Abstract
Background
Gut microbiota plays an important role in the development of atopic dermatitis (AD). We aimed to elucidate research trends in gut microbiota and AD in children, to provide evidence and insights to the clinical prevention and treatment of AD in children.
Methods
A sco** literature review on the studies of gut microbiota and AD were conducted. Two authors independently searched Pubmed et al. databases for studies focused on gut microbiota and AD in children up to January 15, 2022. The literatures were screened and analyzed by two reviewers.
Results
A total of 44 reports were finally included and analyzed. Current researches have indicated that abnormal human microecology is closely associated with AD, and the disturbance of intestinal microbiota plays an important role in the occurrence and development of AD. Probiotics can correct the microbiota disorder, have the functions of regulating immunity, antioxidant, and help to restore the microecological homeostasis. However, there is still a lack of high-quality research reports on the efficacy and safety of probiotics in the prevention and treatment of AD in children.
Conclusions
The changes of gut microbiota are essential to the development of AD in children, which may be an effective target for the prevention and treatment of AD. Future studies with larger sample size and rigorous design are needed to elucidate the effects and safety of probiotics in AD.
Similar content being viewed by others
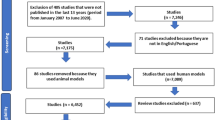
Introduction
Atopic dermatitis (AD) is a chronic, recurring, itchy skin disease with an incidence of 0.2% to 25% [1, 2]. Recent epidemiological survey data in China [ This sco** review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis(PRISMA) extension for Sco** Reviews [16]. The methodological framework for sco** reviews developed by Arksey et al. was used, which comprises five stages: identifying the research question, identifying relevant studies, selecting studies, charting the data and collating, summarizing and reporting the results. Ethical approval was not necessary for this sco** review because a literature review did not require ethical approval based on the related research guidelines. The research question was constructed and developed according to the PICO framework (Population, Intervention, Comparison, Outcome). To be eligible for inclusion in this study, the reports had to meet following criteria: the study focused on the AD Children with age < 16 years old; studies focused on the preventions or treatments associated with gut microbiota; randomized controlled trials (RCTs), quasi experimental or experimental studies, cohort designs; the paper was reported in English or Chinese. We excluded narrative reviews, experts’ opinions and papers published in other languages. Two authors independently searched following databases: Pubmed, CINAHL (EBSCO), MEDLINE, Cochrane library, Cochrane central register of controlled trials, China National Knowledge Infrastructure (CNKI) and Wanfang Database, China Biomedical Literature Database (CBM) for studies focused on gut microbiota and AD in children. The search time limit was from the inception of databases to January 15, 2022. Besides, we searched Google Scholar for potentially related reports. The reference lists of included papers were also screened out to identify any additional relevant papers. The following Boolean search strategy was used to search above databases (Ti: atopic dermatitis ∗ OR dermatitis ∗) AND (Ti: microbiota ∗ OR gut microbiota ∗ OR probiotics ∗ or gastrointestinal ∗) AND (Ti: children ∗ OR child ∗ or pediatric ∗) OR (Ti: prevention ∗ OR treatment ∗). The keywords used were synonyms generated by the databases used and MeSH terms. Two authors independently screened the initial papers identified through the search. The title and abstract of these papers were evaluated based on the inclusion and exclusion criteria to judge their suitability for further stages of review. The retained papers following this process were rescreened by full text to assess the eligibility. A template was developed to allow extraction of the characteristics of each of the studies. This template also allowed recording of the decision made by each reviewer as to whether the paper should be included or excluded or if the decision was uncertain. The result achieved by the two reviewers was evaluated and compared. Any disagreements were resolved by further discussion and reaching consensus. The extracted data included the name of first author, author affiliation information, year of publication, report title, study purpose, study design, sample size, the characteristics of participant, outcome indicators, results and main conclusions. We conducted thematic synthesis proposed by Thomas et al. to answer this sco** review's research question. There are three stages in the thematic synthesis: the free line-by-line coding of the findings of primary literature; the organization of these “free codes” into related areas to construct “descriptive” themes; the development of “analytical” themes. In develo** the theme of this co** review, each reviewer first performed line-by-line coding and descriptive theme generation. Furthermore, two reviewers discussed and integrated the patterns that appear in those themes to generate categories as themes as interpretations beyond the original literature's content. The first database search yielded 465 reports for possible inclusion. Title and abstract review identified 128 articles for full-text review. After full-text review, we removed 84 additional articles for failure to meet inclusion criteria. Finally, 44 studies were included in this sco** review (Fig. 1). Of the included 44 reports, the authors were from 19 countries, the United States was the country with the most authors, followed by the United Kingdom and China. In terms of regions, Europe had the largest number of researchers, followed by Asia and south America. There were 30 observation studies and 14 intervention studies. The species and quantity of human microorganisms are abundant, and as symbiotic organisms, they form the human micro-ecosystem together with the host of human body. Under normal circumstances, the microbiota adheres, colonizes, and reproduces in specific parts of the human body, forming a biofilm barrier, thereby inhibiting the invasion of pathogenic bacteria and maintaining the dynamic balance between the human body and microorganisms [17, 18]. The classic “hygiene hypothesis” holds that urbanized lifestyles, excessive hygiene, and the widespread use of antibiotics can lead to imbalances in human microecology and induce or aggravate allergic diseases such as AD [19]. The skin and the gut are the two main micro-ecosystems of the human body. Some studies have found that compared with the normal population, the diversity of skin and intestinal microbiota in children with AD is reduced before the onset of the disease, which can lead to a shift in the immune response to the T helper 2 (Th2) and increase the possibility of AD onset [20, 21]. During the investigation of Swedish and Estonian children [22, 23], Estonian children had very few allergies, and their intestinal microbiota was dominated by Lactobacillus. However, the main intestinal microbiota of Swedish children was Clostridium, so there were many allergic symptoms. For non-allergic individuals, allergic infants had low levels of lactobacilli in their guts, along with a large number of aerobic bacteria. The results of in vitro and in vivo experiments [24, 25] showed that Lactobacillus could regulate the immune system and resist allergy. Elevated fecal caproic acid levels suggest altered gut microbial communities in infants with allergic symptoms [26, 27]. Experimental data [28] had showed that the intestinal bifidobacteria, gram-positive aerobic bacteria and enterococci were relatively few, but children with a high number of Clostridium and Staphylococcus aureus are prone to allergic symptoms. Probiotics are live microorganisms that help to promote the balance of microbiota, have immune regulation, antioxidant and antibacterial effects, and have been widely used in the prevention and treatment of allergic diseases in children [29]. Th2-type inflammation is the basic feature of AD [30]. On the one hand, oral probiotics can inhibit the Th2 response and make the immune response develop in the direction of Th1 [31]. On the other hand, it can induce immune tolerance and maintain the balance of Th1/Th2, so as to achieve the effect of treating AD [32, 33]. Previous studies have mostly focused on oral probiotics, and topical and oral probiotics are the new hotspots in the research on the relationship between microbiota and AD in recent years. It has been found that oral probiotics can reduce the abnormal colonization of Staphylococcus aureus and other bacteria, restore the diversity of microbiota, help the recovery of intestinal barrier function, and alleviate the clinical symptoms of AD [34]. Epidemiological surveys [35, 36] showed that the proportions of Clostridium, Clostridium difficile, Escherichia coli and Staphylococcus aureus in the gut microbiota of AD patients increased, while the proportions of Bifidobacterium and Bacteroides decreased, suggesting the pathogenesis of AD is closely related to the disturbance of intestinal microbiota. In 2020, Jiang et al. [56, 57]. The influence of various factors changes the changes in the microbiota in the human body, and the composition of intestinal microbes will also be changed if drugs are used for treatment [58]. In terms of dermatopathology, Luo et al. [59] have treated 80 AD children with randomized, double-blind, placebo trial, and the results have showed that after probiotic treatment, their intestinal bacteria were significantly reduced. The group was tested, and the beneficial bacteria were significantly improved, and the allergy symptoms also improved. Probiotics can effectively promote the formation of endogenous barrier mechanisms in children with atopic dermatitis, reduce intestinal inflammation, and avoid allergic symptoms. IL-10 levels increased in the children after continued treatment. Previous study [60] report randomized, double-blind, placebo trial to treat 90 children with moderate and severe atopic dermatitis as experimental subjects, and have used probiotics for adjuvant treatment, and scored itching after 16 weeks. The results have showed that, the degree of itching in children who received probiotic adjuvant therapy is significantly reduced, which have indicated that probiotic adjuvant therapy had a certain effect on the sensitivity of children's skin. At present, there are not many RCTs with rigorous design that can show that probiotics can reduce the severity of AD, and its exact therapeutic effects and safety remain to be further investigated [61]. AD is a Th2-biased inflammatory disorder caused by a combination of complex factors [62]. If the intestinal mucosal immune system is not stimulated by intestinal microorganisms in the early stage of development, the natural development of the immune system will be inhibited, and Treg cells will not be mature enough to regulate certain balances such as Th1/Th2 [11, 63]. By comparing the intestinal microbiota of infants with atopic dermatitis and healthy infants, study [64] has found that the content of Clostridium in the intestine of the former is relatively high, while the content of Bifidobacterium and Lactobacillus in the intestine of healthy infants is higher. It’s been reported that indole-3-carbaldehyde, which is a tryptophan product metabolized by gut microbiota, can alleviate skin inflammation in a mouse model of atopic dermatitis by inhibiting Th2-type cytokine secretion and IgE production. Previous study [65] has pointed out that Bifidobacterium Lactobacillus triple viable bacteria tablets (the ingredients are Bifidobacterium longum, Lactobacillus bulgaricus and Streptococcus thermophilus, 4 tablets/time, 3 times a day for a total of 12 weeks) can increase the number of AD children. These attempts to improve the disease and delay the recurrence of intestinal bifidobacteria and lactobacilli microbiota have brought new hope for the management of chronic disease in children with AD. It is worth noting that the balance of human microbiota is easily affected by various factors such as age, immune status, and external environment, and the therapeutic effect of probiotics is closely related to the strain, timing and dose of administration [66,67,68]. Large-scale randomized, double-blind, placebo-controlled clinical trials are still needed to determine the effective strains, appropriate doses, and duration of treatment of probiotics for AD prevention and treatment. Several review articles about probiotics and gut microbiota with AD were already published and should be considered. Fang et al. [28] have focused on the the potential mechanisms of probiotics on alleviating AD via upregulation of epidermal barrier and regulation of immune signaling, and the possible effective substances on AD, which provide the supports for targeting gut microbiota to AD prevention and treatment. Disamantiaji et al. [69] have included a total of 5 studies, and have concluded that probiotics supplementation in the management of eczema in children does not show a clinically relevant difference vs. standard treatment in reducing eczema severity. We focused on the relationship of gut microbiota and AD in the children population, with more studies included, we have found that probiotics may be an effective and safe treatment option for AD. Still, the current research results have great heterogeneity on the role of probiotics for AD treatment. Therefore, the results of this review must be considered with some limitations. The most significant limitation was that the language of searching databases were limited to English and Chinese, there can be many studies on the gut microbiota and AD in children reported in other language. Besides, the clinical trials with rigorous design are very few, future studies with larger sample size and rigorous design are needed to elucidate the role of gut microbiota in AD development and treatment. In conclusion, the current research evidences have showed that the gut microbiota is closely related to the occurrence and development of AD. Probiotics can help immune cells to exert anti-allergic effects on AD, including enhanced antigen degradation and pro-inflammatory immune responses. Intestinal probiotics can regulate immune cells and immune factors, inhibit the reproduction of pathogens in the intestinal tract, and enhance the intestinal epithelial barrier function. It is worth noting that the specific mechanism of action related to the occurrence and development of intestinal microbiota and AD is not yet clear. Besides, the therapeutic effect and safety of probiotics in children with AD still need to be further confirmed.Methods
Eligibility criteria
Search strategy
Data processing and extraction
Data analysis
Results
Study inclusions
Characteristics of included literature
Gut microbiota and AD development
Probiotics and AD
Conclusions
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40.
Kiiski V, Salava A, Susitaival P, Barnhill S, Remitz A, Heliovaara M. Atopic dermatitis in adults: a population-based study in Finland. Int J Dermatol. 2022;61(3):324–30.
**aoyan L, Quan G, Caibin G. The correlation between the severity of atopic dermatitis in children and NLR. Modern Med Health. 2021;37(7):3–6.
Sulian Y, Yang X, Guoxing Z. Clinical analysis of serum specific IgE and patch test results in children with atopic dermatitis. Chinese J Immunol. 2021;37(1):5–7.
Yanlin Z, Jianbo W. Expression of serum 25-hydroxyvitamin D in children with atopic dermatitis and its clinical significance. Jilin Medicine. 2020;41(10):3–4.
Hu Y, Jiang F, Tan J, Liu S, Li S, Wu M, Yan C, Yu G, Hu Y, Yin Y, et al. Environmental exposure and childhood atopic dermatitis in Shanghai: a season-stratified time-series analysis. Dermatology. 2022;238(1):101–8.
Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16.
Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60.
Chen SY, Chen QW, Shou LM, Pan H, Ruan SM, Liang ZH, Shu QJ. Stevens-Johnson syndrome/toxic epidermal necrolysis successfully treated with Chinese herbal medicine Pi-Yan-Ning: a case report. J Integr Med. 2021;19(6):555–60.
Melli L, Carmo-Rodrigues MSD, Araujo-Filho HB, Mello CS, Tahan S, Pignatari ACC, Sole D, Morais MB. Gut microbiota of children with atopic dermatitis: Controlled study in the metropolitan region of Sao Paulo. Brazil Allergol Immunopathol (Madr). 2020;48(2):107–15.
Reddel S, Del Chierico F, Quagliariello A, Giancristoforo S, Vernocchi P, Russo A, Fiocchi A, Rossi P, Putignani L, El Hachem M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci Rep. 2019;9(1):4996.
Klewicka E, Cukrowska B, Libudzisz Z, Slizewska K, Motyl I. Changes in gut microbiota in children with atopic dermatitis administered the bacteria Lactobacillus casei DN–114001. Pol J Microbiol. 2011;60(4):329–33.
Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy. 2020;75(1):63–74.
Huidrom S. Therapeutic approach of probiotics in children with atopic dermatitis. Antiinflamm Antiallergy Agents Med Chem. 2021;20(1):2–9.
Li BR, Shao SY, Yuan L, Jia R, Sun J, Ji Q, Sui H, Zhou LH, Zhang Y, Liu H, et al. Effects of mild moxibustion on intestinal microbiome and NLRP3 inflammasome in rats with 5-fluorouracil-induced intestinal mucositis. J Integr Med. 2021;19(2):144–57.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA extension for sco** reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Yu L, Deng YH, Huang YH, Ke HJ, Guo Y, Wu JL. Comparison of gut microbiota between infants with atopic dermatitis and healthy controls in Guangzhou. China J Asthma Allergy. 2021;14:493–500.
Zhu LQ, Zhang L, Zhang J, Chang GL, Liu G, Yu DD, Yu XM, Zhao MS, Ye B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J Integr Med. 2021;19(1):56–65.
Alghamdi HA, Behieldin A, Edris S. Gut microbiome skin axis in the development of atopic dermatitis. J Pak Med Assoc. 2021;71(4):1221–7.
Yoon W, Park SH, Lee JS, Byeon JH, Kim SH, Lim J, Yoo Y. Probiotic mixture reduces gut inflammation and microbial dysbiosis in children with atopic dermatitis. Australas J Dermatol. 2021;62(3):e386–92.
Kim JE, Kim HS. Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. 2019;8(4):444.
Sandin A, Annus T, Bjorksten B, Nilsson L, Riikjarv MA, van Hage-Hamsten M, Braback L. Prevalence of self-reported food allergy and IgE antibodies to food allergens in Swedish and Estonian schoolchildren. Eur J Clin Nutr. 2005;59(3):399–403.
Voor T, Julge K, Bottcher MF, Jenmalm MC, Duchen K, Bjorksten B. Atopic sensitization and atopic dermatitis in Estonian and Swedish infants. Clin Exp Allergy. 2005;35(2):153–9.
Rocha-Ramirez LM, Perez-Solano RA, Castanon-Alonso SL, Moreno Guerrero SS, Ramirez Pacheco A, Garcia Garibay M, Eslava C. Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. J Immunol Res. 2017;2017:4607491.
Mohammadian T, Jangaran-Nejad A, Mesbah M, Shirali T, Malekpouri P, Tabandeh MR. Effect of lactobacillus casei on innate immunity responses and aeromonas hydrophila resistance in Shabot. Tor grypus Probiotics Antimicrob Proteins. 2020;12(1):224–35.
Thomas CL, Fernandez-Penas P. The microbiome and atopic eczema: more than skin deep. Australas J Dermatol. 2017;58(1):18–24.
Paller AS, Kong HH, Seed P, Naik S, Scharschmidt TC, Gallo RL, Luger T, Irvine AD. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):26–35.
Fang Z, Li L, Zhang H, Zhao J, Lu W, Chen W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: a review. Front Immunol. 2021;12: 720393.
Huang R, Ning H, Shen M, Li J, Zhang J, Chen X. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.
Los-Rycharska E, Golebiewski M, Sikora M, Grzybowski T, Gorzkiewicz M, Popielarz M, Gawryjolek J, Krogulska A. A combined analysis of gut and skin microbiota in infants with food allergy and atopic dermatitis: a pilot study. Nutrients. 2021;13(5):1682.
Aviles BG, Lozano NM, Contreras-Porta FJ. Could imbalances in the composition of gut microbiota be implicated in the origin of atopic dermatitis? Allergol Immunopathol (Madr). 2020;48(2):105–6.
Ahn SH, Yoon W, Lee SY, Shin HS, Lim MY, Nam YD, Yoo Y. Effects of lactobacillus pentosus in children with allergen-sensitized atopic dermatitis. J Korean Med Sci. 2020;35(18): e128.
Kim JH, Lee SH, Kang MJ, Hwang SG, Park YM, Kim BS, Lee SY, Kim SA, Park MJ, Song KB et al: Host-microbial interactions between PTGR2 and Bifidobacterium in the early life gut of atopic dermatitis children. Pediatr Allergy Immunol 2021.
Climent E, Martinez-Blanch JF, Llobregat L, Ruzafa-Costas B, Carrion-Gutierrez MA, Ramirez-Bosca A, Prieto-Merino D, Genoves S, Codoner FM, Ramon D, et al. Changes in gut microbiota correlates with response to treatment with probiotics in patients with atopic dermatitis a post hoc analysis of a clinical trial. Microorganisms. 2021;9(4):854.
Hu C, van Meel ER, Medina-Gomez C, Kraaij R, Barroso M, Kiefte-de Jong J, Radjabzadeh D, Pasmans S, de Jong NW, de Jongste JC, et al. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J Allergy Clin Immunol. 2021;148(2):612–20.
Jungles K, Tran TDB, Botha M, Rasmussen HE, Teixeira-Reis V, Sodergren E, Gray C, Lunjani N, Hlela C, Basera W et al: Association of gut microbiota and environment in children with AD, comparison of three cohorts of children. Clin Exp Allergy 2021.
Jiang W, Ni B, Liu Z, Liu X, **e W, Wu IXY, Li X. The role of probiotics in the prevention and treatment of atopic dermatitis in children: an updated systematic review and meta-analysis of randomized controlled trials. Paediatr Drugs. 2020;22(5):535–49.
Navarro-Lopez V, Ramirez-Bosca A, Ramon-Vidal D, Ruzafa-Costas B, Genoves-Martinez S, Chenoll-Cuadros E, Carrion-Gutierrez M, Horga de la Parte J, Prieto-Merino D, Codoner-Cortes FM. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154(1):37–43.
Tan-Lim CSC, Esteban-Ipac NAR, Mantaring JBV 3rd. Chan Shih Yen E, Recto MST, Sison OT, Alejandria MM: Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: a systematic review and network meta-analysis. Pediatr Allergy Immunol. 2021;32(1):124–36.
Navarro-Lopez V, Nunez-Delegido E, Ruzafa-Costas B, Sanchez-Pellicer P, Aguera-Santos J, Navarro-Moratalla L. Probiotics in the therapeutic arsenal of dermatologists. Microorganisms. 2021;9(7):1513.
Trikamjee T, Comberiati P, D’Auria E, Peroni D, Zuccotti GV. Nutritional factors in the prevention of atopic dermatitis in children. Front Pediatr. 2020;8:577413.
Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, Indramaya DM, Kusumowidagdo ER, Surono IS. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8(5):833–40.
Kim J, Lee BS, Kim B, Na I, Lee J, Lee JY, Park MR, Kim H, Sohn I, Ahn K. Identification of atopic dermatitis phenotypes with good responses to probiotics (Lactobacillus plantarum CJLP133) in children. Benef Microbes. 2017;8(5):755–61.
D’Elios S, Trambusti I, Verduci E, Ferrante G, Rosati S, Marseglia GL, Drago L, Peroni DG. Probiotics in the prevention and treatment of atopic dermatitis. Pediatr Allergy Immunol. 2020;31(Suppl 26):43–5.
Baldassarre ME, Di Mauro A, Mastromarino P, Fanelli M, Martinelli D, Urbano F, Capobianco D, Laforgia N. Administration of a multi-strain probiotic product to women in the perinatal period differentially affects the breast milk cytokine profile and may have beneficial effects on neonatal gastrointestinal functional symptoms a randomized clinical trial. Nutrients. 2016;8(11):677.
Wickens K, Barthow C, Mitchell EA, Kang J, van Zyl N, Purdie G, Stanley T, Fitzharris P, Murphy R, Crane J. Effects of Lactobacillus rhamnosus HN001 in early life on the cumulative prevalence of allergic disease to 11 years. Pediatr Allergy Immunol. 2018;29(8):808–14.
Park YM, Lee SY, Kang MJ, Kim BS, Lee MJ, Jung SS, Yoon JS, Cho HJ, Lee E, Yang SI, et al. Imbalance of gut streptococcus, clostridium, and akkermansia determines the natural course of atopic dermatitis in infant. Allergy Asthma Immunol Res. 2020;12(2):322–37.
da Costa Baptista IP, Accioly E, de Carvalho PP. Effect of the use of probiotics in the treatment of children with atopic dermatitis; a literature review. Nutr Hosp. 2013;28(1):16–26.
Zhao Y, Qi C, Li X, Lu M, Zhang H, Zhou J, Dang H, Chen J, Li S, Sun J, et al. Prevention of atopic dermatitis in mice by lactobacillus reuteri Fn041 through induction of regulatory T cells and modulation of the gut microbiota. Mol Nutr Food Res. 2021;66(6):e2100699.
Marrs T, Jo JH, Perkin MR, Rivett DW, Witney AA, Bruce KD, Logan K, Craven J, Radulovic S, Versteeg SA, et al. Gut microbiota development during infancy: Impact of introducing allergenic foods. J Allergy Clin Immunol. 2021;147(2):613-621 e619.
Cabridain C, Aubert H, Kaeffer B, Badon V, Boivin M, Dochez V, Winer N, Faurel-Paul E, Planche L, Riochet D, et al. Effectiveness of an antenatal maternal supplementation with prebiotics for preventing atopic dermatitis in high-risk children (the PREGRALL study): protocol for a randomised controlled trial. BMJ Open. 2019;9(4): e024974.
Schneider AM, Nelson AM. Skin microbiota: Friend or foe in pediatric skin health and skin disease. Pediatr Dermatol. 2019;36(6):815–22.
Fanfaret IS, Boda D, Ion LM, Hosseyni D, Leru P, Ali S, Corcea S, Bumbacea R. Probiotics and prebiotics in atopic dermatitis: pros and cons (review). Exp Ther Med. 2021;22(6):1376.
Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Br J Dermatol. 2020;182(1):39–46.
Mahdavinia M, Rasmussen HE, Engen P, Van den Berg JP, Davis E, Engen K, Green SJ, Naqib A, Botha M, Gray C, et al. Atopic dermatitis and food sensitization in South African toddlers: role of fiber and gut microbiota. Ann Allergy Asthma Immunol. 2017;118(6):742-743e743.
Golebiewski M, Los-Rycharska E, Sikora M, Grzybowski T, Gorzkiewicz M, Krogulska A. Mother’s milk microbiome sha** fecal and skin microbiota in infants with food allergy and atopic dermatitis: a pilot analysis. Nutrients. 2021;13(10):3600.
Kim IS, Lee SH, Kwon YM, Adhikari B, Kim JA, Yu DY, Kim GI, Lim JM, Kim SH, Lee SS, et al. Oral administration of beta-glucan and lactobacillus plantarum alleviates atopic dermatitis-like symptoms. J Microbiol Biotechnol. 2019;29(11):1693–706.
Lopez-Santamarina A, Gonzalez EG, Lamas A, Mondragon ADC, Regal P, Miranda JM. Probiotics as a possible strategy for the prevention and treatment of allergies a narrative review. Foods. 2021;10(4):701.
Rui**g L, Yingjie W, Jie L. The composition ratio of probiotics in intestinal microbiota of children with atopic dermatitis. Chinese J Leprosy Dermatol. 2018;34(11):31–4.
**g L, Qiaoying Y: The role of probiotics in the prevention and treatment of allergic diseases in children. **njiang Medicine 2017, 47(6):652 - 653, 647.
Li W, Xu X, Wen H, Wang Z, Ding C, Liu X, Gao Y, Su H, Zhang J, Han Y, et al. Inverse association between the skin and oral microbiota in atopic dermatitis. J Invest Dermatol. 2019;139(8):1779-1787 e1712.
Galli E, Cinicola B, Carello R, Caimmi S, Brindisi G, De Castro G, Zicari AM, Tosca MA, Manti S, Martelli A, et al. Atopic dermatitis. Acta Biomed. 2020;91(11-S):e2020011.
Lehtimaki J, Thorsen J, Rasmussen MA, Hjelmso M, Shah S, Mortensen MS, Trivedi U, Vestergaard G, Bonnelykke K, Chawes BL, et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J Allergy Clin Immunol. 2021;148(1):234–43.
Yangyang L, Kayao L, **angyu L. Clinical analysis of 145 cases of childhood atopic dermatitis. J Pract Dermatol. 2020;13(1):3–6.
Liya, Zhen G, **gbin D: Efficacy evaluation of probiotics in the treatment of adult atopic dermatitis Chinese Journal of Leprosy and Dermatology. 2014;1(4):205–207
Avershina E, Cabrera Rubio R, Lundgard K, Perez Martinez G, Collado MC, Storro O, Oien T, Dotterud CK, Johnsen R, Rudi K. Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J Allergy Clin Immunol. 2017;139(4):1399–402.
Mashiah J, Karady T, Fliss-Isakov N, Sprecher E, Slodownik D, Artzi O, Samuelov L, Ellenbogen E, Godneva A, Segal E et al: Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun Inflamm Dis 2021.
Zhao H, Zhou J, Lu H, ** A, Luo M, Wang K, Lv H, Wang H, Wang P, Miao J, et al. Azithromycin pretreatment exacerbates atopic dermatitis in trimellitic anhydride-induced model mice accompanied by correlated changes in the gut microbiota and serum cytokines. Int Immunopharmacol. 2022;102:108388.
Disamantiaji AP, Izza EF, Soelaeman MF, Sembiring T, Louisa M. Probiotics in the management of atopic dermatitis for children: a case-based review. Dermatol Res Pract. 2020;2020:4587459.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
W L designed research; Y L, X D, S Z, X T, C L conducted research; Y L, X D, S Z analyzed data; W L wrote the first draft of manuscript; Y L, X D had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this study, all methods were performed in accordance with the relevant guidelines and regulations. Ethical approval is necessary for this sco** review because a literature review did not require ethical approval based on the related research guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Du, X., Zhai, S. et al. Gut microbiota and atopic dermatitis in children: a sco** review. BMC Pediatr 22, 323 (2022). https://doi.org/10.1186/s12887-022-03390-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03390-3