Abstract
Background
Foot-and-mouth disease (FMD), which is caused by foot-and-mouth disease virus (FMDV), is a highly contagious tansboundary disease of cloven-hoofed animals and causes devastating economic damages. Accurate, rapid and simple detection of FMDV is critical to containing an FMD outbreak. Recombinase polymerase amplification (RPA) has been explored for detection of diverse pathogens because of its accuracy, rapidness and simplicity. A visible and equipment-free reverse-transcription recombinase polymerase amplification assay combined with lateral flow strip (LFS RT-RPA) was developed to detect the FMDV using primers and LF probe specific for the 3D gene.
Results
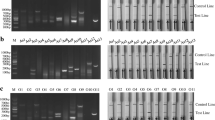
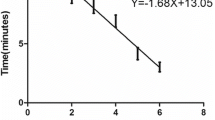
The FMDV LFS RT-RPA assay was performed successfully in a closed fist using body heat for 15 min, and the products were visible on the LFS inspected by the naked eyes within 2 min. The assay could detect FMDV serotypes O, A and Asia1, and there were no cross-reactions with vesicular stomatitis virus (VSV), encephalomyocarditis virus (EMCV), classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV), porcine circovirus 2 (PCV2) and pseudorabies virus (PRV). The analytical sensitivity was 1.0 × 102 copies in vitro transcribed FMDV RNA per reaction, which was the same as a real-time RT-PCR. For the 55 samples, FMDV RNA positive rate was 45.5% (25/55) by LFS RT-RPA and 52.7% (29/55) by real-time RT-PCR. For the LFS RT-RPA assay, the positive and negative predicative values were 100% and 80%, respectively.
Conclusions
The performance of the LFS RT-RPA assay was comparable to real-time RT-PCR, while the LFS RT-RPA assay was much faster and easier to be performed. The developed FMDV LFS RT-RPA assay provides an attractive and promising tool for rapid and reliable detection of FMDV in under-equipped laboratory and at point-of-need facility, which is of great significance in FMD control in low resource settings.
Similar content being viewed by others
Background
Foot-and-mouth disease (FMD) is a highly contagious viral disease of wild and domesticated cloven-hoofed animals. The causative agent, foot-and-mouth disease virus (FMDV), a non-enveloped, single-stranded positive-sense RNA virus, belongs to the genus Aphthovirus within the family Picornaviridae [1]. FMDV exists in seven distinct serotypes comprising O, A, C, Asia1 and South African Territories (SAT) serotypes SAT1, SAT2 and SAT3 and multiple subtypes due to the high mutational rate of the virus [1]. Although the mortality rate of FMD is generally low, the disease can be economically devastating due to production losses in endemic countries and trade restrictions in FMD-free countries. It is estimated that annual global impact of FMD in endemic regions alone is between US$ 6.5 and 21 billion [2].
The above facts clearly indicate that the early, rapid and robust diagnosis of FMD is imperative in the prevention and control of the disease. FMD is characterized by vesicular lesions and ulcerations on the tongue, mouth, nasal region and coronary bands of infected animals [3]. Nevertheless, reliable diagnosis based on clinical signs alone can sometimes be difficult because the clinical signs are often mild in adult sheep and goats [4] and a number of viral diseases clinically mimic FMD, including vesicular stomatitis (VS), swine vesicular disease (SVD), vesicular exanthema of swine (VES), and Senecavirus A (SVA) infection. Therefore, laboratory diagnostic tools for detection of FMDV are imperative for the effective control and elimination of the disease. Currently, several conventional methods are available for the detection of FMDV, including virus isolation (VI), antigen-capture ELISA (Ag-ELISA), and immunochromatographic lateral flow device (Ag-LFD) [5, 6]. VI is a relatively laborious and time-consuming method that must be performed in a high-containment biosafety laboratory. Ag-ELISA has a limited sensitivity and also requires skilled technicians to perform and interpret the assays. Ag-LFD has only been validated for use with epithelial samples [5]. Molecular diagnostic assays are now recognized as reliable detection methods for FMDV. A number of reverse transcription polymerase chain reaction (RT-PCR) assays have been reported and accepted widely for the detection of FMDV RNA, such as RT-PCR and real-time RT-PCR [7,8,9]. The RT-PCR assays are designed for use in well-equipped laboratories with reliable electrical supply and highly trained technicians, and unsuitable for being used in under-equipped laboratories and in field. Although several real-time RT-PCR assays have been transferred onto a portable platform and trialled successfully in field settings [7, 10, 11], expensive high precision instrumentation and consistent electrical power are still needed. When compared to current RT-PCR assays, the use of isothermal technologies reduces the need for high precision instrumentation, consistent electrical power and complex sample preparation [12]. Recently, several field-deployable isothermal DNA amplification assays including the reverse transcription insulated isothermal PCR (RT-iiPCR), reverse transcription loop-mediated isothermal amplification (RT-LAMP), nucleic acid sequence based amplification (NASBA) and reverse transcription helicase dependent amplification (RT-HDA) have been developed for FMDV detection [13, A rapid, visible and equipment-free method using body heat is developed successfully for PON diagnosis of FMD. The good specificity, sensitivity, and easy sample-to-answer protocol make the developed LFS RT-RPA assay ideal for the accurate and rapid detection of FMDV RNA in under-equipped laboratory and at PON facility, especially in low resource settings.Conclusions
Abbreviations
- CSFV:
-
Classical swine fever virus
- CT:
-
Cycle threshold
- EMCV:
-
Encephalomyocarditis virus
- FMDV:
-
Foot-and-mouth disease virus
- LFS:
-
Lateral flow strip
- MMLV:
-
Moloney Murine Leukemia virus
- PCV2:
-
Porcine circovirus 2
- PON:
-
Point-of-need
- PRRSV:
-
Porcine reproductive and respiratory syndrome virus
- PRV:
-
Pseudorabies virus
- RPA:
-
Recombinase polymerase amplification
- SVA:
-
Senecavirus A
- TT:
-
Threshold time
- VSV:
-
Vesicular stomatitis virus
References
Alexandersen S, Zhang Z, Donaldson AI, Garland AJ. The pathogenesis and diagnosis of foot-and-mouth disease. J Comp Pathol. 2003;129(1):1–36.
Knight-Jones TJ, Rushton J. The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev Vet Med. 2013;112(3–4):161–73.
Alexandersen S, Mowat N. Foot-and-mouth disease: host range and pathogenesis. Curr Top Microbiol Immunol. 2005;288:9–42.
Donaldson AI, Sellers RF: Foot-and-mouth disease. In: Martin WB, Aitken ID, editors. Diseases of Sheep, 3 edn. Oxford: Blackwell Science; 2000. p. 254–8.
Ferris NP, Nordengrahn A, Hutchings GH, Reid SM, King DP, Ebert K, Paton DJ, Kristersson T, Brocchi E, Grazioli S, et al. Development and laboratory validation of a lateral flow device for the detection of foot-and-mouth disease virus in clinical samples. J Virol Methods. 2009;155(1):10–7.
Jamal SM, Belsham GJ. Foot-and-mouth disease: past, present and future. Vet Res. 2013;44:116.
Callahan JD, Brown F, Osorio FA, Sur JH, Kramer E, Long GW, Lubroth J, Ellis SJ, Shoulars KS, Gaffney KL, et al. Use of a portable real-time reverse transcriptase-polymerase chain reaction assay for rapid detection of foot-and-mouth disease virus. J Am Vet Med Assoc. 2002;220(11):1636–42.
Reid SM, Ferris NP, Hutchings GH, Zhang Z, Belsham GJ, Alexandersen S. Diagnosis of foot-and-mouth disease by real-time fluorogenic PCR assay. Vet Rec. 2001;149(20):621–3.
Reid SM, Ferris NP, Hutchings GH, Zhang Z, Belsham GJ, Alexandersen S. Detection of all seven serotypes of foot-and-mouth disease virus by real-time, fluorogenic reverse transcription polymerase chain reaction assay. J Virol Methods. 2002;105(1):67–80.
Howson ELA, Armson B, Madi M, Kasanga CJ, Kandusi S, Sallu R, Chepkwony E, Siddle A, Martin P, Wood J, et al. Evaluation of two lyophilized molecular assays to rapidly detect foot-and-mouth disease virus directly from clinical samples in field settings. Transbound Emerg Dis. 2017;64(3):861–71.
Madi M, Hamilton A, Squirrell D, Mioulet V, Evans P, Lee M, King DP. Rapid detection of foot-and-mouth disease virus using a field-portable nucleic acid extraction and real-time PCR amplification platform. Vet J. 2012;193(1):67–72.
LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Weigl B. A simple, inexpensive device for nucleic acid amplification without electricity-toward instrument-free molecular diagnostics in low-resource settings. PLoS One. 2011;6(5):e19738.
Ambagala A, Fisher M, Goolia M, Nfon C, Furukawa-Stoffer T, Ortega Polo R, Lung O. Field-deployable reverse transcription-insulated isothermal PCR (RT-iiPCR) assay for rapid and sensitive detection of foot-and-mouth disease virus. Transbound Emerg Dis. 2017;64(5):1610–23.
Collins RA, Ko LS, Fung KY, Lau LT, **ng J, Yu AC. A method to detect major serotypes of foot-and-mouth disease virus. Biochem Biophys Res Commun. 2002;297(2):267–74.
Dukes JP, King DP, Alexandersen S. Novel reverse transcription loop-mediated isothermal amplification for rapid detection of foot-and-mouth disease virus. Arch Virol. 2006;151(6):1093–106.
**gwei J, Baohua M, Suo** Q, Binbing L, He L, **%2CQ&author=Binbing%2CL&author=He%2CL&author=**aobing%2CH&author=Yongchang%2CC&author=Chunyi%2CX"> Google Scholar
Aebischer A, Wernike K, Hoffmann B, Beer M. Rapid genome detection of Schmallenberg virus and bovine viral diarrhea virus by use of isothermal amplification methods and high-speed real-time reverse transcriptase PCR. J Clin Microbiol. 2014;52(6):1883–92.
Daher RK, Stewart G, Boissinot M, Bergeron MG. Recombinase polymerase amplification for diagnostic applications. Clin Chem. 2016;62(7):947–58.
Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4(7):e204.
Abd El Wahed A, El-Deeb A, El-Tholoth M, Abd El Kader H, Ahmed A, Hassan S, Hoffmann B, Haas B, Shalaby MA, Hufert FT, et al. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS One. 2013;8(8):e71642.
Yang Y, Qin X, Song Y, Zhang W, Hu G, Dou Y, Li Y, Zhang Z. Development of real-time and lateral flow strip reverse transcription recombinase polymerase amplification assays for rapid detection of peste des petits ruminants virus. Virol J. 2017;14(1):24.
Yang Y, Qin X, Zhang W, Li Y, Zhang Z. Rapid and specific detection of porcine parvovirus by isothermal recombinase polymerase amplification assays. Mol Cell Probes. 2016;30(5):300–5.
Hou P, Wang H, Zhao G, He C, He H. Rapid detection of infectious bovine Rhinotracheitis virus using recombinase polymerase amplification assays. BMC Vet Res. 2017;13(1):386.
Hou P, Zhao G, Wang H, He C, Huan Y, He H. Development of a recombinase polymerase amplification combined with lateral-flow dipstick assay for detection of bovine ephemeral fever virus. Mol Cell Probes. 2017.
Wang J, Wang J, Li R, Liu L, Yuan W. Rapid and sensitive detection of canine distemper virus by real-time reverse transcription recombinase polymerase amplification. BMC Vet Res. 2017;13(1):241.
Liu L, Wang J, Geng Y, Wang J, Li R, Shi R, Yuan W. Equipment-free recombinase polymerase amplification assay using body heat for visual and rapid point-of-need detection of canine parvovirus 2. Mol Cell Probes. 2018;39:41–6.
Wang J, Wang J, Li R, Shi R, Liu L, Yuan W. Evaluation of an incubation instrument-free reverse transcription recombinase polymerase amplification assay for rapid and point-of-need detection of canine distemper virus. J Virol Methods. 2018;260:56–61.
Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG. Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol Cell Probes. 2015;29(2):116–21.
Lillis L, Lehman D, Singhal MC, Cantera J, Singleton J, Labarre P, Toyama A, Piepenburg O, Parker M, Wood R, et al. Non-instrumented incubation of a recombinase polymerase amplification assay for the rapid and sensitive detection of proviral HIV-1 DNA. PLoS One. 2014;9(9):e108189.
Wu YD, Xu MJ, Wang QQ, Zhou CX, Wang M, Zhu XQ, Zhou DH. Recombinase polymerase amplification (RPA) combined with lateral flow (LF) strip for detection of toxoplasma gondii in the environment. Vet Parasitol. 2017;243:199–203.
Wang J, Wang J, Liu L, Li R, Yuan W. Rapid detection of porcine circovirus 2 by recombinase polymerase amplification. J Vet Diagn Invest. 2016;28(5):574–8.
Crannell ZA, Rohrman B, Richards-Kortum R. Equipment-free incubation of recombinase polymerase amplification reactions using body heat. PLoS One. 2014;9(11):e112146.
Wang R, Zhang F, Wang L, Qian W, Qian C, Wu J, Ying Y. Instant, visual, and instrument-free method for on-site screening of GTS 40-3-2 soybean based on body-heat triggered recombinase polymerase amplification. Anal Chem. 2017;89(8):4413–8.
Acknowledgements
The authors thank the laboratory staff in the animal hospital of Agricultural University of Hebei and the staff in the Hebei Animal Disease Control Center for samples collection.
Funding
This work was supported by Natural Science Foundation Youth Project of Hebei Province (C2017325001), Science and Technology Project Foundation of Hebei Province (16226604D), Earmarked Fund foe Hebei Sheep&Goat Innovation team of Modern Agro-industry technology Research System (HBCT2018140204) and partially funded by the Fund for One-hundred Outstanding Innovative Talents from Hebei Institution of Higher Learning (SLRC2017039). The funding agencies had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Availability of data and materials
The dataset analyzed during the current study is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
JCW and WZY conceived and designed the study. LBL, JFW and RXZ developed the LFS RT-RPA assay and analyzed the data. ML, RSH and QAH made the spiked samples, performed the clinical samples testing. ML, RSH and QAH helped in the data analysis and manuscript revision. JCW and WZY wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Animal Care and Ethics Committee of Agricultural University of Hebei (approval no. IACECHEBAU20110509).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, L., Wang, J., Zhang, R. et al. Visual and equipment-free reverse transcription recombinase polymerase amplification method for rapid detection of foot-and-mouth disease virus. BMC Vet Res 14, 263 (2018). https://doi.org/10.1186/s12917-018-1594-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-018-1594-x