial damage and mortality after coronary artery occlusion. Type 2 diabetes mellitus (T2DM) represents an important risk factor for impaired collateral vessel growth. However, the mechanism of reduced coronary collateralization in type 2 diabetic patients remains unclear.
Methods
With the reference to the recent researches, this review article describes the pathogenic effects of T2DM on collateral development and outlines possible clinical and biochemical markers associated with reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion (CTO).
Results
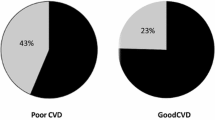
Diffuse coronary atherosclerosis in T2DM reduces pressure gradient between collateral donor artery and collateral recipient one, limiting collateral vessel growth and function. An interaction between advanced glycation end-products and their receptor activates several intracellular signaling pathways, enhances oxidative stress and aggravates inflammatory process. Diabetic condition decreases pro-angiogenic factors especially vascular endothelial growth factor and other collateral vessel growth related parameters. Numerous clinical and biochemical factors that could possibly attenuate the development of coronary collaterals have been reported. Increased serum levels of glycated albumin, cystatin C, and adipokine C1q tumor necrosis factor related protein 1 were associated with poor coronary collateralization in type 2 diabetic patients with stable coronary artery disease and CTO. Diastolic blood pressure and stenosis severity of the predominant collateral donor artery also play a role in coronary collateral formation.
Conclusions
T2DM impairs collateral vessel growth through multiple mechanisms involving arteriogenesis and angiogenesis, and coronary collateral formation in patients with T2DM and CTO is influenced by various clinical, biochemical and angiographic factors. This information provides insights into the understanding of coronary pathophysiology and searching for potential new therapeutic targets in T2DM.
Similar content being viewed by others
Background
In the human heart, vascular channels with lumen diameter ranging from 40 to 200 μm link large conductance coronary arteries to one another. These interconnecting arteriolar networks are called coronary collaterals [1, 2]. Abundant evidence indicates that when the proximal part of a major epicardial coronary artery is transiently or permanently occluded, the development of coronary collateral circulation serves as a natural conduit system bridging the occluded coronary vessels [3, 4]. Although these anastomoses are often incapable of restoring flow to normal levels, well-developed coronary collaterals could, at least partially, supplies the downstream perfusion area via the arteriolar connection (Fig. 1), thereby preventing or alleviating myocardial ischemia, reducing infarct size, protecting left ventricular function, and even decreasing mortality [5, 6].
Epidemiological data frequently demonstrate that type 2 diabetes mellitus (T2DM) is increasingly prevalent and represents an important risk factor for cardiovascular disease involving arteries and/or capillaries [7]. Compared with non-diabetic patients, those with T2DM often have more severe and diffuse coronary atherosclerosis, more complicated revascularization procedures (percutaneous coronary intervention [PCI] or coronary bypass grafting surgery), and less favorable long-term outcomes (e.g., higher rates of in-stent restenosis, stent thrombosis, and coronary atherosclerotic disease progression) [8]. In fact, cardiovascular disease remains the major cause of death for almost three quarters of type 2 diabetic patients, in which impaired coronary collateral formation may play a role [9].
In this review, we will describe the effects of T2DM on collateral vessel growth and discuss the role of clinical and biochemical factors as possible markers of reduced coronary collateralization and their clinical relevance in type 2 diabetic patients with stable coronary artery disease and chronic total occlusion (CTO), with the reference to the recent researches.
Potential mechanisms of impaired collateral growth in diabetes
In adult organisms, the compensatory growth of blood vessels under ischemic conditions is an appreciated response, which can be achieved in two distinctive ways (i.e., arteriogenesis and angiogenesis) [10]. The process of arteriogenesis is stimulated by physical force and accompanied with enlargement and maturation of pre-existing arterioles (i.e., arterialization of the capillary bed). Briefly, when a coronary artery becomes completely occluded, the pressure gradient across the collaterals is elevated, which results in an increase in blood flow velocity and tangential fluid shear stress imposed on the endothelium, leading to a series of cellular response, including modulation of cell adhesion molecules that would in turn facilitate adhesion and transmigration of circulating mononuclear cells to sites of arterial formation. These cells then become activated and secrete matrix-degrading proteinases, leading to outward arterial remodeling. They also release other cytokines that contribute to the growth of arteriolar collaterals. Recent studies have shown that many genes related to inflammation, transcription, and neovascularization are significantly upregulated in the ischemic regions and associated with collateral growth [11]. The oxygen gradient over the collateral vessels is increased in patients with a less matured collateral circulation and related to local levels of pro-arteriogenic cytokines [12, 13]. Angiogenesis, sprouting of new capillaries from the pre-existing vessels, is induced by hypoxia-inducible factor 1-α and driven largely by vascular endothelial growth factor (VEGF) released either by ischemic tissues or by inflammatory cells. Angiogenesis is entirely regulated by a balance of pro- and anti-angiogenic factors [3]. Although formation and maturation of blood vessels are dependent on endothelial and vascular smooth muscle cells, and affected by various growth factors and inflammatory cytokines, the increase in diameter via arteriogenesis weights much more than the number of newly formed capillaries via angiogenesis [1,2,3].
The mechanism of reduced coronary collateralization in T2DM remains unclear and is likely multifactorial. Although the presence of a chronic coronary total occlusion would be expected to significantly decrease intracoronary pressure distal to the occluded segment which could promote arteriogenesis, there exists a trend towards severe coronary atherosclerosis in type 2 diabetic patients as manifested by long and diffuse lesions and small vessel disease, which could reduce pressure gradient between the collateral donor artery and collateral recipient one, and therefore, limits collateral vessel growth and function. The PROSPECT study with gray-scale and radiofrequency intravascular ultrasound showed that type 2 diabetic patients often have coronary atherosclerotic lesions characterized by a large necrotic core, thin-cap fibroatheroma, and high calcium content, especially for those with a longer duration of T2DM and poorer glycemic control [14]. These coronary lesion characteristics favor plaque instability and degradation, and predict future major adverse cardiac events independent of myocardial ischemia [15]. Previous studies have revealed that glucose fluctuations provoke oxidative stress that leads to endothelial cell dysfunction, progression of coronary atherosclerosis, and plaque vulnerability [16, 17]. These results suggest that there may be a symbiotic relationship between vulnerable plaque and T2DM. Recently, Hinkel et al. reported that diabetic human myocardial explants revealed capillary rarefaction and pericyte loss compared to nondiabetic explants. Moreover, they found that in a diabetic pig model, hyperglycemia induced microvascular rarefaction in the myocardium even without ischemia, and capillary density further decreased in chronic ischemia hearts [18]. These observations highlight that T2DM destabilized microvascular vessels of the heart and may impair the responsiveness of ischemic myocardium to proangiogenic factors. Data from prior studies have also shown a pronounced increase of collateral resistance, adverse functional and structural remodeling of the coronary arterioles, and obliteration of pre-existing blood vessels in T2DM [19,20,21]. All these changes jointly lead to reduced arteriogenic property in type 2 diabetic patients.
Chronic hyperglycemia and altered redox state in diabetes increase the formation and accumulation of advanced glycation endproducts (AGEs). Binding of AGEs to receptor for AGEs (RAGE) activates several intracellular signaling pathways including activation of mitogen activated protein kinase (MAPK), p21ras and NF-κB translocation, resulting in enhanced oxidative stress and upregulation of many inflammatory genes [22]. Furthermore, overexpression of RAGE has negative effects on endothelial function, neointima formation, and angiogenesis [23]. Additionally, in a diabetic setting, pro-angiogenic factors including VEGF, fibroblast growth factor (FGF), and other collateral vessel growth related parameters are altered. There exists endothelial dysfunction characterized by decreased synthesis of nitric oxide, increased expression of endothelin-1 and adhesion molecules, elevated basal oxidative stress or more oxidative redox state, and increased vascular permeability [24]. Impaired monocyte/macrophage recruitment has been shown to be responsible for reduced collateral growth under diabetic conditions [25], and cytokines (e.g., transforming growth factor β, tumor necrosis factor [TNF]-α, monocyte chemotactic protein [MCP]-1, C-reactive protein [CRP], interleukin-8 and interleukin 20) and cell-extracellular matrix interaction may also play a role [24]. It has been shown that downstream VEGF receptors (VEGFRs) and Rho/Rho kinase pathway are important in the regulation of collateral development. Soluble VEGFR-1 is a negative endogenous modulator of angiogenesis by binding and sequestering VEGF. Increased expression and secretion of soluble VEGFR-1 prevents in vivo and in vitro capillary growth and angiogenesis [26]. The expression of soluble VEGFR-1 is decreased in hypoxia status and this protein molecule is degraded by local matrix metalloproteinase-7 to allow VEGF to escape sequestration in ischemic lesions [27]. In addition, soluble VEGFR-2 exhibits anti-lymphangiogenic property and its serum levels are related to insulin resistance in patients with metabolic syndrome, whereas the biological effect of soluble VEGFR-3 remains unclear [28]. These results suggest that VEGF-soluble VEGFR-1 mechanism is crucial to physiological homeostasis of vasculature and modulation of pro- and anti-angiogenesis. We have demonstrated elevated serum soluble VEGFR-1 and soluble VEGFR-2 levels and remarkably reduced VEGF and placenta growth factor levels in type 2 diabetic patients with CTO and low coronary collateralization [29], indicating a linkage of this negative regulator of angiogenesis to impaired coronary collateral formation. Previous studies have observed that myocardial expression of VEGFR-2 is reduced along with a down-regulation of its signal transduction in type 2 diabetic patients [30] and that AGEs inhibit VEGFR-1-mediated chemotaxis in diabetic monocytes [31]. Serum soluble VEGFR-1 level is increased in patients with T2DM [32], and diabetic condition aggravates vascular inflammation through amplifying RAGE-mediated mechanism [33]. In patients with T2DM, glycation of apoprotein A attenuates atheroprotective function of high-density lipoprotein (HDL), and, in contrast, glycation of apoprotein B reinforces low-density lipoprotein-induced inflammatory response [34, 35]. Thus, diabetic pathophysiology promotes an anti-angiogenic process and meanwhile mitigates pro-angiogenic factors in coronary vasculature during ischemia, jointly leading to impaired collateral growth.
Metabolic syndrome characterized by a cluster of risk components including abdominal obesity, insulin resistance, hyperglycemia, dyslipidemia and hypertension has been considered as one of the significant factors affecting adversely the development of coronary collateral vessels. In fact, this syndrome remained an independent risk factor for poor coronary collateralization even after adjusting for T2DM, and approximately 30–40% of these patients show little to no coronary collateral growth [36]. Previous studies indicated that an increasing number of component pathologies of the metabolic syndrome correlated with increasingly poorer coronary collateral development by angiographic grading systems [24]. Metabolic syndrome compromises vascular adaptations to ischemia, resulting in impaired coronary collateral growth. Central to this inadequate adjustment is impairment in endothelial function produced by oxidative stress, which also corrupts the signal transduction of growth factors [36].
Factors influencing coronary collateralization in diabetes
Besides severity of coronary obstruction [37, 38], numerous factors that could possibly attenuate the development and biological function of coronary collaterals have been reported such as old age [39], traditional risk factors for coronary artery disease [40,41,42,43,44], hyperlipoprotein (a) [89]. Emerging evidence suggests that microRNAs are implicated in a variety of physiological processes, including glucose homeostasis [90]. Circulating microRNAs in the plasma of patients with stable coronary artery disease and CTO may provide information about the coronary collateral capacity. For example, elevated miR-320 and miR-221 levels were indicative of endothelial dysfunction and accompanied with impaired angiogenesis in diabetes, whereas microRNA-126 was increased in patients with well-developed collateral circulation, along with VEGF levels [91]. In addition, although serum HDL was associated with the development of coronary collateral circulation [92], coronary atherosclerosis is more influenced by HDL quality than by its quantity in the diabetic condition. Glycation of apolipoprotein A-I and A-IV has been shown to be related to the presence and severity of coronary artery disease and plaque progression in T2DM [93,94,95]. Their effects on coronary collateral vessel growth in T2DM are currently investigated.
Conclusions
T2DM adversely affects coronary collateral development through multiple cellular mechanisms on arteriogenesis and angiogenesis, and the formation of coronary collaterals in patients with T2DM and CTO is influenced by various clinical, biochemical and angiographic factors. Therefore, studies on the relationship between T2DM and coronary collateral circulation are clinically relevant in terms of understanding coronary pathophysiology in diabetes and searching for potential new therapeutic target in future.
Abbreviations
- AGEs:
-
advanced glycation end products
- CRP:
-
C-reactive protein
- CTRP:
-
C1q tumor necrosis factor related protein
- FGF:
-
fibroblast growth factor
- GA:
-
glycated albumin
- GFR:
-
glomerular filtration rate
- HbA1c:
-
glycated hemoglobin
- HDL:
-
high-density lipoprotein
- MAPK:
-
mitogen activated protein kinase
- MCP:
-
monocyte chemotactic protein
- MDRD:
-
modification of Diet in Renal Disease
- NF:
-
nuclear factor
- PCI:
-
percutaneous coronary intervention
- PPAR-γ:
-
peroxisome proliferator-activated receptor-gamma
- RAGE:
-
receptor for advanced glycation end products
- ROS:
-
reactive oxide spices
- T2DM:
-
type 2 diabetes mellitus
- VEGF:
-
vascular endothelial growth factor
- VEGFR:
-
VEGF receptor
References
Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34(34):2674–82.
Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med. 2013;11:143.
Zimarino M, D’Andreamatteo M, Waksman R, Epstein SE, De Caterina R. The dynamics of the coronary collateral circulation. Nat Rev Cardiol. 2014;11(4):191–7.
Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010;122(12):1210–20.
Jang WJ, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Kim WS, Lee YT, Gwon HC. Long-term survival benefit of revascularization compared with medical therapy in patients with coronary chronic total occlusion and well-developed collateral circulation. JACC Cardiovasc Intv. 2015;8(2):271–9.
Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33(5):614–21.
Winer N, Sowers JR. Epidemiology of diabetes. J Clin Pharmacol. 2004;44(4):397–403.
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014;5(4):444–70.
Werner GS, Richartz BM, Heinke S, Ferrari M, Figulla HR. Impaired acute collateral recruitment as a possible mechanism for increased cardiac adverse events in patients with diabetes mellitus. Eur Heart J. 2003;24(12):1134–42.
Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95.
Millard RW, Wang Y. Milieu interieur: the search for myocardial arteriogenesis signals. J Am Coll Cardiol. 2009;53(23):2148–9.
Schirmer SH, van Royen N, Moerlan PD, Fledderus JO, Henriques JP, van der Schaaf R, Vis MM, Baan J Jr, Koch KT, Horrevoets AJ, et al. Local cytokine concentration and oxygen pressure are related to maturation of collateral circulation in humans. J Am Coll Cardiol. 2009;53(23):2141–7.
Werner GS, Ferrari M, Richartz BM, Gastmann O, Figulla HR. Microvascular dysfunction in chronic total coronary occlusions. Circulation. 2001;104(10):1129–34.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marson SP, et al. A prospective, natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Kennedy MW, Fabris E, Suryapranata H, Kedhi E. Is ischemia the only factor predicting cardiovascular outcomes in all diabetes mellitus patients? Cardiovasc Diabetol. 2017;16:15.
Kuroda M, Shinke T, Otake H, Sugiyama D, Takaya T, Takahashi H, Terashita D, Uzu K, Tahara N, Kashiwagi D, et al. Effects of daily glucose fluctuations on the healing response to everolimus-eluting stent implantation as assessed using continuous glucose monitoring and optical coherence tomography. Cardiovasc Diabetol. 2016;15:79.
Yoshida N, Yamamoto H, Shinke T, Otake H, Kuroda M, Terashita D, Takahashi H, Sakaguchi K, Hirota Y, Emoto T, et al. Impact of CD14++CD16+ monocytes on plaque vulnerability in diabetic and non-diabetic patients with asymptomatic coronary artery disease: a cross-sectional study. Cardiovasc Diabetol. 2017;16(1):96.
Hinkel R, Howe A, Renner S, Ng J, Lee S, Klett K, Kaczmarek V, Moretti A, Laugwitz KL, Skroblin P, et al. Diabetes mellitus–induced microvascular destabilization in the myocardium. J Am Coll Cardiol. 2017;69(2):131–43.
De Bruyne B, Hersbach F, Pijls NHJ, Bartunek J, Bech JW, Heyndrickx GR, Gould KL, Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but ‘normal’ coronary angiography. Circulation. 2001;104(2):2401–6.
Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. 2015;18(3):265–81.
Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605.
Hansen L, Gupta D, Joseph G, Weiss D, Taylor WR. The receptor for advanced glycation end products impairs collateral formation in both diabetic and non-diabetic mice. Lab Invest. 2017;97(1):34–42.
Simons M. Diabetic monocyte and vascular endothelial growth factor signaling impairment. Circulation. 2009;120(2):104–5.
Rocic P. Why is coronary collateral growth impaired in type II diabetes and the metabolic syndrome? Vascul Pharmacol. 2012;57(5–6):179–86.
Ito WD, Lund N, Sager H, Becker W, Wenzel U. Different impact of diabetes mellitus type II and arterial hypertension on collateral artery growth and concomitant macrophage accumulation. Vasa. 2015;44(1):31–41.
Kaza E, Ablasser K, Poutias D, Griffiths ER, Saad FA, Hofstaetter JG, del Nido PJ, Friehs I. Up-regulation of soluble vascular endothelial growth factor receptor-1 prevents angiogenesis in hypertrophied myocardium. Cardiovasc Res. 2011;89(2):410–8.
Ikeda T, Sun L, Tsuruoka N, Ishigaki Y, Yoshitomi Y, Yoshitake Y, Yonekura H. Hypoxia down-regulates sFlt-1 (sVEGFR-1) expression in human microvascular endothelial cells by a mechanism involving mRNA alternative processing. Biochem J. 2011;436(2):399–407.
Wada H, Satoh N, Kitaoka S, Ono K, Morimoto T, Kawamura T, Nakano T, Fujita M, Kita T, Shimatsu A, et al. Soluble VEGF receptor-2 is increased in sera of subjects with metabolic syndrome in association with insulin resistance. Atherosclerosis. 2010;208(2):512–7.
Sun Z, Shen Y, Lu L, Zhang RY, Pu LJ, Zhang Q, Yang ZK, Hu J, Chen QJ, Shen WF. Increased serum level of soluble vascular endothelial growth factor receptor-1 is associated with poor coronary collateralization in patients with stable coronary artery disease. Circ J. 2014;78(5):1191–6.
Sasso FC, Torella D, Carbonara O, Ellison GM, Torella M, Scardone M, Marra C, Nasti R, Marfella R, Cozzolino D, et al. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. J Am Coll Cardiol. 2005;46(5):827–34.
Tchaikovski V, Olieslagers S, Bohmer F, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120(2):150–9.
Nandy D, Mukhopadhyay D, Basu A. Both vascular endothelial growth factor and soluble Flt-1 are increased in type 2 diabetes but not in impaired fasting glucose. J Investig Med. 2010;58(6):804–6.
Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complication. Ann N Y Acad Sci. 2011;1243:88–102.
Hedrick CC, Thorpe SR, Fu MX, Harper CM, Yoo J, Kim SM, Wong H, Peters AL. Glycation impairs high-density lipoprotein function. Diabetologia. 2000;43(3):312–20.
Younis N, Sharma R, Soran H, Charlton-Menys V, Elseweidy M, Durrington PN. Glycation as an atherogenic modification of LDL. Curr Opin Lipidol. 2008;19(4):378–84.
Pung YF, Chilian WM. Corruption of coronary collateral growth in metabolic syndrome: role of oxidative stress. World J Cardiol. 2010;2(12):421–7.
Werner GS. The role of coronary collaterals in CTO. Curr Cardiol Rev. 2014;10(1):57–64.
Borekci A, Gur M, Seker T, Baykan AO, Ozaltun B, Karakoyun S, Karakurt A, Turkoglu C, Makca I, Cayli M. Coronary collateral circulation in patients with chronic coronary total occlusion: the relationship with cardiac risk markers and SYNTAX score. Perfusion. 2015;30(6):457–64.
Epstein SE, Lassance-Soares RM, Faber JE, Burnett MS. Effects of aging on the collateral circulation, and therapeutic implications. Circulation. 2012;125(25):3211–9.
Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y, SMART Study Group. Coronary collateral circulation: the effects of smoking and alcohol. Atherosclerosis. 2007;191(1):191–8.
Koerselman J, de Jaegere PP, Verhaar MC, Grobbee DE, van der Graaf Y, SMART Study Group. High blood pressure is inversely related with the presence and extent of coronary collaterals. J Hum Hypertens. 2005;19(10):809–17.
Yetkin E, Topal E, Erguzel N, Senen K, Heper G, Waltenberger J. Diabetes mellitus and female gender are the strongest predictors of poor collateral vessel development in patients with severe coronary artery stenosis. Angiogenesis. 2015;18(2):201–7.
Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49(3):554–60.
Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia. Circulation. 2000;102(suppl III):III370–3.
Fan Y, Hu JS, Guo F, Lu ZB, **a H. Lipoprotein (a) as a predictor of poor collateral circulation in patients with chronic stable coronary heart disease. Braz J Med Biol Res. 2017;50:e5979.
Uysal OK, Sahin DY, Duran M, Turkoglu C, Yildirim A, Elbasan Z, Ozkan B, Tekin K, Kunak AU, Yilmaz Y, et al. Association between uric acid and coronary collateral circulation in patients with stable coronary artery disease. Angiology. 2014;65(3):227–31.
Gulec S, Ozdemir AO, Maradit-Kremers H, Dincer I, Atmaca Y, Erol C. Elevated levels of C-reactive protein are associated with impaired coronary collateral development. Eur J Clin Invest. 2006;16(6):369–75.
Seiler C, Pohl T, Billinger M, Meier B. Tumor necrosis factor alpha concentration and collateral flow in patients with coronary artery disease and normal systolic left ventricular function. Heart. 2003;89(1):96–7.
Shen Y, Ding FH, Zhang RY, Zhang Q, Lu L, Shen WF. Association of serum mimecan with angiographic coronary collateralization in patients with stable coronary artery disease and CTO. Atherosclerosis. 2016;252:75–81.
Akm F, Ayca B, Celik O, Sahin C. Predictors of poor coronary collateral development in patients with stable coronary artery disease: neutropil-to-lymphocyte ratio and platelets. Anatol J Cardiol. 2015;15(3):218–23.
Sahinarslan A, Kocaman SA, Topal S, Ercin U, Bukan N, Yalcin R, Timurkaynak T. Relation between serum monocyte chemoattractant protein-1 and coronary collateral development. Coron Artery Dis. 2010;21(8):455–9.
Akboga MK, Akyel A, Sahinarslan A, Demirtas CY, Yayla C, Boyaci B, Yalcin R. Relationship between plasma apelin level and coronary collateral circulation. Atherosclerosis. 2014;235(2):289–94.
Keeley EC, Moorman JR, Liu L, Gimple LW, Gimple LW, Lipson LC, Ragosta M, Taylor AM, Lake DE, Burdick MD, et al. Plasma chemokine levels are associated with the presence and extent of angiographic coronary collaterals in chronic ischemic heart disease. PLoS ONE. 2011;6:e21174.
Ozeke O, Gungor M, Topalouglu S, Aras D, Ozer C. Chronic total artery occlusions in noninfarct-related coronary arteries. Int J Angiol. 2014;23(17):17–22.
Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Glycated albumin is superior to heamoglobin A1c for evaluating the presence and severity of coronary artery disease in type 2 diabetic patients. Cardiology. 2012;123(2):84–90.
Shen Y, Pu LJ, Lu L, Zhang Q, Zhang RY, Shen WF. Serum advanced glycation end-products and receptors as prognostic biomarkers in diabetics undergoing coronary artery stent implantation. Can J Cardiol. 2012;28(6):737–43.
Shen Y, Lu L, Ding FH, Sun Z, Zhang RY, Zhang Q, Yang ZK, Hu J, Chen QJ, Shen WF. Association of increased serum glycated albumin levels with low coronary collateralization in type 2 diabetic patients with stable angina and CTO. Cardiovasc Diabetol. 2013;12:165.
Cohen MP, Shea E, Chen S, Shearman CW. Glycated albumin increases oxidase stress, activates NF-κB and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-beta 1 production in macrophage RAW cells. J Lab Clin Med. 2003;141(4):242–9.
Li H, Zhang X, Guan X, Cui X, Wang Y, Chu H, Cheng M. Advanced glycation end products impair the migration, adhesion and secretion potentials of late endothelial progenitor cells. Cardiovasc Diabetol. 2012;11:46.
Angelidis C, Deftereos S, Giannopoulos G, Anatoliotakis N, Bouras G, Hatzis G, Panagopoulou V, Pyrgakis V, Cleman MW. Cystatin C: an emerging biomarker in cardiovascular disease. Curr Top Med Chem. 2013;13(2):164–79.
Shen Y, Ding FH, Zhang RY, Zhang Q, Lu L, Shen WF. Serum cystatin C reflects angiographic coronary collateralization in stable coronary artery disease patients with CTO. PLoS ONE. 2015;10:e0137253.
Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem. 2009;55(11):1932–43.
Shen Y, Ding FH, Wu F, Sun Z, Zhang RY, Zhang Q, Lu L, Wu ZG, Shen WF. Cystatin C-versus creatinine- based definition of renal dysfunction for detecting low coronary collateralization in patients with stable angina and CTO. J Diabetes Metab. 2014;5:453–9.
**e SL, Li HY, Deng BQ, Luo NS, Geng DF, Wang JF, Nie RQ. Poor coronary collateral vessel development in patients with mild to moderate renal insufficiency. Clin Res Cardiol. 2011;100(3):227–33.
Schotiker B, Brenner H, Herder C, Rothenbacher D, Muller H. Clinical utility of creatinine- and cystatin C-based definition of renal function for risk prediction of primary cardiovascular events in patients with diabetes. Diabetes Care. 2012;35(4):879–86.
Lin TH, Wang CL, Su HM, Hsu PC, Juo SH, Voon WC, Shin SJ, Lai WT, Sheu SH. Functional vascular endothelial growth factor gene polymorphisms and diabetes: effect on coronary collaterals in patients with significant coronary artery disease. Clin Chim Acta. 2010;411(21–22):1688–93.
Ouchi N, Walsh K. Cardiovascular and metabolic regulation by the adiponectin/CTRP family of proteins. Circulation. 2012;125(25):3066–8.
Siasos G, Tousoulis D, Kollia C, Oikonomou E, Siasou Z, Stefanadis C, Papavassiliou AG. Adiponectin and cardiovascular disease: mechanisms and new therapeutic approaches. Curr Med Chem. 2012;19(8):1193–209.
Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, et al. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125(25):3159–69.
Shen Y, Lu L, Liu ZH, Zhu JZ, Sun Z, Zhang RY, Zhang Q, Hu J, Chen QJ, Wu ZG, et al. Increased serum level of CTRP1 is associated with low coronary collateralization in stable angina patients with CTO. Int J Cardiol. 2014;174(1):2013–6.
Lu L, Zhang RY, Wang XQ, Liu ZH, Shen Y, Ding FH, Meng H, Wang LJ, Yan XX, Yang K, et al. C1q/TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J. 2016;37(22):1762–71.
Shen Y, Ding FH, Wu F, Lu L, Zhang RY, Zhang Q, Wu ZG, Shen WF. Association of blood pressure and coronary collateralization in type 2 diabetic and nondiabetic patients with stable angina and CTO. J Hypertens. 2015;33(3):621–6.
Vamos EP, Harris M, Millett C, Pape UJ, Khunti K, Curcin V, Curcin V, Molokhia M, Majeed A. Association of systolic and diastolic blood pressure and all cause mortality in patients newly diagnosed type 2 diabetes: retrospective cohort study. BMJ. 2012;345:e5567.
Shen Y, Ding FH, Zhang RY, Yang ZK, Hu J, Shen WF. Impact of diabetes and stenosis of donor artery on pressure-derived coronary collateral flow in patients with stable coronary artery disease and CTO. Eur Heart J. 2017;38(suppl-1):1752.
Biscetti F, Straface G, Arena V, Stigliano E, Pecorini G, Rizzo P, et al. Pioglitazone enhances collateral blood flow in ischemic hindlimb of diabetic mice through an Akt-dependent VEGF-mediated mechanism, regardless of PPARγ stimulation. Cardiovasc Diabetol. 2009;8:49.
ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85.
Ladwiniec A, Hoye A. The hemodynamic effects of collateral donation to a CTO: implications for patient management. Int J Cardiol. 2015;196:159–66.
Seiler C, Fleisch M, Meier B. Direct intracoronary evidence of collateral steal in humans. Circulation. 1997;96(12):4261–7.
Dincer I, Ongun A, Turhan S, Ozdol C, Kumbasar D, Erol C. Association between the dosage and duration of statin treatment with coronary collateral development. Coron Artery Dis. 2006;17(6):561–6.
van der Heoven NW, Teunissen PF, Werner GS, Delewi R, Schirmer SH, Traupe T, van der Laan AM, Tijssen JG, Piek JJ, Seiler C, van Royen N. Clinical parameters associated with collateral development in patients with chronic coronary total occlusion. Heart. 2013;99(15):1100–5.
Schirmer SH, Degen A, Baumhakel M, Custodis F, Schuh L, Kohlhaas M, Friedrich E, Bahlmann F, Kappl R, Maack C, et al. Heart rate reduction by If-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur Heart J. 2012;33(10):1223–31.
Altin T, Kilickap M, Tutar E, Turhan S, Atmaca Y, Gulec S, Oral D, Erol C. The relationship of chronic angiotensin enzyme inhibitor use and coronary collateral vessel development. Int Heart J. 2007;48(4):435–42.
Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of CTOs on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for CTO. Catheter Cardiovasc Interv. 2013;82(1):95–107.
Kennedy MW, Fabris E, Suryapranata H, Kedhi E. Is ischemia the only factor predicting cardiovascular outcomes in all diabetes mellitus patients? Cardiovasc Diabetol. 2017;16:15.
Blundhun PK, Wu ZJ, Chen MH. Coronary artery bypass surgery compared with percutaneous coronary interventions in patients with insulin-treated type 2 diabetes mellitus: a systemic review and meta-analysis of 6 randomized controlled trials. Cardiovasc Diabetol. 2016;15:2.
Choi KH, Yang JH, Song YB, Hahn JY, Choi JH, Gwon HC, Lee SH, Choi SH. Long-term clinical outcomes of patients with coronary CTO treated with percutaneous coronary intervention versus medical therapy according to presence of diabetes mellitus. EuroIntervention. 2017;13(8):970–7.
Sanguineti F, Garot P, O’Connor S, Watanabe Y, Spaziano M, Lefèvre T, Hovasse T, Benamer H, Unterseeh T, Chevalier B, et al. Chronic total coronary occlusion treated by percutaneous coronary intervention: long-term outcome in patients with and without diabetes. EuroIntervention. 2017;12(15):e1889–97.
Ladwiniee A, Connington MS, Rossington J, Mather AN, Alahmar A, Oliver RM, Nijjer SS, Davies JE, Thackray S, Alamgir F, et al. Collateral donor artery physiology and the influence if a CTO on fractional flow reserve. Circ Cardiovasc Interv. 2015;8(7):e002219.
Lu L, Wang YN, Li MC, Wang HB, Pu LJ, Niu WQ, Meng H, Yang EL, Zhang RY, Zhang Q, et al. Reduced serum levels of vasostatin-2, an antiinflammatory peptide derived from chromogranin A, are associated with the presence and severity of coronary artery disease. Eur Heart J. 2012;23(18):2297–306.
Shantikumar S, Caporali AC, Emanueli C. Role of microRNAs in diabetes and its cardiovascular complications. Cardiovasc Res. 2012;93(4):583–93.
Papageorgiou N, Zacharia E, Tousoulis D. Association between microRNAs and coronary collateral circulation: is there a new role for the small non-coding RNAs? Ann Transl Med. 2016;4(11):223.
Kadi H, Ozyurt H, Ceyhan K, Koc F, Celik A, Burucu T. The relationship between high-density lipoprotein cholesterol and coronary collateral circulation in patients with coronary artery disease. J Investig Med. 2012;60(5):808–12.
Pu LJ, Lu L, Du R, Wang XQ, Zhang RY, Zhang Q, Yang ZK, Chen QJ, Shen WF. Glycation of apoprotein A-I is associated with coronary artery plaque progression in type 2 diabetes mellitus. Diabetes Care. 2013;36(5):1312–20.
Shen Y, Lu L, Sun JT, Zhang RY, Ding FH, Pu LJ, Chen QJ, Shen WF, Lu L. Glycation of apoA-I is associated with reduced levels and activities of paraoxonase 1 and 3 in serum and in HDL, and with severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:52.
Dai Y, Shen Y, Li QR, Ding FH, Wang XQ, Sun JT, Yan XX, Wang LJ, Yang K, Wang HB, et al. Association of apolipoprotein A-IV glycation with the severity of coronary artery disease in type 2 diabetic patients: glycated apolipoprotein A-IV promoting atherogenesis through mediation of nuclear receptor NR4A3. Am Coll Cardiol. 2017;70(16):2006–19.
Authors’ contributions
YS, FHD wrote the article, substantially contributed to discussion of the content, and edited the manuscript. YD, XQW researched data for the article. RYZ, LL substantially contributed to discussion of the content and reviewed the manuscript. WFS reviewed the manuscript before submission. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable. No new datasets were generated for this manuscript.
Consent for publication
All authors consent this manuscript for publication.
Ethics approval and consent to participate
The study protocol was approved by the Ethics committee of Rui ** Hospital, Shanghai Jiao tong University School of Medicine.
Funding
This study was supported in part by the Research Foundation of Chinese National Natural Science (81400327), Shanghai Science & Technology Committee (14ZR1425800) and Medico-engineering Project (GY2016MS66) and Talent Young Investigators (17XJ11009) of Shanghai Jiao Tong University School of Medicine.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, Y., Ding, F.H., Dai, Y. et al. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol 17, 26 (2018). https://doi.org/10.1186/s12933-018-0671-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-018-0671-6