Abstract
Transposable elements (TEs) have been consistently underestimated in their contribution to genetic instability and human disease. TEs can cause human disease by creating insertional mutations in genes, and also contributing to genetic instability through non-allelic homologous recombination and introduction of sequences that evolve into various cis-acting signals that alter gene expression. Other outcomes of TE activity, such as their potential to cause DNA double-strand breaks or to modulate the epigenetic state of chromosomes, are less fully characterized. The currently active human transposable elements are members of the non-LTR retroelement families, LINE-1, Alu (SINE), and SVA. The impact of germline insertional mutagenesis by TEs is well established, whereas the rate of post-insertional TE-mediated germline mutations and all forms of somatic mutations remain less well quantified. The number of human diseases discovered to be associated with non-allelic homologous recombination between TEs, and particularly between Alu elements, is growing at an unprecedented rate. Improvement in the technology for detection of such events, as well as the mounting interest in the research and medical communities in resolving the underlying causes of the human diseases with unknown etiology, explain this increase. Here, we focus on the most recent advances in understanding of the impact of the active human TEs on the stability of the human genome and its relevance to human disease.
Similar content being viewed by others
Introduction to mammalian transposable elements
Transposable elements (TEs) occupy almost half, 46%, of the human genome, making the TE content of our genome one of the highest among mammals, second only to the opossum genome with a reported TE content of 52% [1, 2]. The total representation of TE-related sequences in the human genome is probably even higher, as many of the sequences of the most ancient TEs have deteriorated beyond recognition [3]. The human genome contains two major classes of TEs, DNA and RNA transposons, defined by the type of molecule used as an intermediate in their mobilization.
DNA TEs encode a transposase that re-enters the nucleus to specifically recognize transposon sequences in chromosomal DNA. The transposase excises these sequences from their genomic location and inserts them into a new genomic site (reviewed in [4]); this is also referred to as 'cut and paste' transposition. Human DNA TE activity subsided over 37 million years ago [5]; as a result, DNA TEs no longer contribute significantly to the ongoing mutagenesis in humans.
Retrotransposons or retroelements make use of an RNA-mediated transposition process. Retroelements are subdivided into two major groups: those containing long-terminal repeats, LTR retroelements, and all others, lumped into the category of non-LTR retroelements. Although inactive in humans for millions of years, the best known LTR retrotransposons, the endogenous retroviruses, make up approximately 8% of the human genome [1]. This contrasts with rodent genomes, in which LTR elements continue to contribute a high proportion of the germline TE-associated mutations (reviewed in [6]).
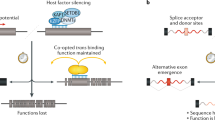
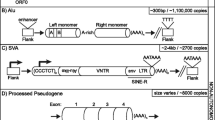
Non-LTR retrotransposons include autonomous and non-autonomous members. The autonomous long interspersed element-1 (LINE-1 or L1), and its non-autonomous partners, such as 'SINER, VNTR, and Alu' (SVA) and the short interspersed element (SINE) Alu, are the only mobile elements with clear evidence of current retrotrans-positional activity in the human genome [7] and will therefore be the primary focus of this article.
The human L1 is about 6 kb long and encodes two open reading frames, ORF1 and ORF2, which are both required for L1 retrotransposition (Figure 1a) [8]. ORF2 encodes endonuclease and reverse transcriptase activities that are crucial for the insertion mechanism [8, 9]. SINEs and SVA elements do not encode any proteins [10], instead they depend on the presence of the functional L1s, and they are therefore often referred to as L1 parasites [11]. In contrast to L1, Alu elements require only ORF2 of L1 for their mobilization [11, 12]. Alu elements are transcribed by RNA polymerase III and encode a variable length adenosine-rich region at their 3' end, a critical feature for retro-transposition [10]. SVA is a composite element containing a complex sequence composed of a (CCCTCT)n hexamer repeat region, an Alu-derived region, a variable number tandem repeat (VNTR) region and a retroviral-derived sequence (Figure 1a) [13]. The requirements for SVA mobilization are still poorly understood [13, TE activity can generate a wide-spectrum of genomic mutations, ranging from point mutations to gross rearrangements with gain of genomic information, as well as interference with normal gene processing and expression after insertion. These mutations contribute to idiopathic human disease. Because of the intimate relationship between L1 activity and multiple cellular processes, it is likely that people with genetic backgrounds that produce defects in any of the pathways influencing the L1 lifecycle are more vulnerable to insult from TEs. Thus, to evaluate the impact of these elements on the stability of the human genome and human disease, it is crucial to take into account their cumulative activity in a specific genetic background as well as the potential modulating effects of the extracellular environment. The increasing ease of sequencing genomes is likely to help clarify the extent of the contribution of mobile elements to genetic instability in many human diseases. This information is critical in determining the full spectrum of mutations contributing to human disease. However, the full impact of these ubiquitous, high-copy-number elements on the biology of the cell may remain elusive for some time.Conclusions
Abbreviations
- AML:
-
acute myelogenous leukemia
- BRCA1 :
-
breast cancer-1 gene
- CGH:
-
comparative genomic hybridization
- DSB:
-
double-strand break
- LINE-1:
-
L1, long interspersed element-1
- LTR:
-
long terminal repeat
- NAHR:
-
non-allelic homologous recombination
- NHEJ:
-
non-homologous end joining
- ORF:
-
open reading frame
- SINE:
-
short interspersed element
- SVA:
-
SINE-R, VNTR, and Alu element
- TE:
-
transposable element.
References
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, et al: Initial sequencing and analysis of the human genome. Nature. 2001, 409: 860-921. 10.1038/35057062.
Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, Belov K, Miller W, Clarke L, Chinwalla AT, Yang SP, Heger A, Locke DP, Miethke P, Waters PD, Veyrunes F, Fulton L, Fulton B, Graves T, Wallis J, Puente XS, Lopez-Otin C, Ordonez GR, Eichler EE, Chen L, Cheng Z, Deakin JE, Alsop A, Thompson K, Kirby P, et al: Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008, 453: 175-183. 10.1038/nature06936.
Gu W, Castoe TA, Hedges DJ, Batzer MA, Pollock DD: Identification of repeat structure in large genomes using repeat probability clouds. Anal Biochem. 2008, 380: 77-83. 10.1016/j.ab.2008.05.015.
Sinzelle L, Izsvak Z, Ivics Z: Molecular domestication of transposable elements: from detrimental parasites to useful host genes. Cell Mol Life Sci. 2009, 66: 1073-1093. 10.1007/s00018-009-8376-3.
Pace JK, Feschotte C: The evolutionary history of human DNA transposons: evidence for intense activity in the primate lineage. Genome Res. 2007, 17: 422-432. 10.1101/gr.5826307.
Maksakova IA, Romanish MT, Gagnier L, Dunn CA, Lagemaat van de LN, Mager DL: Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet. 2006, 2: e2-10.1371/journal.pgen.0020002.
Chen JM, Stenson PD, Cooper DN, Ferec C: A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum Genet. 2005, 117: 411-427. 10.1007/s00439-005-1321-0.
Moran JV, Holmes SE, Naas TP, DeBerardinis RJ, Boeke JD, Kazazian HH: High frequency retrotransposition in cultured mammalian cells. Cell. 1996, 87: 917-927. 10.1016/S0092-8674(00)81998-4.
Feng Q, Moran JV, Kazazian HH, Boeke JD: Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996, 87: 905-916. 10.1016/S0092-8674(00)81997-2.
Belancio VP, Hedges DJ, Deininger P: Mammalian non-LTR retrotransposons: for better or worse, in sickness and in health. Genome Res. 2008, 18: 343-358. 10.1101/gr.5558208.
Dewannieux M, Esnault C, Heidmann T: LINE-mediated retrotransposition of marked Alu sequences. Nat Genet. 2003, 35: 41-48. 10.1038/ng1223.
Wallace N, Wagstaff BJ, Deininger PL, Roy-Engel AM: LINE-1 ORF1 protein enhances Alu SINE retrotransposition. Gene. 2008, 419: 1-6. 10.1016/j.gene.2008.04.007.
Ostertag EM, Goodier JL, Zhang Y, Kazazian HH: SVA elements are nonautonomous retrotransposons that cause disease in humans. Am J Hum Genet. 2003, 73: 1444-1451. 10.1086/380207.
Wang H, **ng J, Grover D, Hedges DJ, Han K, Walker JA, Batzer MA: SVA elements: a hominid-specific retroposon family. J Mol Biol. 2005, 354: 994-1007. 10.1016/j.jmb.2005.09.085.
Branciforte D, Martin SL: Developmental and cell type specificity of LINE-1 expression in mouse testis: implications for transposition. Mol Cell Biol. 1994, 14: 2584-2592.
Ergun S, Buschmann C, Heukeshoven J, Dammann K, Schnieders F, Lauke H, Chalajour F, Kilic N, Stratling WH, Schumann GG: Cell type-specific expression of LINE-1 open reading frames 1 and 2 in fetal and adult human tissues. J Biol Chem. 2004, 279: 27753-27763. 10.1074/jbc.M312985200.
Martin SL: Ribonucleoprotein particles with LINE-1 RNA in mouse embryonal carcinoma cells. Mol Cell Biol. 1991, 11: 4804-4807.
Martin SL, Branciforte D: Synchronous expression of LINE-1 RNA and protein in mouse embryonal carcinoma cells. Mol Cell Biol. 1993, 13: 5383-5392.
An W, Han JS, Schrum CM, Maitra A, Koentgen F, Boeke JD: Conditional activation of a single-copy L1 transgene in mice by Cre. Genesis. 2008, 46: 373-383. 10.1002/dvg.20407.
Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, Kazazian HH: L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009, 23: 1303-1312. 10.1101/gad.1803909.
Babushok DV, Ostertag EM, Courtney CE, Choi JM, Kazazian HH: L1 integration in a transgenic mouse model. Genome Res. 2006, 16: 240-250. 10.1101/gr.4571606.
Miki Y, Nishisho I, Horii A, Miyoshi Y, Utsunomiya J, Kinzler KW, Vogelstein B, Nakamura Y: Disruption of the APC gene by a retrotransposal insertion of L1 sequence in a colon cancer. Cancer Res. 1992, 52: 643-645.
Dombroski BA, Mathias SL, Nanthakumar E, Scott AF, Kazazian HH: Isolation of an active human transposable element. Science. 1991, 254: 1805-1808. 10.1126/science.1662412.
Babushok DV, Kazazian HH: Progress in understanding the biology of the human mutagen LINE-1. Hum Mutat. 2007, 28: 527-539. 10.1002/humu.20486.
Belancio VP, Hedges DJ, Deininger P: LINE-1 RNA splicing and influences on mammalian gene expression. Nucleic Acids Res. 2006, 34: 1512-1521. 10.1093/nar/gkl027.
Belancio VP, Roy-Engel AM, Deininger P: The impact of multiple splice sites in human L1 elements. Gene. 2008, 411: 38-45. 10.1016/j.gene.2007.12.022.
Perepelitsa-Belancio V, Deininger P: RNA truncation by premature polyadenylation attenuates human mobile element activity. Nat Genet. 2003, 35: 363-366. 10.1038/ng1269.
Wheelan SJ, Aizawa Y, Han JS, Boeke JD: Gene-breaking: a new paradigm for human retrotransposon-mediated gene evolution. Genome Res. 2005, 15: 1073-1078. 10.1101/gr.3688905.
Han JS, Szak ST, Boeke JD: Transcriptional disruption by the L1 retrotransposon and implications for mammalian transcriptomes. Nature. 2004, 429: 268-274. 10.1038/nature02536.
Matlik K, Redik K, Speek M: L1 antisense promoter drives tissue-specific transcription of human genes. J Biomed Biotechnol. 2006, 2006: 71753-10.1155/JBB/2006/71753.
Speek M: Antisense promoter of human L1 retrotransposon drives transcription of adjacent cellular genes. Mol Cell Biol. 2001, 21: 1973-1985. 10.1128/MCB.21.6.1973-1985.2001.
Chen C, Ara T, Gautheret D: Using Alu elements as polyadenylation sites: a case of retroposon exaptation. Mol Biol Evol. 2009, 26: 327-334. 10.1093/molbev/msn249.
Lee YK, Chew A, Phan H, Greenhalgh DG, Cho K: Genome-wide expression profiles of endogenous retroviruses in lymphoid tissues and their biological properties. Virology. 2008, 373: 263-273. 10.1016/j.virol.2007.10.043.
Sorek R, Ast G, Graur D: Alu-containing exons are alternatively spliced. Genome Res. 2002, 12: 1060-1067. 10.1101/gr.229302.
Deininger PL, Batzer MA: Alu repeats and human disease. Mol Genet Metab. 1999, 67: 183-193. 10.1006/mgme.1999.2864.
Deininger PL, Batzer MA: Mammalian retroelements. Genome Res. 2002, 12: 1455-1465. 10.1101/gr.282402.
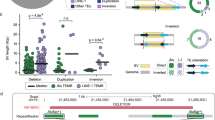
Han K, Sen SK, Wang J, Callinan PA, Lee J, Cordaux R, Liang P, Batzer MA: Genomic rearrangements by LINE-1 insertion-mediated deletion in the human and chimpanzee lineages. Nucleic Acids Res. 2005, 33: 4040-4052. 10.1093/nar/gki718.
Han K, Lee J, Meyer TJ, Wang J, Sen SK, Srikanta D, Liang P, Batzer MA: Alu recombination-mediated structural deletions in the chimpanzee genome. PLoS Genet. 2007, 3: 1939-1949. 10.1371/journal.pgen.0030184.
Han K, Lee J, Meyer TJ, Remedios P, Goodwin L, Batzer MA: L1 recombination-associated deletions generate human genomic variation. Proc Natl Acad Sci USA. 2008, 105: 19366-19371. 10.1073/pnas.0807866105.
Bailey JA, Liu G, Eichler EE: An Alu transposition model for the origin and expansion of human segmental duplications. Am J Hum Genet. 2003, 73: 823-834. 10.1086/378594.
Hedges DJ, Deininger PL: Inviting instability: transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutat Res. 2006, 616: 46-59.
Strout MP, Marcucci G, Bloomfield CD, Caligiuri MA: The partial tandem duplication of ALL1 (MLL) is consistently generated by Alu-mediated homologous recombination in acute myeloid leukemia. Proc Natl Acad Sci USA. 1998, 95: 2390-2395. 10.1073/pnas.95.5.2390.
Franke G, Bausch B, Hoffmann MM, Cybulla M, Wilhelm C, Kohlhase J, Scherer G, Neumann HP: Alu-Alu recombination underlies the vast majority of large VHL germline deletions: molecular characterization and genotype-phenotype correlations in VHL patients. Hum Mutat. 2009, 30: 776-786. 10.1002/humu.20948.
Mazoyer S: Genomic rearrangements in the BRCA1 and BRCA2 genes. Hum Mutat. 2005, 25: 415-422. 10.1002/humu.20169.
Casarin A, Martella M, Polli R, Leonardi E, Anesi L, Murgia A: Molecular characterization of large deletions in the von Hippel-Lindau (VHL) gene by quantitative real-time PCR: the hypothesis of an Alu-mediated mechanism underlying VHL gene rearrangements. Mol Diagn Ther. 2006, 10: 243-249.
Kim SH, Bae JH, Chae JJ, Kim UK, Choe SJ, Namkoong Y, Kim HS, Park YB, Lee CC: Long-distance PCR-based screening for large rearrangements of the LDL receptor gene in Korean patients with familial hypercholesterolemia. Clin Chem. 1999, 45: 1424-1430.
Font-Llitjos M, Rodriguez-Santiago B, Espino M, Sillue R, Manas S, Gomez L, Perez-Jurado LA, Palacin M, Nunes V: Novel SLC7A7 large rearrangements in lysinuric protein intolerance patients involving the same AluY repeat. Eur J Hum Genet. 2009, 17: 71-79. 10.1038/ejhg.2008.145.
Schnittger S, Wormann B, Hiddemann W, Griesinger F: Partial tandem duplications of the MLL gene are detectable in peripheral blood and bone marrow of nearly all healthy donors. Blood. 1998, 92: 1728-1734.
Belgnaoui SM, Gosden RG, Semmes OJ, Haoudi A: Human LINE-1 retrotransposon induces DNA damage and apoptosis in cancer cells. Cancer Cell Int. 2006, 6: 13-10.1186/1475-2867-6-13.
Farkash EA, Kao GD, Horman SR, Prak ET: Gamma radiation increases endonuclease-dependent L1 retrotransposition in a cultured cell assay. Nucleic Acids Res. 2006, 34: 1196-1204. 10.1093/nar/gkj522.
Gasior SL, Wakeman TP, Xu B, Deininger PL: The human LINE-1 retrotransposon creates DNA double-strand breaks. J Mol Biol. 2006, 357: 1383-1393. 10.1016/j.jmb.2006.01.089.
Wallace NA, Belancio VP, Deininger PL: L1 mobile element expression causes multiple types of toxicity. Gene. 2008, 419: 75-81. 10.1016/j.gene.2008.04.013.
Brouha B, Schustak J, Badge RM, Lutz-Prigge S, Farley AH, Moran JV, Kazazian HH: Hot L1s account for the bulk of retrotransposition in the human population. Proc Natl Acad Sci USA. 2003, 100: 5280-5285. 10.1073/pnas.0831042100.
Lutz SM, Vincent BJ, Kazazian HH, Batzer MA, Moran JV: Allelic heterogeneity in LINE-1 retrotransposition activity. Am J Hum Genet. 2003, 73: 1431-1437. 10.1086/379744.
Seleme MC, Vetter MR, Cordaux R, Bastone L, Batzer MA, Kazazian HH: Extensive individual variation in L1 retrotransposition capability contributes to human genetic diversity. Proc Natl Acad Sci USA. 2006, 103: 6611-6616. 10.1073/pnas.0601324103.
Comeaux MS, Roy-Engel AM, Hedges DJ, Deininger PL: Diverse cis factors controlling Alu retrotransposition: what causes Alu elements to die?. Genome Res. 2009, 19: 545-555. 10.1101/gr.089789.108.
Roy-Engel AM, Salem AH, Oyeniran OO, Deininger L, Hedges DJ, Kilroy GE, Batzer MA, Deininger PL: Active Alu element "A-tails": size does matter. Genome Res. 2002, 12: 1333-1344. 10.1101/gr.384802.
Bennett EA, Keller H, Mills RE, Schmidt S, Moran JV, Weichenrieder O, Devine SE: Active Alu retrotransposons in the human genome. Genome Res. 2008, 18: 1875-1883. 10.1101/gr.081737.108.
Hata K, Sakaki Y: Identification of critical CpG sites for repression of L1 transcription by DNA methylation. Gene. 1997, 189: 227-234. 10.1016/S0378-1119(96)00856-6.
Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, Ehrlich M, Laird PW: Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005, 33: 6823-6836. 10.1093/nar/gki987.
Yang N, Zhang L, Zhang Y, Kazazian HH: An important role for RUNX3 in human L1 transcription and retrotransposition. Nucleic Acids Res. 2003, 31: 4929-4940. 10.1093/nar/gkg663.
Yang N, Kazazian HH: L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in human cultured cells. Nat Struct Mol Biol. 2006, 13: 763-771. 10.1038/nsmb1141.
Yu F, Zingler N, Schumann G, Stratling WH: Methyl-CpG-binding protein 2 represses LINE-1 expression and retrotransposition but not Alu transcription. Nucleic Acids Res. 2001, 29: 4493-4501. 10.1093/nar/29.21.4493.
Gasior SL, Roy-Engel AM, Deininger PL: ERCC1/XPF limits L1 retrotransposition. DNA Repair (Amst). 2008, 7: 983-989. 10.1016/j.dnarep.2008.02.006.
Suzuki J, Yamaguchi K, Kajikawa M, Ichiyanagi K, Adachi N, Koyama H, Takeda S, Okada N: Genetic evidence that the non-homologous end-joining repair pathway is involved in LINE retrotransposition. PLoS Genet. 2009, 5: e1000461-10.1371/journal.pgen.1000461.
Hulme AE, Bogerd HP, Cullen BR, Moran JV: Selective inhibition of Alu retrotransposition by APOBEC3G. Gene. 2007, 390: 199-205. 10.1016/j.gene.2006.08.032.
Stenglein MD, Harris RS: APOBEC3B and APOBEC3F inhibit L1 retrotransposition by a DNA deamination-independent mechanism. J Biol Chem. 2006, 281: 16837-16841. 10.1074/jbc.M602367200.
Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, Taccioli GE, Batzer MA, Moran JV: DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002, 31: 159-165. 10.1038/ng898.
Bourc'his D, Bestor TH: Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004, 431: 96-99. 10.1038/nature02886.
Belancio V, Roy-Engel AM: Xenobiotics-modulation of human mobile elements and genetic instability. Encyclopedia of Environmental Health. Edited by: Nriagu JO. 2009, Amsterdam: Elsevier, 1-10.
Cost GJ, Feng Q, Jacquier A, Boeke JD: Human L1 element target-primed reverse transcription in vitro. EMBO J. 2002, 21: 5899-5910. 10.1093/emboj/cdf592.
Acknowledgements
This article was made possible by grants P20RR020152 (PLD, VPB, and AMR-E), R01GM45668 (PLD), and R01GM079709A (AMR-E) from the National Institutes of Health (NIH) and an EPSCOR grant from the National Science Foundation (PLD). VPB is supported by NIH/NIA grant 5K01AG030074 and an Ellison Medical Foundation New Scholar in Aging award (AG-NS-0447-08). The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the NIH. Competitive Advantage Funds (2006) from the Louisiana Cancer Research Consortium (LCRC) were also awarded to AMR-E.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors participated equally in the conception and writing of this article.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Belancio, V.P., Deininger, P.L. & Roy-Engel, A.M. LINE dancing in the human genome: transposable elements and disease. Genome Med 1, 97 (2009). https://doi.org/10.1186/gm97
Published:
DOI: https://doi.org/10.1186/gm97