Abstract
Background
Community-acquired bacterial pneumonia (CABP) can lead to sepsis and is associated with high mortality rates in patients presenting with shock and/or respiratory failure and who require mechanical ventilation and admission to intensive care units, thus reflecting the limited effectiveness of current therapy. Preclinical studies support the efficacy of expanded allogeneic adipose-derived mesenchymal stem cells (eASCs) in the treatment of sepsis. In this study, we aim to test the safety, tolerability and efficacy of eASCs as adjunctive therapy in patients with severe CABP (sCABP).
Methods
In addition to standard of care according to local guidelines, we will administer eASCs (Cx611) or placebo intravenously as adjunctive therapy to patients with sCABP. Enrolment is planned for approximately 180 patients who will be randomised to treatment groups in a 1:1 ratio according to a pre-defined randomization list. An equal number of patients is planned for allocation to each group. Cx611 will be administered on Day 1 and on Day 3 at a dose of 160 million cells (2 million cells / mL, total volume 80 mL) through a 20–30 min (240 mL/hr) intravenous (IV) central line infusion after dilution with Ringer Lactate solution. Placebo (Ringer Lactate) will also be administered through a 20–30 min (240 mL/hr) IV central line infusion at the same quantity (total volume of 80 mL) and following the same schedule as the active treatment. The study was initiated in January 2017 and approved by competent authorities and ethics committees in Belgium, Spain, Lithuania, Italy, Norway and France; monitoring will be performed at regular intervals. Funding is from the European Union’s Horizon 2020 Research and Innovation Program.
Discussion
SEPCELL is the first trial to assess the effects of eASCs in sCABP. The data generated will advance understanding of the mode of action of Cx611 and will provide evidence on the safety, tolerability and efficacy of Cx611 in patients with sCABP. These data will be critical for the design of future confirmatory clinical investigations and will assist in defining endpoints, key biomarkers of interest and sample size determination.
Trial registration
NCT03158727, retrospectively registered on 9 May 2017.
Similar content being viewed by others
Background
Community-acquired pneumonia (CAP) is an acute lung infection and the leading cause of mortality among infectious diseases [1]. CAP is a substantial public health issue, with an overall annual incidence ranging from 1.6–10.6/1000 adult population in Europe [2]. Community-acquired bacterial pneumonia (CABP), where CAP is triggered by bacterial pathogens such as Streptococcus pneumoniae and gram-negative organisms, is also a significant cause of disease complications, particularly sepsis [3]. Almost half (48%) of patients hospitalised due to CABP develop organ dysfunction during the disease course [4]. In severe CABP (sCABP), patients may experience respiratory failure, necessitating invasive mechanical ventilation, and/or hypotension, which may be refractory to intravascular volume expansion, thus requiring the use of vasopressors [3].
CAP is commonly caused by bacterial pathogens, and treated accordingly with antibiotic and supportive therapy [3]. However, despite advances in critical care management, the mortality rate of CAP has remained unacceptably high [5]. In cases where patients require intensive care unit (ICU) admission for additional care, mortality rates of approximately 25–50% have been reported [5]. Early treatment with appropriate antibiotics, usually a β-lactam plus a macrolide, may improve patient outcomes, particularly in those at a higher risk of death [3]. To date, the application of corticosteroid combination therapy in CAP/CABP has remained controversial, with some studies demonstrating reduced mortality [6,7,8], but others report a minimal influence on outcomes [9] and an increase in adverse effects [10]. Given the limited armamentarium for CAP management, there exists a clear unmet need for new therapeutic strategies to improve outcomes for patients with sCABP.
Recently, several non-antibiotic therapies have been explored as adjuvant treatments, including neutralising antibodies against bacterial toxins, immunoglobulins, growth factors and mesenchymal stem cells (MSCs). MSCs have both immunomodulatory and anti-microbial effects, and possess the ability to modulate the phenotype and function of a range of immune cells through direct cell-to-cell interactions, immunomodulatory factors and secretion of growth factors, making them attractive candidates to treat diseases associated with a defective inflammatory response (Fig. 1) [11]. MSCs have received wide attention as a novel therapeutic candidate for various inflammatory medical conditions including graft versus host disease, consequences of myocardial infarction, and perianal fistula in Crohn’s disease [12,13,14], and several preclinical studies have examined the effectiveness of MSCs for the treatment of sepsis [15,16,17,18,19,20,21].
Adipose-derived mesenchymal stem cells are sampled from human adipose tissue and expanded ex vivo. Their immunomodulatory and anti-microbial effects are utilised for the treatment of sCABP. eASC MoA: eASCs modulate inflammation through the generation of regulatory immune cells (e.g. Tregs, M2 Mph) by reducing pro-inflammatory cytokines (e.g. TNFα, IL-6, IL-8); increasing the release of the anti-inflammatory cytokine IL-10; inhibiting apoptosis of immune cells; and reducing lymphocyte, neutrophil and macrophage infiltration. eASCs also have anti-microbial effects as they release peptides with antimicrobial properties (e.g. LL-37) and increase the phagocytic capacity of monocytes, macrophages and neutrophils. Due to these properties, eASCs can reduce organ injury and increase functionality, thus conferring a therapeutic benefit [11]. eASC expanded adipose-derived mesenchymal stem cell, IFN interferon, IL interleukin, KGF keratinocyte growth factor, M2 Mph macrophages, MoA mode of action, sCABP severe community-acquired bacterial pneumonia, Treg regulatory T cell, TNFα tumour necrosis factor alpha
Although MSCs from different sources share many characteristics, significant variability in their immunomodulatory properties exist [22]. In contrast to bone-marrow-derived MSCs (BM-MSCs), adipose-derived mesenchymal stem cells (ASCs) can be obtained from human adipose tissue via liposuction and constitute an easily accessible source of stem cells [23]. Furthermore, ASCs display a reduced susceptibility to natural killer cell-mediated lysis compared to BM-MSCs, and may, therefore, remain in tissues long enough to balance the immune response before being cleared [24]. Additionally, compared with BM-MSCs, ASCs have been described as having greater immunomodulatory effects [25,26,27,28,29].
A previous phase I trial to investigate the safety and demonstrate the anti-inflammatory effect of expanded ASCs (eASCs) for treating sepsis-like clinical symptoms reported no serious adverse events (SAEs) nor respiratory complications in particular [19]. Here, we describe the study design and methodology for SEPCELL (NCT03158727), an ongoing phase Ib/IIa study to assess the safety, tolerability and efficacy of eASCs (Cx611) as adjunctive therapy in patients with sCABP.
Objectives
The primary objective of SEPCELL is to determine the safety profile of two central line infusions of Cx611 administered within 3 days (on days 1 and 3) at a dose of 160 million cells each to monitor any AE and potential immunological host responses against the administered cells during the follow-up period. The secondary objectives are to explore the clinical efficacy of Cx611 in terms of a reduction of the duration of mechanical ventilation and/or the need for vasopressors and/or improved survival and/or clinical cure of the sCABP, as well as other efficacy-related endpoints. A further objective is to understand the mode of action (MoA) of Cx611 in patients with sCABP by identifying the pro-inflammatory and anti-inflammatory pathways through which Cx611 may affect the underlying processes of sepsis.
Methods
Design
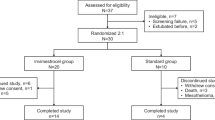
This protocol (version 1, July 2015) follows guidance from the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [30]. A SPIRIT schedule of enrolment, interventions and assessment is provided in Fig. 2. SEPCELL is a phase Ib/IIa, randomised, double-blind, parallel group, placebo-controlled, multicentre trial that planned to enrol 180 patients with sCABP. The study will be conducted from January 2017 to December 2021 in Belgium, France, Lithuania and Spain in over 20 centres. Once eligibility is confirmed, subjects treated in an ICU for sCABP will receive standard-of-care (SoC) according to local guidelines plus two 80 mL intravenous (IV) central line infusions of Cx611 at a fixed dose of 160 million cells (240 mL/hr), or placebo (two 80 mL IV central line infusions [240 mL/hr] of Ringer lactate solution). The randomisation and the first infusion of Cx611 or placebo will be performed as early as possible within the first 18 h of patients fulfilling at least one of the two major criteria of severity for CAP (i.e. from the initiation of invasive mechanical ventilation or vasopressors) (Fig. 3a, b). The day of administration of the first dose of Cx611 (or placebo) will be considered day 1 of the study. The maximum screening duration will be 18 h and treatment duration will be 3 days (Fig. 3a, b). The study will permit concomitant SoC, including antibiotic and other therapies in the ICU, in an add-on design. Patient stratification will be considered based on CABP severity criteria at inclusion: shock requiring vasopressors or invasive mechanical ventilation, or both.
a Study design for SEPCELL. A phase Ib/IIa, randomised, multicentre, double-blind, placebo-controlled study to assess the safety, tolerability and efficacy of eASCs (Cx611) administered intravenously, in addition to SoC therapy, to patients with sCABP. An amendment to the study protocol extended the follow-up period to 2 years (Table 2). b Trial flow diagram for SEPCELL. BL baseline, CABP community-acquired bacterial pneumonia, SPIRIT Standard Protocol Items: Recommendations for Interventional Trials, eASC expanded allogeneic adipose-derived mesenchymal stem cell, IV intravenous, SAE serious adverse event, sCABP severe community-acquired bacterial pneumonia, SoC standard of care. *From initiation of mechanical ventilation and/or vasopressors administration; **Screening procedures will start as soon as informed consent is signed
Study population
The reference population will consist of adult patients admitted to the ICU with sCABP. Eligible patients will include those with clinical diagnosis of acute CABP (developed within ≤21 days past).
Patients and public involvement
Although patients were not involved in the conception or design of the study, the study concept was approved by a public grant from the EU Horizon Grant commission.
Participant eligibility
Inclusion criteria
Inclusion criteria consist of: age between 18 and 80 years; body weight between 50 and 100 kg; clinical diagnosis of acute CABP (developed within ≤21 past days) based upon the presence of two relevant symptoms (fever, tachypnoea, leukocytosis or hypoxaemia) and radiographic findings of new pulmonary infiltrate(s); having sufficiently severe pneumonia necessitating ICU management, and requiring either invasive mechanical ventilation or treatment with vasopressors for at least 2 h; female subjects must either have no childbearing potential or have negative serum or urine pregnancy test; sexually active subjects (of both sexes) must agree to use contraception for the entire duration of the study, or for 3 months after the last dose of the investigational medicinal product, whichever occurs later; signed informed consent provided by the subject (or relatives or legal representative). The informed consent form includes information that data will be recorded, collected, processed and may be transferred to European Economic Area (EEA) or non-EEA countries in accordance with the European Union Data Protection Regulation (2016/679).
Exclusion criteria
Exclusion criteria consist of: hospital-acquired, healthcare-associated or ventilator-associated pneumonia; pneumonia of exclusively viral or fungal origin; known or suspected Pneumocystis jirovecii pneumonia; aspiration pneumonia; known active tuberculosis; a history of post-obstructive pneumonia; cystic fibrosis; any chronic lung disease requiring oxygen therapy at home; infection in another organ caused by the same pathogen; expectation for development of a rapidly fatal disease within 72 h after randomisation; inability to maintain a mean arterial pressure ≥ 50 mmHg prior to screening despite utilisation of vasopressors and IV fluids; not being expected to survive for 3 months due to other medical conditions or diseases; subjects with primary or metastatic lung cancer or with anticipated chemotherapy within the next 90 days; haematopoietic and lymphoreticular malignancies, unless in remission; a known primary immunodeficiency disorder or acquired immune deficiency syndrome with CD4 count < 200 cells/mm3 or not receiving highly active antiretroviral therapy; receiving immunosuppressant therapy or chronic high doses of steroids; granulocytopoenia (not due to sepsis); receiving any stem cell, organ or bone marrow transplant within the past 6 months; undergoing treatment with a biological agent, or plasma exchange treatment within the last 8 weeks; receiving or having received another investigational medication within 90 days prior to the start of the study; allergies or hypersensitivity to antibiotics and/or any component of CryoStor CS10®; a known liver function deficiency; hospitalisation in the preceding 15 days; conditions resulting in a New York Heart Association Class IV functional status; end-stage neuromuscular disorders that impair weaning; subjects with complete quadriplegia.
Interventions
Subjects will receive two 80 mL central line infusions (240 mL/hr. over 20–30 min) of IV allogeneic Cx611, at a fixed dose of 160 million cells on day 1 and day 3, respectively; or placebo. Placebo (Ringer Lactate) will be given also through a 20–30 min (240 mL/hr) IV central line infusion at the same quantity (total volume of 80 mL) and following the same schedule than the active treatment.
Follow-up
All patients will be followed up for a period of at least 3 months.
Withdrawal
In accordance with the Declaration of Helsinki, patients, or upon their legally authorised representatives’ decision, will be free to withdraw from the study at any time if they wish to, for any reason specified or unspecified. Withdrawal from the study will not affect or detriment the patient’s further care or treatment. Patients have the right to withdraw their consent at any time, partially or totally; however, all data collected until the time of withdrawal will be used in the analyses. Potential criteria for discontinuing or modifying allocated interventions include: occurrence of adverse events that justify the withdrawal from the study; the decision of a subject to withdraw from the study; major protocol deviations; pregnancy.
Outcome measures
The safety endpoints assessed throughout the study will include incidence of treatment-emergent AEs, including changes in vital signs and signs of allergic reactions such as anaphylaxis (Table 1). Changes in 12-lead electrocardiograms (ECGs) will be assessed from screening, day 1 and day 3 (both post-dose). All AEs will be judged as being related or not to the study treatment.
Efficacy endpoints measured will include mechanical ventilator and vasopressor treatment-free days over 28 days. Subjects’ clinical response will be assessed during visits on days 8–10, 14 and 29. Response will be determined as cure, non-response or indeterminate. Indications of non-response related to pneumonia will include persistence/progression of baseline signs/symptoms; indications of non-response unrelated to pneumonia will include any other cause of clinical response failure that in the investigator’s judgement is unrelated to the index pneumonia (e.g. myocardial infarction, pulmonary thromboembolism). Time to clinical cure, duration of antibiotic treatment, rate of pneumonia recurrence/re-infection after clinical cure, and time to recurrence/re-infection of pneumonia after clinical cure at clinical response assessment will be recorded.
Participant timeline
Once a suitable patient has been identified and following informed consent, the maximum screening duration will be 18 h (Fig. 3a). However, initiation of treatment with Cx611/placebo should be performed as early as possible within this 18 h window. The treatment period (day 1 and day 3) starts with the administration of the first Cx611/placebo dose and ends with administration of the second dose. Premature discontinuation can occur if the patient withdraws from the study before day 90 and will lead to an early termination visit.
Sample size
The results from this study will be exploratory in nature; hence, there is no hypothesis testing comparing outcomes between treatment arms. As little is presently known about the outcome measures used in the study of stem cells, SEPCELL will provide the initial dataset necessary for determining endpoints and enable preliminary estimates of effect size for the design of future efficacy-finding studies of Cx611 used as add-on therapy in patients with sCABP requiring mechanical ventilation and/or vasopressor administration.
Recruitment
Since patients with sCABP require prompt treatment, study enrolment will take place at each study site during a short time window (up to 18 h), starting from when the patient is first identified to fulfil the appropriate severity criteria and ending with the administration of the first dose of study treatment. Each participating centre will keep a log of all screened patients with sCABP, and investigators will be requested to record the reason(s) for not including patients who were screened but not enrolled.
Randomisation and allocation
After performing the screening visit and verifying inclusion and exclusion criteria and subject’s eligibility, investigators will place a screening and approval call to the clinical coordinating centre (CCC) physicians. The CCC will provide a randomisation authorisation number, which will allow the patient to be randomised within the study. Patients will then be randomised in a 1:1 ratio to receive Cx611 or placebo by interactive response technology (IRT). The IRT will assign a randomisation number to the patient, which will be used to link the patient to a treatment arm and will specify a unique medication number for the package of the investigational medicinal product (a pack containing two vials of Cx611 or placebo properly labelled) to be dispensed to the patient at each administration date. The IRT will produce a patient randomisation list via a validated system that automates the random assignment of patient numbers to randomisation numbers. A separate medication list will be produced via the IRT provider also using a validated, automated method to randomise the assignment of medication numbers to packs containing the investigational medicinal product.
Blinding
Both patients and investigators (or designated personnel) who collect the AE data and evaluate the clinical outcomes of sCABP will be blinded to treatments. Treatments will be prepared by unblinded personnel for administration in a blinded location, and this individual(s) will not be allowed to participate in any efficacy assessment of the sCABP during the study. The unblinded personnel will not be permitted to share information about the treatment with any member of the blinded team. To ensure double blinding, the primary packaging of Cx611 and placebo will be identical. Additionally, a specific blinding plan at each site will document all personnel involved in the trial and their responsibilities.
Data management
Data management and handling will be conducted according to the study-specific Data Management Plan in accordance with International Conference on Harmonization guidelines [31] and clinical research organisation standard operating procedures (SOPs). Data entry, validation and data queries will be handled by the Trial Form Support (TFS) data management team. Data will be subjected to validation according to TFS SOPs in order to ensure accuracy in the collected case report form data. Before database closure, reconciliation will be performed between the SAEs entered in the safety database and the study database. Any deviations, i.e., discrepancies and additions from the process defined in the Data Management Plan, will be described in a study-specific data management report. When data for the primary endpoint are available and before database lock, a blinded adjudication committee will review subject evaluability, sCABP clinical response assessment and patient assignment processes.
General statistical methods
In general, data will be reported by means of summary statistics. Continuous data will be presented with the number of observations, mean value, standard deviation, and minimum, median and maximum value. Categorical data will be presented as counts and percentages when applicable. Individual patient data will be listed. The results of all laboratory test results, physical examination findings, ECGs and vital signs will be presented in data listings; safety laboratory data will be presented by absolute and changes from baseline values by visit. All abnormalities will be assessed for potential clinical relevance.
Protocol amendments
Protocol amendments are summarised in Table 2.
Confidentiality
All study-related information and participant information will be stored securely at the study site in areas with limited access. Password-protected access systems will be used to ensure the confidentiality of local databases.
Publications
The list of authors of the publication will be defined according to International Committee of Medical Journal Editors criteria, involvement in trial design, oversight, number of evaluable patients enrolled, analysis and interpretation of data, and preparation of manuscript. The study will only be published once it has been finished and the final analysis is completed; the final manuscript must be approved by all the authors before publication [32]. Medical writing support will be utilised as required.
Ethics and dissemination
If the patient is unable to comprehend the scope of the trial prior to enrolment due to altered mental status associated with the underlying pneumonia (or any other disease), written informed consent to participate in the study must be obtained from the patient’s legally acceptable representative, as required by national laws, respective regulations and institutional review boards/independent ethics committees/regional ethics boards. Written informed consent will be sought from the patient as soon as he/she becomes capable of comprehending the scope of the trial. The study results (publications, conference presentations) will be published in peer-reviewed, open access journals and conferences.
Discussion
SEPCELL is the first trial to assess the effects of eASCs in sCABP. Due to the associated complications and high mortality rate of sCABP, treatment remains a key challenge and a global public health issue [1]. With current therapies ineffective for many patients [3], there is an unmet need for novel therapies to address these challenges.
In animal models, the immunomodulatory properties of MSCs were found to significantly reduce mortality via a combination of reduced inflammation, production of anti-microbial effectors and increased phagocytosis [11, 33]. To date, there are no large-scale clinical trials demonstrating efficacy of stem cell therapy in sCABP. However, results from a phase I dose-escalation clinical trial of MSCs in septic shock provided data suggesting MSCs in doses up to 250 million cells are to be deemed safe and well-tolerated (NCT02421484 [34];). A phase II trial to assess the efficacy and safety of MSCs in the treatment of septic shock is now planned (NCT03369275). Furthermore, results from a phase I and phase IIa clinical trial (NCT01775774; NCT02097641) have shown stem cell therapy to be well tolerated in patients with acute respiratory distress syndrome (ARDS) [35, 36], with two phase II trials currently ongoing in ARDS in the US and the Republic of Korea (NCT03818854, STAT; NCT02112500, STELLAR, respectively).
The current trial will help advance our knowledge of the anti-inflammatory effects of eASCs, such as effects on T-cell response, RNA expression profiles of blood leukocytes and plasma concentration of biomarkers in this patient population, building on the promising results provided by preclinical and phase I data for Cx611 [19,20,21]. SEPCELL will also provide insights on the efficacy of Cx611, including its impact on the need for use of mechanical ventilation and/or vasopressors, time to clinical cure and rate of pneumonia recurrence/re-infection after clinical cure in patients with sCABP. Although mortality will not be assessed during the SEPCELL study, data on the safety and tolerability of eASCs in patients with sCABP will provide key information to facilitate the design of subsequent clinical trials.
To conclude, SEPCELL is a multicentre, double blind, randomised controlled trial assessing the safety, tolerability and efficacy of eASCs (Cx611) as adjunctive therapy for patients with sCABP. The novel MoA offered by eASCs may address the underlying immune dysregulation caused by sCABP, and, thereby, offer another treatment option for patients with this disease and ultimately reduce patient mortality [37]. In addition to advancing our understanding of the MoA of Cx611, data from this completed trial will provide evidence on the safety, tolerability and efficacy of Cx611 in patients with sCABP. Analysis of study results and outcomes will be critical for the design of further confirmatory clinical investigations in terms of definition of endpoints, key biomarkers of interest and sample size determination.
Availability of data and materials
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results of the study, will be made available after the publication of study results within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization. A completed SPIRIT checklist is available in Additional file 1; the World Health Organization Trial Registration Data Set is available in Additional file 2.
Abbreviations
- AE:
-
Adverse event
- ARDS:
-
Acute respiratory distress syndrome
- ASC:
-
Adipose-derived mesenchymal stem cell
- BM-MSC:
-
Bone-marrow-derived mesenchymal stem cell
- CABP:
-
Community-acquired bacterial pneumonia
- CAP:
-
Community-acquired pneumonia
- CCC:
-
Clinical coordinating centre
- eASC:
-
Expanded ASC
- ECG:
-
Electrocardiograms
- IRT:
-
Interactive response technology
- MoA:
-
Mode of action
- MSC:
-
Mesenchymal stem cell
- SAE:
-
Serious adverse event
- sCABP:
-
Severe CABP
- SoC:
-
Standard of care
- SOP:
-
Standard operating procedure
References
Leoni D, Rello J. Severe community-acquired pneumonia: optimal management. Curr Opin Infect Dis. 2017;30:240–7.
Liapikou A, Cilloniz C. Severe community-acquired pneumonia: severity and management. Community Acquir Infect. 2015;2:3.
Morgan AJ, Mrcp M, Fficm F, Glossop Bmedsci AJ, Bs BM, Frca M, et al. Severe community-acquired pneumonia. BJA Educ. 2015;16:167–72.
Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–78.
Restrepo MI, Faverio P, Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis. 2013;26:151–8.
Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–86.
Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–8.
Marti C, Grosgurin O, Harbarth S, Combescure C, Abbas M, Rutschmann O, et al. Adjunctive Corticotherapy for community acquired pneumonia: a systematic review and meta-analysis. PLoS One. 2015;10:e0144032.
Wiersinga WJ, Bonten MJ, Boersma WG, Jonkers RE, Aleva RM, Kullberg BJ, et al. Management of community-acquired pneumonia in adults: 2016 guideline update from the Dutch working party on antibiotic policy (SWAB) and Dutch Association of Chest Physicians (NVALT). Neth J Med. 2018;76:4–13.
Horita N, Otsuka T, Haranaga S, Namkoong H, Miki M, Miyashita N, et al. Adjunctive systemic corticosteroids for hospitalized community-acquired pneumonia: systematic review and meta-analysis 2015 update. Sci Rep. 2015;5:14061.
Lombardo E. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells. 2015;7:379.
Bernardo ME, Ball LM, Cometa AM, Roelofs H, Zecca M, Avanzini MA, et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46:200–7.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human Mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86.
Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–90.
Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9.
Pedrazza L, Cubillos-Rojas M, De Mesquita FC, Luft C, Cunha AA, Rosa JL, et al. Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res Ther. 2017;8:289.
Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39.
Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Phys Lung Cell Mol Phys. 2012;302:L1003–13.
Perlee D, van Vught LA, Scicluna BP, Maag A, Lutter R, Kemper EM, et al. Intravenous infusion of human adipose Mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group, Placebo Controlled Trial. Stem Cells. 2018;36:1778–88.
Perlee D, de Vos AF, Scicluna BP, Mancheño P, de la Rosa O, Dalemans W, et al. Human adipose-derived Mesenchymal stem cells modify lung immunity and improve antibacterial defense in Pneumosepsis caused by Klebsiella pneumoniae. Stem Cells Transl Med. 2019;8:785–96.
Perlee D, De Vos AF, Scicluna BP, Maag A, Mancheño P, De La Rosa O, et al. Role of tissue factor in the procoagulant and antibacterial effects of human adipose-derived mesenchymal stem cells during pneumosepsis in mice. Stem Cell Res Ther. 2019;10:286.
Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother. 2008;35:272–7.
Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg. 2015;42:169–79.
DelaRosa O, Sánchez-Correa B, Morgado S, Ramírez C, Del Río B, Menta R, et al. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2012;21:1333–43.
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6.
Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher Immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–63.
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–52.
Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42.
Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008;32:384–93.
Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
Guideline for Industry Text on Validation of Analytical Procedures. 1995.
Battisti WP, Wager E, Baltzer L, Bridges D, Cairns A, Carswell CI, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163:461–4.
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with Mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559.
McIntyre LA, Stewart DJ, Mei SHJ, Courtman D, Watpool I, Granton J, et al. Cellular immunotherapy for septic shock: a phase I clinical trial. Am J Respir Crit Care Med. 2018;197:337–47.
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32.
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–62.
Laroye C, Gibot S, Reppel L, Bensoussan D. Concise review: Mesenchymal stromal/stem cells: a new treatment for Sepsis and septic shock? Stem Cells. 2017;35:2331–9.
Knaus W, Draper E, Wagner D, Zimmerman J. APACHE II: a severity of disease classification system - PubMed. Crit Care Med. 1985;13:818–29.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10.
Acknowledgements
Medical writing support was provided by Eoin Duffy at Syneos Health Communications and funded by Takeda Pharmaceuticals.
Funding
Authors are partners of the SEPCELL Consortium. This study is funded by the European Union’s Horizon 2020 Research and Innovation Program under grant agreement number 681031; funding from this grant covered approximately one third of the total estimated costs of the SEPCELL trial. Peer-review of the study protocol was performed by experts from the Horizon 2020 Research and Innovation Program. The study sponsor is TiGenix SAU (a wholly-owned subsidiary of Takeda Pharmaceutical Company Limited) who funded approximately two thirds of the total estimated trial costs. The funding from TiGenix SAU/Takeda Pharmaceuticals covered costs associated with (but not limited to) appointing the contract research organization, the manufacturing and ship** of Cx611, rental and ship** of cryofreezers for storage of Cx611 at study sites, internal personnel costs for all departments involved in the SEPCELL trial, regulatory submissions and interactions, and performing non-clinical studies. The SEPCELL Study Consortium is coordinated by Takeda Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
TvdP, PFL, MS, BF, KAC, OdlR, and EL contributed to the design of the study protocol. TvdP and OdlR designed and conducted the biomarker and immunogenicity studies, and generated supportive data for designing the protocol. PFL, MS, BF and KAC managed patient recruitment and the national coordinators. EL generated supportive data for the study protocol design. KAC was the clinical trial lead and coordinator. PFL acted as the principal investigator of the trial. EL is the SEPCELL Consortium Coordinator and obtained EU funding with support from TvdP, PFL, MS, BF, KAC and OdlR. All authors participated in drafting the manuscript, contributed to critical revisions for important intellectual content and read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study will be conducted in compliance with the protocol; regulatory requirements; good clinical practice (GCP); consolidated guidelines Committee for Proprietary Medicinal Products (CPMP)/ICH/135/95 (July 1996), adopted in the EU by CPMP EU Commission Directive 2005/28/EC (GCP, April 2005); and the ethical principles of the latest revision of the Declaration of Helsinki as adopted by the World Medical Association. As SEPCELL will be performed across several European countries ethics approval was obtained from ethics review committees at the participating institution in each country. In France, the Comité de Protection des Personnes (CPPSOOM4), at Cabanis Haut, Centre Hospitalier Esquirol. In Spain, The Independent Ethics Committee for Research Involving Medicinal Products (47/864244.9/15) on behalf of The Secretary of the Regional IECm of the Community of Madrid. In Belgium, The Medical Ethics Committee (015/35) for The Vrije Universiteit Brussel. In Italy, Sapienza University Ethics Committee (39 SA_2018) for Sapienza University. In Norway, the Regional Committee for Medical Health Research Ethics (2017/1768/REK midt) at St. Olavs Hospital, Trondheim. In Lithuania, the Lithuanian Bioethics Committee (P-17-50) for the State Budget Institution with Klaipeda Republican Hospital as the designated study site. Consent to participate in SEPCELL was confirmed by subjects (or relatives or legal representative) through a signed informed consent form. The informed consent form includes information that data will be recorded, collected, processed and may be transferred to European Economic Area (EEA) or non-EEA countries in accordance with the European Union Data Protection Regulation (2016/679).
Consent for publication
Not applicable.
Competing interests
TvdP, PFL, MS and BF are members of the SEPCELL Consortium. OdlR, K-AC and EL are employees of Takeda. The institutions of PFL, MS and BF are study sites. In addition to funding from the European Union, Takeda Pharmaceuticals provided additional funding for the SEPCELL study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
SPIRIT checklist.
Additional file 2.
World Health Organization Trial Registration Data Set is available.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Laterre, PF., Sánchez-García, M., van der Poll, T. et al. A phase Ib/IIa, randomised, double-blind, multicentre trial to assess the safety and efficacy of expanded Cx611 allogeneic adipose-derived stem cells (eASCs) for the treatment of patients with community-acquired bacterial pneumonia admitted to the intensive care unit. BMC Pulm Med 20, 309 (2020). https://doi.org/10.1186/s12890-020-01324-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-020-01324-2