Abstract
Background
Acute respiratory infections (ARIs) are common in children and mostly caused by viruses, but the significance of the detection of multiple viruses in ARIs is unclear. This study investigated 14 respiratory viruses in ARIs among children and associated meteorological factors in Shantou, southern China.
Methods
Paired nasal/throat-flocked swabs collected from 1,074 children with ARIs, who visited outpatient walk-in clinics in a tertiary hospital between December 2010 and November 2011, were examined for fourteen respiratory viruses - influenza viruses (FluA, FluB), respiratory syncytial viruses (RSV A and B), human coronaviruses (hCoV: 229E, OC43, HKU1, NL63), human metapneumoviruses (hMPV A and B), parainfluenza viruses (PIV1-4), human rhinoviruses (HRV A, B, C), enteroviruses (EV), adenoviruses (ADV), human bocavirus (hBoV), and human parechoviruses (hPeV) - by multiplex real-time PCR.
Results
We identified at least one virus in 82.3% (884/1,074) and multiple viruses in 38.6% (415/1,074) of patients. EV and HRV were the most frequently detected single viruses (42.3%, 374/884 and 39.9%, 353/884 respectively) and co-detected pair (23.1%, 96/415). Overlap** seasonal trends of viruses were recorded over the year, with dual peaks for EV and single peaks for the others. By logistic regression analysis, EV was positively associated with the average temperature and humidity, hCoV, and PIV4, but negatively with HRV, PIV3, and hBoV. HRV was inversely associated with EV and PIV3.
Conclusions
This study reports high viral detection and co-detection rates in pediatric ARI cases mainly due to EV and HRV. Many viruses circulated throughout the year with similar seasonal trends in association with temperature, humidity, and wind velocity. Statistically significant associations were present among the viruses. Understanding the polyviral etiology and viral interactions in the cases with multiple viruses warrants further studies.
Similar content being viewed by others
Background
Acute respiratory infections (ARIs) are one of the illnesses of highest morbidity and mortality in children worldwide [1-3]. The pathogens causing ARIs vary geographically and by season, but globally viruses play a major role. Respiratory syncytial virus (RSV) is by far the most common pathogen associated with severe respiratory diseases as bronchiolitis, exacerbation of asthma, or pneumonia in early life, and is a leading cause of hospitalization in children under two [4]. Influenza viruses have the greatest potential to cause severe respiratory diseases in the very young, the elderly and those with underlying chronic conditions [5]. Enteroviruses including human rhinoviruses (HRV) and human enteroviruses (EV), previously identified in childhood upper respiratory tract infections, are commonly associated with milder ARIs and have been suspected as major etiological agents of lower respiratory tract infections leading to bronchiolitis and pneumonia in infants [6]. It has also been reported that human metapneumovirus (hMPV) causes approximately 5-10% of all ARIs in children and adults [7] and adenoviruses (ADV) account for 5-15% of respiratory infections in children [8]. Respiratory illnesses can be attributable to other viruses such as parainfluenza viruses (PIV) and human coronaviruses hCoV-229E, OC43 [7]. With rapid progress in molecular diagnostics, newly discovered viruses including human bocavirus (hBoV), human coronaviruses (hCoV-NL63, hCoV-HKU1), human parechoviruses (hPeV), and polyomaviruses WU (WUPyV) and KI (KIPyV) have also been detected in children with respiratory infections, with varying levels of proof of causation [9].
Hospital-based studies in children published over the last decade worldwide have identified viruses in up to 95% of ARI episodes, with a single virus found in 40-60% and multiple viruses in 1-40% of infected patients [7,8,10]. Co-infection is reportedly related to the time of year when circulations of multiple viruses occur [11]. Some studies have shown that the prevalence of co-infections is not related to the absolute prevalence of individual viruses [12]. Factors such as young age, male gender, and history of immunosuppression are associated with an increased chance of viral co-infections [11,13,14]. There could be likely interactions between climatic, environmental, and behavioral factors, and complex interplay between circulating viruses and population-level immunity regarding viral co-infections. Understanding these factors may help us prevent transmission of these infections.
Recent etiologic studies on pediatric respiratory infections mostly report the prevalence in hospitalized children and the seasonality of viruses without elaborating viral co-infection. Therefore, the significance of the detection of multiple viral pathogens in ARIs is unclear. Here, we investigated fourteen common respiratory viruses among pediatric outpatients in southern China during 2010–2011 and their associations with meteorological factors.
Methods
Study location
This study was conducted at the Pediatric Outpatient Walk-in Clinics, the First Affiliated Hospital of Shantou University Medical College. The Pediatric Department provides both primary and tertiary care (common practice in China) for approximately 35,000 children per year in the Chaoshan region of southern China. The Chaoshan region is in the subtropical zone with an average annual temperature of 21.3°C and excellent to lightly polluted air quality levels (air quality index, AQI: 17–142, in 2012–2013).
Study design
Based on modified WHO standard case definition of ARIs [7], eligible participants were defined as a child 0–16 years of age presenting within 3 days of onset of illness with at least two of the following: fever, sore throat, cough, rhinorrhea, nasal congestion, and hoarseness of voice. Patients with any condition preventing swab collection were excluded. We recruited eligible patients in the morning, during which approximately 70% of patient visits are made, on a daily basis except public holidays from December 2010 to November 2011. Participants’ demographic details and clinical features are shown in Table 1. Paired nose and throat-flocked swabs (Copan, Brescia, Italy, Cat. no. 503CS01 and 502CS01) were collected from each participant, combined in one tube, and stored within 3 h of collection at −80°C until further processing.
Laboratory procedure
Multiplex real-time PCR was performed using Roche, Lightcycler 480 II (Roche Diagnostics, Penzberg, Germany) to identify the following 14 respiratory viruses: influenza A (FluA), influenza B (FluB), respiratory syncytial viruses A and B (RSV), human coronaviruses 229E, OC43, HKU1 and NL63 (hCoV), human metapneumoviruses A and B (hMPV), human parainfluenza virus types 1, 2 , 3, and 4 (PIV1, PIV2, PIV3, and PIV4), human rhinoviruses A, B, and C (HRV), human enteroviruses (EV), human adenoviruses (ADV), human bocavirus (hBoV), and human parechoviruses (hPeV).
Nucleic acid extraction was performed using the QIAamp Viral RNA Mini Kit (QIAGEN GmbH, Hilden, Germany, Cat. no. 52906). Reverse transcription and Real-time PCR assays were performed as described previously [15], except for the primers and/or probes for HRV, hPeV, and internal control equine arteritis virus (EAV, see the sequences of 14 viruses in Additional file 1). Due to known cross-reactivity between enteroviruses [16-18], HRV was detected using two sets of primers and probes: HRV-v1 (version 1) for screening and HRV-v2 (version 2) for confirmation. Real-time PCR results were interpreted as described previously [15]. The PCR was considered positive or negative when the Cp value was less than 40 cycles or exceeded 40 cycles, respectively, and the positive control showed the expected Cp value, negative control was negative, and internal control showed the expected Cp value. A negative internal control signal was accepted in case of a positive target sequence with correct positive and negative control signals.
Meteorological data
Meteorological data, including the average daily temperature (°C), the average daily humidity (%), and the average daily wind velocity (km/h), were collected from the official website of Shantou Meteorology, TuTiempo.net (http://www.tutiempo.net/en/Climate/Shantou/2011/593160.htm).
Statistical analysis
We used Chi-square test to compare differences in the distribution of categorical variables, ANOVA and Kruskall Wallis tests to compare medians, and the Pearson correlation analysis to evaluate the associations between the meteorological factors and viruses and among viruses. The variables with significant associations were further analyzed in multivariate logistic regression models, in which symptoms and positivity of viruses were treated as dependent and independent variables to assess virus-symptom associations; and individual viruses were treated as dependent variables with meteorological factors or other viruses as independent variables to investigate meteorological factor-virus and virus-virus associations. A two-tailed p-Value of <0.05 was considered significant. All these analyses were performed with SPSS Statistics version 17.0.
Ethics
The study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College and the Oxford University Tropical Research Ethical Committee (OxTREC). Written informed consent was obtained from parents or legal guardians of children enrolled in the study.
Results
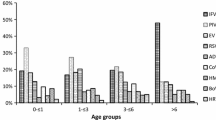
Of 1,074 children (62.3% male) recruited, 43.6% (468/1,074) were >2-5 years old (Table 1). At least one virus was identified in 82.3% (884/1,074) of the patients, with single virus in 43.7% (469/1,074) and multiple viruses in 38.6% (415/1,074). hPeV was not detected. Compared with virus-negative patients, virus-positive patients were less likely to have fever (OR: 0.7, 95% CI: 0.5-1.0, p = 0.05). Patients with multiple viruses were more likely to have rhinorrhea than those with single virus (OR: 1.4, 95% CI: 1.1-1.9, p < 0.05, Table 1). hCoV (OR: 1.6, 95% CI: 1.0-2.4) and PIV4 (OR: 1.6, 95% CI: 1.0-2.4) were more prevalent in the >5 year age group than in the ≤5 year group (all p ≤ 0.05), while hBoV (OR: 0.3, 95% CI: 0.1-0.7) and RSV (OR: 0.4, 95% CI: 0.2-1.0) were less frequently found in the >5 year group (all p < 0.05). Chi-square test and multivariate logistic regression analysis showed that cough was positively associated with HRV and RSV, and negatively with EV; rhinorrhea was positively associated with HRV, PIV3, and hBoV, and negatively with EV; fever was positively associated with EV, and negatively with HRV and PIV3; and nasal congestion was positively associated with RSV, and negatively with EV and hCoV (all p < 0.05, Table 2).
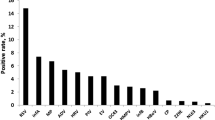
Viruses detected alone or co-detected with other viruses are shown in Table 3. The most frequently detected virus was EV (42.3%, 374/884), followed by HRV (39.9%, 353/884), and hCoV (17.5%, 155/884). EV and HRV were most commonly co-detected with other viruses (Table 3) and also the most commonly co-detected pair of viruses (23.1%, 96/415, see the distribution pattern of viruses in Additional file 2). Screening with HRV-v1 identified 298 cases co-positive for HRV and EV, and subsequent confirmation with HRV-v2 primers/3 probes [15] resulted in only 96 positive cases (32.2%, 96/298).
Seasonality and meteorological factors
The temporal circulation and co-circulation patterns of viruses are shown in Figures 1 and 2. There were overlap** seasonal trends of many viruses throughout the year, with dual peaks for EV in July and September and single peaks for the other viruses. Both EV and HRV circulated throughout the year. hCoV and PIV4 circulated predominantly between April and May but sporadically throughout the year. PIV3, RSV, FluA, and ADV peaked in January, while hBoV peaked in March. FluB circulated mostly from February to July with a peak in April. Co-detection of 5–7 viruses occurred all in May (see Additional file 3). The optimal average daily temperature, humidity, and wind velocity for these viruses are shown in Table 4.
Temporal circulation pattern of respiratory viruses (n = 1,074). EV, enterovirus; HRV, human rhinovirus; hCoV, human coronavirus; PIV1-4, parainfluenza 1–4; hBoV, human bocavirus; RSV, respiratory syncytial virus; FluA, influenza A; FluB, influenza B; ADV, adenovirus. % = individual-virus-positive cases/total cases tested.
Temporal co-circulation pattern of respiratory viruses (n = 1,074). EV, enterovirus; HRV, human rhinovirus; hCoV, human coronavirus; PIV1-4, parainfluenza 1–4; hBoV, human bocavirus; RSV, respiratory syncytial virus; FluA, influenza A; FluB, influenza B; ADV, adenovirus. % = individual-virus-positive cases/total cases tested.
Virus-meteorological and virus-virus associations
Table 5 shows the multivariate logistic regression models for independent associations between the viruses and meteorological factors and between the viruses. EV was positively associated with the average temperature and humidity and the presence of hCoV and PIV4, but negatively with HRV, PIV3, and hBoV. HRV was negatively associated with the presence of EV and PIV3. hCoV was positively associated with the average temperature and humidity and the presence of EV and PIV4. PIV3 was positively associated with the average humidity and the presence of RSV and FluA, but negatively with the average temperature and wind velocity, and the presence of EV, HRV, and hBoV. PIV4 was positively associated with the average temperature and the presence of hCoV and RSV, however, negatively with the wind velocity. hBoV was positively associated with RSV and FluA, but negatively with the average temperature and humidity and the presence of EV and PIV3. RSV was positively associated with the presence of PIV3-4, hBoV, and FluA, but negatively with the average temperature and wind velocity. FluA was positively associated with the presence of PIV3-4, hBoV, and RSV, but negatively with the average temperature.
Discussion
This is the first prospective study reporting the associations between meteorological parameters and co-circulation patterns of 14 common respiratory viruses. The viral detection rate among pediatric outpatients with ARIs in this study (82.3%, 884/1,074) was higher than those reported from Nan**g, China (16 viruses, 50.6%, 248/490) [19] and other countries, including Honduras (16 viruses, 75.4%, 260/345) [20] and Greece (17 viruses, 70.0%, 428/611) [6] in the same study period. Enteroviruses (EV, 34.8% and HRV, 32.9%) were most frequently detected in our outpatient children. Influenza viruses and RSV, the leading pathogens in pediatric outpatients in similar studies [6,21-23], were detected in 10.4% and 7.0% of our cases, with hCoVs (229E, OC43, HKU1, and NL63) in 14.4%, and relatively recently discovered viral pathogens hBoV and hMPV in 7.3% and 0.3% of cases, respectively (Table 3).
The viral co-detection rate (38.6%, 415/1,074) was also high among our study population. Reported rates of co-detection vary widely, from 6.1% among pediatric patients with influenza-like illness [19] to 62% among infants with acute bronchiolitis [24]. Detection of dual viruses is common, and co-detection of five [25] or even six viruses [26] is not anecdotal. All the cases with 5–7 viruses in this study were in May, the end of the cold season in the Chaoshan region. This may be in part due to past viral infections, as some viruses can still be detectable by PCR several weeks after infection [15,16]. Most studies have shown that RSV is the predominant respiratory pathogen co-detected in hospitalized children, followed by HRV, PIV, hMPV, hBoV, and FluA [25,27]. In this study, EV, HRV, hCoV, and PIV 3–4 were involved in the majority of co-detections, with EV-HRV as the most frequently co-detected pair (23% of co-detections). EV and HRV were included in the panels in many studies globally [6,7,10,11,24,28-34], and the EV-HRV pair was the most commonly detected pair among outpatient children with ARIs in Finland (19.6% of co-detections) [28] and infants with acute bronchiolitis in Brazil [10]. The co-detection rate of EV-HRV in this study is similar to that in Finland [28].
Varying detection rates of multiple viruses in different studies may reflect the differences in the study period and location, study population, environmental factors, the number of respiratory pathogens tested, and/or the diagnostic methods/techniques used. Likely reasons behind high detection rates of single and multiple viruses in this study could be due to improved recovery of viruses by using flocked swabs [35] and/or combined nasal and throat swabs [16].
There are advantages and disadvantages of multiplex PCR technique in diagnosing respiratory viral infections. While its high sensitivity and specificity facilitate simultaneous detection of a large spectrum of viruses, including those difficult to be identified by traditional methods [32], its capacity to detect low amounts of viral nucleic acids in some cases during viral incubation period, asymptomatic infection, or post-infectious shedding makes it difficult to interpret the results [30,32]. The development and validation of standardized quantitative PCR with clinically relevant cutoff values [30] or combining qPCR with serology could be helpful for etiologic understanding of simultaneous presence of multiple viruses.
Certain host-specific risk factors may predispose a child to respiratory co-infection. Younger age [11, Acute respiratory infections Influenza A Influenza B Respiratory syncytial virus Human coronavirus Human metapneumovirus Parainfluenza virus Human rhinovirus Enteroviruses Adenoviruses Human bocavirus Human parechoviruses Lin YK, Chang CK, Chang SC, Chen PS, Lin C, Wang YC. Temperature, nitrogen dioxide, circulating respiratory viruses and acute upper respiratory infections among children in Taipei, Taiwan: a population-based study. Environ Res. 2013;120:109–18. Feldman RA, Kamath KR, Rao PS, Webb JK. Infection and disease in a group of South Indian families. I. Introduction, methods, definitions and general observations in a continuing study. Am J Epidemiol. 1969;89(4):364–74. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164–9. Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Van Caeseele P, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135(7):1077–90. Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–30. Kouni S, Karakitsos P, Chranioti A, Theodoridou M, Chrousos G, Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013;19(8):772–7. Razanajatovo NH, Richard V, Hoffmann J, Reynes JM, Razafitrimo GM, Randremanana RV, et al. Viral etiology of influenza-like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS One. 2011;6(3):e17579. Zou L, Zhou J, Li H, Wu J, Mo Y, Chen Q, et al. Human adenovirus infection in children with acute respiratory tract disease in Guangzhou. China APMIS. 2012;120(8):683–8. Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9(1):247. Nascimento MS, Souza AV, Ferreira AV, Rodrigues JC, Abramovici S, Silva Filho LV. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics (Sao Paulo). 2010;65(11):1133–7. Cilla G, Onate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J Med Virol. 2008;80(10):1843–9. Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respi Viruses. 2013;7(1):18–26. Peng D, Zhao D, Liu J, Wang X, Yang K, **cheng H, et al. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155. Chorazy ML, Lebeck MG, McCarthy TA, Richter SS, Torner JC, Gray GC. Polymicrobial Acute Respiratory Infections in a Hospital-Based Pediatric Population. Pediatr Infect Dis J. 2013;32(5):460–6. Jansen RR, Schinkel J, Koekkoek S, Pajkrt D, Beld M, de Jong MD, et al. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J Clin Virol. 2011;51(3):179–85. Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46(2):533–9. Centers for Disease C Prevention. Clusters of acute respiratory illness associated with human enterovirus 68–Asia, Europe, and United States 2008–2010. MMWR Morb Mortal Wkly Rep. 2011;60(38):1301–4. Jaramillo-Gutierrez G, Benschop KS, Claas EC, de Jong AS, van Loon AM, Pas SD, et al. September through October 2010 multi-centre study in the Netherlands examining laboratory ability to detect enterovirus 68, an emerging respiratory pathogen. J Virol Methods. 2013;190(1–2):53–62. Huo X, Qin Y, Qi X, Zu R, Tang F, Li L, et al. Surveillance of 16 respiratory viruses in patients with influenza-like illness in Nan**g, China. J Med Virol. 2012;84(12):1980–4. Schlaudecker EP, Heck JP, Macintyre ET, Martinez R, Dodd CN, McNeal MM, et al. Etiology and seasonality of viral respiratory infections in rural honduran children. Pediatr Infect Dis J. 2012;31(11):1113–8. Vidaurreta SM, Marcone DN, Ellis A, Ekstrom J, Cukier D, Videla C, et al. [Acute viral respiratory infection in children under 5 years: Epidemiological study in two centers in Buenos Aires, Argentina]. Arch Argent Pediatr. 2011;109(4):296–304. Bharaj P, Sullender WM, Kabra SK, Mani K, Cherian J, Tyagi V, et al. Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89. Mathisen M, Strand TA, Sharma BN, Chandyo RK, Valentiner-Branth P, Basnet S, et al. RNA viruses in community-acquired childhood pneumonia in semi-urban Nepal; a cross-sectional study. BMC Med. 2009;7:35. Huguenin A, Moutte L, Renois F, Leveque N, Talmud D, Abely M, et al. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol. 2012;84(6):979–85. Stefanska I, Romanowska M, Donevski S, Gawryluk D, Brydak LB. Co-infections with influenza and other respiratory viruses. Adv Exp Med Biol. 2013;756:291–301. Peng J, Kong W, Guo D, Liu M, Wang Y, Zhu H, et al. The epidemiology and etiology of influenza-like illness in Chinese children from 2008 to 2010. J Med Virol. 2012;84(4):672–8. Paranhos-Baccala G, Komurian-Pradel F, Richard N, Vernet G, Lina B, Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43(4):407–10. Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O. Viral etiology of common cold in children, Finland. Emerg Infect Dis. 2009;15(2):344–6. van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, et al. Associations between Pathogens in the Upper Respiratory Tract of Young Children: Interplay between Viruses and Bacteria. PLoS One. 2012;7(10):e47711. Jansen RR, Wieringa J, Koekkoek SM, Visser CE, Pajkrt D, Molenkamp R, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49(7):2631–6. Pierangeli A, Gentile M, Di Marco P, Pagnotti P, Scagnolari C, Trombetti S, et al. Detection and ty** by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007;79(4):463–8. Calvo C, Garcia-Garcia ML, Blanco C, Vazquez MC, Frias ME, Perez-Brena P, et al. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42(3):268–72. Njouom R, Yekwa EL, Cappy P, Vabret A, Boisier P, Rousset D. Viral etiology of influenza-like illnesses in cameroon, january-december 2009. J Infect Dis. 2012;206 Suppl 1:S29–35. Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23(11):1003–7. Debyle C, Bulkow L, Miernyk K, Chikoyak L, Hummel KB, Hennessy T, et al. Comparison of nasopharyngeal flocked swabs and nasopharyngeal wash collection methods for respiratory virus detection in hospitalized children using real-time polymerase chain reaction. J Virol Methods. 2012;185(1):89–93. Brunstein JD, Cline CL, McKinney S, Thomas E. Evidence from multiplex molecular assays for complex multipathogen interactions in acute respiratory infections. J Clin Microbiol. 2008;46(1):97–102. du Prel JB, Puppe W, Grondahl B, Knuf M, Weigl JA, Schaaff F, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49(6):861–8. Ji W, Chen ZR, Guo HB, Wang MJ, Yan YD, Zhang XL, et al. Characteristics and the prevalence of respiratory viruses and the correlation with climatic factors of hospitalized children in Suzhou children’s hospital. Zhonghua Yu Fang Yi Xue Za Zhi. 2011;45(3):205–10. Chan PK, Mok HY, Lee TC, Chu IM, Lam WY, Sung JJ. Seasonal influenza activity in Hong Kong and its association with meteorological variations. J Med Virol. 2009;81(10):1797–806. Tang JW, Lai FY, Nymadawa P, Deng YM, Ratnamohan M, Petric M, et al. Comparison of the incidence of influenza in relation to climate factors during 2000–2007 in five countries. J Med Virol. 2010;82(11):1958–65. Khor CS, Sam IC, Hooi PS, Quek KF, Chan YF. Epidemiology and seasonality of respiratory viral infections in hospitalized children in Kuala Lumpur, Malaysia: a retrospective study of 27 years. BMC Pediatr. 2012;12:32. Nascimento-Carvalho CM, Cardoso MR, Barral A, Araujo-Neto CA, Oliveira JR, Sobral LS, et al. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scand J Infect Dis. 2010;42(11–12):839–44. Urashima M, Shindo N, Okabe N. A seasonal model to simulate influenza oscillation in Tokyo. Jpn J Infect Dis. 2003;56(2):43–7. Chen Z, Zhu Y, Wang Y, Zhou W, Yan Y, Zhu C, et al. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: an analysis over 11 years. Arch Virol. 2014;159(4):631–9. Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiol Infect. 1998;121(1):121–8. Chan PK, Sung RY, Fung KS, Hui M, Chik KW, Adeyemi-Doro FA, et al. Epidemiology of respiratory syncytial virus infection among paediatric patients in Hong Kong: seasonality and disease impact. Epidemiol Infect. 1999;123(2):257–62. Omer SB, Sutanto A, Sarwo H, Linehan M, Djelantik IG, Mercer D, et al. Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiol Infect. 2008;136(10):1319–27. Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76(9):2712–7. Tang JW, Lai FY, Wong F, Hon KL. Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol Infect. 2010;138(2):226–35. Fang LQ, Wang LP, de Vlas SJ, Liang S, Tong SL, Li YL, et al. Distribution and risk factors of 2009 pandemic influenza A (H1N1) in mainland China. Am J Epidemiol. 2012;175(9):890–7. Landry ML, Garner R, Ferguson D. Comparison of the NucliSens Basic kit (Nucleic Acid Sequence-Based Amplification) and the Argene Biosoft Enterovirus Consensus Reverse Transcription-PCR assays for rapid detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 2003;41(11):5006–10. Meijer A, Benschop KS, Donker GA, van der Avoort HG. Continued seasonal circulation of enterovirus D68 in the Netherlands, 2011–2014. Euro Surveill. 2014, 19(42). Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20935. de Almeida MB, Zerbinati RM, Tateno AF, Oliveira CM, Romao RM, Rodrigues JC, et al. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16(6):996–9. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6(1):71–7. We would like to thank the pediatricians from the Pediatric Department, the First Affiliated Hospital of Shantou University Medical College for their generous support, the children and their guardians for participation in this study, Richard Molenkamp at the University of Amsterdam, Academic Medical Center for technique and knowledge transfer to set up the multiplex real-time PCR, Jieling Chen at the Shantou-Oxford Clinical Research Unit for technical assistance, and staff in the International Institute of Infection and Immunity, Shantou University Medical College for their assistance with real-time PCR. This study was supported by the Li Ka Shing Foundation, Shantou University Medical College, and the University of Oxford (grant No. B9RSRT0-14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that they have no competing interests. BLC and HP designed and performed the experiments, analyzed the data, and wrote the paper. DGZ designed and performed the experiments, and analyzed the data. FZ analyzed the data. JF conceived and designed the experiments, and analyzed the data. FL analyzed the data, and facilitated the study. HRvD analyzed the data. BYW facilitated the study. WB-T designed the experiments, analyzed the data, and wrote the paper. All authors read and approved the final manuscript.
Primers and probes of 14 viruses.
The distribution pattern of viruses in pediatric outpatients with acute respiratory infections, ARIs (n=415).
The number of viral co-positive cases by month.
This article is published under an open access license.
Please check the 'Copyright Information' section either on this page or in the PDF
for details of this license and what re-use is permitted.
If your intended use exceeds what is permitted by the license or if
you are unable to locate the licence and re-use information,
please contact the Rights and
Permissions team.
Cui, B., Zhang, D., Pan, H. et al. Viral aetiology of acute respiratory infections among children and associated meteorological factors in southern China.
BMC Infect Dis 15, 124 (2015). https://doi.org/10.1186/s12879-015-0863-6 Received: Accepted: Published: DOI: https://doi.org/10.1186/s12879-015-0863-6Abbreviations
References
Acknowledgements
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Authors’ contributions
Additional files
Additional file 1:
Additional file 2:
Additional file 3:
Rights and permissions
About this article
Cite this article
Keywords