Abstract
Hearing loss is the most common sensory neural disorder in humans, and according to a WHO estimation, 5.5% (466 million) of people worldwide have disabling hearing loss. In this study, a Chinese family with prelingual sensorineural hearing loss was investigated. The affected individuals showed moderately severe hearing loss at all frequencies. Using target genome enrichment and high-throughput sequencing, the homozygous variant c.2372del; p.(Ser791fs) was identified in PDZD7. This variant lies in exon 15 of PDZD7 and results in a frame shift followed by an early stop codon. It is classified as pathogenic according to the ACMG/AMP guidelines and ClinGen specifications. Our study expands the pathogenic variant spectrum of PDZD7 and strengthens the clinical importance of this gene in patients with moderately severe hearing loss.
Similar content being viewed by others
Introduction
Hearing loss is the most common sensory neural disorder in humans, and according to a WHO estimation, 5.5% (466 million) of the world population has disabling hearing loss (https://www.who.int/health-topics/hearing-loss). It is estimated that more than half of neonatal sensorineural hearing loss (SNHL) is caused by genetic factors [1]. Nonsyndromic SNHL, in which no other symptoms occur, accounts for approximately 70% of hereditary SNHL. Syndromic SNHL, which is associated with other symptoms, accounts for approximately 30% of hereditary SNHL [1]. To date, 124 genes have been implicated in nonsyndromic SNHL, and more than 400 syndromic SNHLs have been identified (https://hereditaryhearingloss.org/, data updated on August 30, 2021). In the hearing system, the proteins encoded by most of these deafness genes are located in inner ear hair cells. These are polarized epithelial cells with stereocilia bundles at the top, which translate motion to neuronal signals [2].
PDZD7 encodes a scaffold protein that is expressed in the cortex and inner ear. Pathogenic variants in PDZD7 have been reported to cause autosomal recessive nonsyndromic SNHL [3, 4]. Moreover, PDZD7 has been suggested to be a contributor to digenic Usher syndrome type IIC and a modifier in patients with Usher Syndrome (USH) Type IIA [5]. As the most common cause of deaf blindness, USH is divided into three subtypes (USH1, USH2, and USH3) based on the degree and onset age of hearing loss, onset age of retinitis pigmentosa and involvement of vestibular impairment. Twelve genes have been linked to USH, including 6 USH1 genes (MYO7A, USH1C, CDH23, PCDH15, USH1G and CIB2), 4 USH2 genes (USH2A, ADGRV1, WHRN and PDZD7), and 2 USH3 genes (CLRN1 and HARS) [6]. Eight of these USH genes have been identified to cause both USH and nonsyndromic SNHL, namely, MYO7A, CDH23, USH1C, PCDH15, WHRN, CIB2, USH1G and PDZD7.
In this study, we present the genetic characteristics of a Chinese Han family with congenital SNHL. The affected individuals had moderately severe hearing loss at all frequencies. Using targeted genome enrichment (TGE) and high-throughput sequencing (HTS), we identified a novel homozygous frameshift variant in exon 15 of PDZD7. The variant results in a frame shift followed by an early stop codon and would most likely lead to nonsense-mediated mRNA decay (NMD). Our study enriches the variant spectrum of PDZD7 and suggested that TGE and HTS are reliable tools for genetic testing of hereditary hearing loss for large genes such as PDZD7.
Materials and methods
Subjects
Participants in this study were recruited from the outpatient department of the Affiliated Eye and ENT Hospital of Fudan University, Shanghai, China. All family members were evaluated by audiological tests. Pure tone audiometry at frequencies of 125, 250, 500, 1000, 2000, 4000, and 8000 Hz was performed on family members above the age of 6. Romberg and tandem gait tests were performed to evaluate vestibular functions. Auditory brainstem response (ABR) test was performed on family members under the age of 6. High-resolution computed tomography (HRCT) scans of the temporal bone were obtained to examine inner ear malformations. Written informed consent was obtained from adult participants and parents of all minor participants involved in the study. This study was approved by the ethics committee of the Institutional Review Board of the Eye, Ear, Nose and Throat Hospital affiliated with Fudan University (Shanghai, China).
Targeted exome sequencing
Genomic DNA was extracted from the whole blood from participants using a genomic DNA isolation kit (Qiagen, Hilden, Germany). To screen common pathogenic deafness variants in the GJB2, SLC26A4, and MT-RNR1 genes, the patients were prescreened by PCR amplification and Sanger sequencing. A paired-end sequencing library was prepared using a library preparation kit (New England Biolabs, Ipswich, MA, catalog# E6040). A human deafness gene exon enrichment kit including 168 genes was used to capture target genome intervals (Additional file1: Table S1). High-throughput sequencing was performed using Illumina HiSeq 2000 according to the manufacturer’s instructions (Illumina, Inc., San Diego, CA).
Bioinformatics and validation of the variants
Sequencing reads were generated by the Illumina CASAVA v1.8 pipeline and aligned to the human reference genome (hg19) using the Burrows–Wheeler Aligner (BWA) program. Variants were called using the GATK package v4.1.8.1. All variants were annotated and characterized using ANNOVAR software. To identify pathogenic variants, we filtered out the following: (1) low-quality variants (depth < 10, or genotype quality < 30); (2) variants in the noncoding regions, except for those that might disrupt splicing; (3) synonymous variants in the coding region; (4) variants with minor allele frequency (MAF) > 0.001 in several databases (1000 Genome Project, gnomad v2.1.1 and in-house database); and (5) variants labeled as “benign” in the ClinVar database. The deleterious effect of variants was predicted by SIFT scores, REVEL, and CADD scores. To validate the variants, Sanger sequencing of PDZD7 exon 15 was performed on genomic DNA from all family members and 96 normal hearing controls. PCR and sequencing primers were designed by Primer3 online software. Sanger sequencing was performed on a 3730XL sequencer (Applied Biosystems) according to the manufacturer’s instructions.
Results
Family and clinical presentations
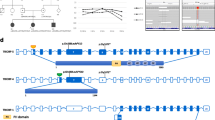
Family D27 is a nonconsanguineous Chinese family that includes three affected siblings and two normal hearing parents (Fig. 1A). This family underwent auditory tests, and the family history was obtained. The affected siblings were 9, 7 and 2 years old during the examination. Audiograms of the two older patients showed bilateral moderate to severe HL with a slightly downward slope (Fig. 1B). The ABR test of II:3 revealed bilateral HL with a threshold of approximately 65 dB (Fig. 1C). Although newborn hearing screenings were not performed, the parents recalled no responses to subtle sounds at 1–2 years of age, suggesting a congenital phenotype. Vestibular functional tests of the two older patients revealed no abnormalities. HRCT scans of II:1 and II:2 revealed no inner ear malformations. We also performed pure tone audiometry on the parents and detected no hearing loss features. Otoscopy and full physical examination with special attention to renal and ophthalmological evaluations revealed no additional abnormalities.
Targeted high-throughput sequencing
Targeted high-throughput sequencing of all exons and exon–intron boundaries for 168 deafness genes was performed on the proband II:1. Sequencing yielded 2.8 million 100 bp paired-end reads. After adaptor trimming and low-quality read filtering, paired-end fastq files were aligned to the human genome (hg19). A mean depth of 118.1 for the targeted exons was achieved, and 97.8% of the targeted genome intervals were covered by at least 10 sequencing reads. After filtering against MAFs from various databases (1000 Genome Project, gnomad v2.1.1 and in-house database), we focused on variants in the coding region and intronic variants that might affect splicing. Based on the assumption of an autosomal recessive mode of inheritance, we focused on genes with homozygous or compound heterozygous variants. A homozygous variant, c.2372del; p.(Ser791Phefs*17); p.(Ser791fs) (NM_001195263.2), was identified in exon 15 of PDZD7. This variant was not present in any of the reference databases.
Genetic analysis of the PDZD7 variant
Sanger sequencing of exon 15 of PDZD7 was performed for all family members (Fig. 2A). Variant interpretation was performed according to the ACMG/AMP guidelines and ClinGen specifications [7,8,9,4]. The variant identified in this study localizes to exon 15 of PDZD7, which is unique to the long isoform (Fig. 2B). Including our research, 3 pathogenic variants have been discovered in domains unique to the long isoform (Fig. 2B). Patients harboring each of these 3 variants (p.Ser703fs, p.Arg781_Ser784del and p.(Ser791fs)) showed the same characteristic auditory phenotype of bilateral moderately severe hearing loss at all frequencies with gentle downward slo** as patients with variants in other parts of the gene [15, 16]. Another variant (p.Cys732fs), cosegregating with biallelic USH2A variants in a patient with Usher syndrome, unique to the long isoform, was identified as a Usher syndrome modifier [5]. A mouse model lacking exons 2–5 of Pdzd7, which disrupts all isoforms, and a mouse model lacking exon 14, which only disrupts the long isoform, both manifest stereocilia disorganization and MET deficits, leading to a similar hearing loss phenotype. Moreover, in mice lacking exon 14 of the Pdzd7 gene, the short isoforms were not detected in the inner ear at the protein level. These findings suggest that the PDZD7 long isoform is indispensable for hair cell function. However, the PDZD7 short isoform may not localize in the stereocilia and therefore make no contribution to stereocilia function.
Hereditary hearing loss is a genetically and phenotypically heterozygous disorder. To date, 124 genes have been identified for nonsyndromic SNHL, and 46 genes have been identified for the nine most common syndromic HLs (https://hereditaryhearingloss.org/, data updated on August 30, 2021). The phenotypes of the patients vary by audiogram, age of onset, progression, vestibular complications, inner ear malformations, retinal complications, etc. [23]. These heterogeneities hindered the genetic diagnosis of hearing loss and call for a more comprehensive variant screening strategy that takes phenotype-genotype correlations into consideration. Since most cases of autosomal recessive nonsyndromic SNHL is characterized by prelingual severe to profound HL, the relatively rare moderately severe audiogram at all frequencies may serve as a reminder for potential causative PDZD7 variants.
We report a novel pathogenic frameshift variant on PDZD7 in a Chinese family with moderately severe HL. This variant lies in exon 15 and is unique to the long isoform of the PDZD7 protein. Our study extends the variant spectrum of the PDZD7 gene in the Chinese population. The relatively uncommon moderately severe audiogram with a slightly downward slope is characteristic of PDZD7 patients. The identification of a novel pathogenic PDZD7 variant may be valuable for genetic consultation and functional research.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Smith RJ, Bale JF Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–90.
Dror AA, Avraham KB. Hearing impairment: a panoply of genes and functions. Neuron. 2010;68(2):293–308.
Booth KT, Azaiez H, Kahrizi K, Simpson AC, Tollefson WTA, Sloan CM, Meyer NC, Babanejad M, Ardalani F, Arzhangi S, et al. PDZD7and hearing loss: more than just a modifier. Am J Med Genet A. 2015;167(12):2957–65.
Schneider E, Märker T, Daser A, Frey-Mahn G, Beyer V, Farcas R, Schneider-Rätzke B, Kohlschmidt N, Grossmann B, Bauss K, et al. Homozygous disruption of PDZD7 by reciprocal translocation in a consanguineous family: a new member of the Usher syndrome protein interactome causing congenital hearing impairment. Hum Mol Genet. 2009;18(4):655–66.
Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schäfer E, Roux A-F, Dafinger C, Bernd A, et al. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Investig. 2010;120(6):1812–23.
Toms M, Pagarkar W, Moosajee M. Usher syndrome: clinical features, molecular genetics and advancing therapeutics. Ther Adv Ophthalmol. 2020;12:2515841420952194.
Zhang J, Yao Y, He H, Shen J. Clinical interpretation of sequence variants. Curr Protoc Hum Genet. 2020;106(1):e98.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Oza AM, DiStefano MT, Hemphill SE, Cushman BJ, Grant AR, Siegert RK, Shen J, Chapin A, Boczek NJ, Schimmenti LA, et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat. 2018;39(11):1593–613.
Peng J, **ang J, ** X, Meng J, Song N, Chen L, Abou Tayoun A, Peng Z. VIP-HL: Semi-automated ACMG/AMP variant interpretation platform for genetic hearing loss. Hum Mutat. 2021;42(12):1567–75.
Zou J, Zheng T, Ren C, Askew C, Liu X-P, Pan B, Holt JR, Wang Y, Yang J. Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice. Hum Mol Genet. 2014;23(9):2374–90.
Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, Harrison SM. ClinGen Sequence Variant Interpretation Working G: recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018;39(11):1517–24.
Supek F, Lehner B, Lindeboom RGH. To NMD or Not To NMD: nonsense-mediated mRNA decay in cancer and other genetic diseases. Trends Genet. 2021;37(7):657–68.
Chen Q, Zou J, Shen Z, Zhang W, Yang J. Whirlin and PDZ domain-containing 7 (PDZD7) proteins are both required to form the quaternary protein complex associated with usher syndrome type 2. J Biol Chem. 2014;289(52):36070–88.
Luo H, Hassan RN, Yan J, **e J, Du P, Hu Q, Zhu Y, Jiang W. Novel recessive PDZD7 biallelic mutations associated with hereditary hearing loss in a Chinese pedigree. Gene. 2019;709:65–74.
Vona B, Lechno S, Hofrichter MA, Hopf S, Laig AK, Haaf T, Keilmann A, Zechner U, Bartsch O. Confirmation of PDZD7 as a nonsyndromic hearing loss gene. Ear Hear. 2016;37(4):e238-246.
Guan J, Wang H, Lan L, Wang L, Yang J, **e L, Yin Z, **ong W, Zhao L, Wang D, et al. Novel recessive PDZD7 biallelic mutations in two Chinese families with non-syndromic hearing loss. Am J Med Genet A. 2018;176(1):99–106.
Lee SY, Han JH, Kim BJ, Oh SH, Lee S, Oh DY, Choi BY. Identification of a potential founder effect of a novel PDZD7 Variant involved in moderate-to-severe sensorineural hearing loss in Koreans. Int J Mol Sci. 2019;20(17):4174.
Le Quesne SP, James C, Ocaka L, Tekman M, Grunewald S, Clement E, Stanescu HC, Kleta R, Morrogh D, Calder A, et al. An example of the utility of genomic analysis for fast and accurate clinical diagnosis of complex rare phenotypes. Orphanet J Rare Dis. 2017;12(1):24.
Fahimi H, Behroozi S, Noavar S, Parvini F. A novel recessive PDZD7 bi-allelic mutation in an Iranian family with non-syndromic hearing loss. BMC Med Genom. 2021;14(1):37.
Wu D, Huang W, Xu Z, Li S, Zhang J, Chen X, Tang Y, Qiu J, Wang Z, Duan X, et al. Clinical and genetic study of 12 Chinese Han families with nonsyndromic deafness. Mol Genet Genom Med. 2020;8(4):e1177.
Kim NK, Kim AR, Park KT, Kim SY, Kim MY, Nam JY, Woo SJ, Oh SH, Park WY, Choi BY. Whole-exome sequencing reveals diverse modes of inheritance in sporadic mild to moderate sensorineural hearing loss in a pediatric population. Genet Med. 2015;17(11):901–11.
Shearer AE, Hildebrand MS, Sloan CM, Smith RJ. Deafness in the genomics era. Hear Res. 2011;282(1–2):1–9.
Acknowledgements
We would like to thank our patients for agreeing to donate their personal data for our study and to have these data published.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82192860, 81620108005, 81500801, 81830029), the National Key R&D Program of China (Nos. 2017YFA0103900, 2016YFC0905200) and the Shanghai Municipal Health Commission (Nos. 201840277).
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were performed in accordance with the Declaration of Helsinki. This study was approved by the Eye, Ear, Nose and Throat Hospital affiliated with Fudan University Review Board of the Office of Research Compliance through protocol 2017044. Written informed consent was obtained from adult participants involved in the study. Written informed consent was obtained from parents/guardians of all minor participants involved in the study. This study was approved by the ethics committee of the Institutional Review Board of the Eye, Ear, Nose and Throat Hospital affiliated with Fudan University (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1.
Supplementary Table S1: Table of genes for the hearing loss sequencing panel.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Du, Q., Sun, Q., Gu, X. et al. Novel homozygous variant in the PDZD7 gene in a family with nonsyndromic sensorineural hearing loss. BMC Med Genomics 15, 135 (2022). https://doi.org/10.1186/s12920-022-01289-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01289-7