Abstract
Bacillus subtilis B25 was isolated from banana rhizosphere soil. It has been confirmed for B25 to have stronger antagonism against Fusarium oxysporum f.sp.cubense, Additionally B25 has good inhibitory to plant pathogens, including Corynespora cassiicola, Alternaria solani, Botrytis cinerea and Colletotrichum gloeosporioides on potato dextrose agar (PDA) plates. The antagonistic substance can be extracted from cell-free culture broth supernatants by 70% (w/v) (NH4)2 SO4 saturation. Clear blank band was observed between the protein and a pathogen. The examination of antagonistic mechanism under light microscope showed that the antifungal protein of B25 appeared to inhibit pathogens by leading to mycelium and spores tumescence, distortion, abnormality. The isolation procedure comprised ion exchange chromatography on DEAE-Sephadex Fast Flow and gel filtration chromatography on SephadexG-100. The purified antifungal fraction showed a single band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The active fraction was identified by NanoLC-ESI-MS/MS The amino acid sequences of 17 peptides segments were obtained. The analysis of the protein suggested that it was a hypothetical protein (gi154685475), with a relative molecular mass of 38708.67 Da and isoelectric point (pI) of 5.63.
Similar content being viewed by others
Introduction
Fusarium wilt, caused by Fusarium oxysporum f.sp.cubense (FOC.), was a devastating disease of banana, which was threatening the safety of banana production in China and the worldwide. At present, this disease have spread to a very large of area. Effective control methods for it are not available. Banana as one of the most important economic crops, its contribution to the overall fruit production reached a very high level, but now many existing banana orchard is being wiped out. It was a potential measure to screen antagonistic bacteria and produce microbial germicide in the control of Fusarium wilt of banana. The chemical control and use of fungicides are the most effective way of preventing the occurrence of plant diseases. However, the use of chemicals is considered undesirable because of concerns over residues, following an increased public health concern. The bio control has become an interesting alternative to conventional methods.
B.subtilis, which distribute in the nature widely as non-pathogenic bacteria, can nonribosomally synthesizes several kinds of small antibiotic peptides (<2000 Da) that have antifungal activities, such as iturin (Delcambe et al. 1977; Tsuge et al. 2001; Stein 2005), surfactin (Peypoux et al. 1999; Carrillo et al. 2003), fengymycin, bacilysin (Loeffler et al. 1986), bacillomycin (Peypoux et al. 1980), mycosubtilin (Peypoux et al. 1986), and mycobacillin (Sengupta et al. 1971). Additionally B. subtilis secretes an abundance of proteins (Liu et al. 2007; Chen et al. 2010; Luo et al. 2013).
Bacillus subtilis B25 was isolated from banana rhizosphere soil in Hainan. It has been confirmed for B25 that antagonism against Fusarium oxysporum f.sp.cubense (FOC) and other plant fungal pathogen can be produced (Liu 2011, China). We found that the antimicrobial substance could tolerate acid and not changed significantly even in 100°C boil 1 hour (Lin 2013, China). The aim of this paper was to extract the antifungal substances of Bacillus subtilis B25 and test it’s activity.
Materials and methods
Chemicals
All chemicals were analytical grade. Bovine serum albumin was from Sigma. Low-molecular weight protein standards were purchased from TaKaRa Biotechnology (Dalin) Co., Ltd. (China), DEAE-Sephadex Fast Flow was obtained from GE, SephadexG-100 from Pharmacia.
Microorganisms and culture conditions
B. subtilis B25 was grown in fermentation medium containing (g/L): glucose, 8.0; peptone, 8.0; yeast extract, 1.0; beef extract, 3.0; NaCl, 10.0; The pH of the medium was adjusted to 7.0 before autoclaving.
Cultivation was performed using 250 ml Erlenmeyer flasks containing 100 ml medium for 48 h at 28°C. As inoculation, 2% (v/v) bacteria grew in Luria-Bertani broth (g/L): peptone, 10.0; yeast extract, 5.0; and NaCl, 10.0; pH 7.0.
Fusarium oxysporum f.sp.cubense was isolated from wilted banana vascular and stored at 4°C in PDA and it was used as the indicator.
B25 was isolated from banana rhizosphere soil and identified as Bacillus subtilis on the basis of its morphological, biochemical and physiological characteristics, and 16S r DNA sequence. It’s capability of controlling Fusarium wilt in green house and in the field was evaluated (Liu et. al. 2011). Also, the characteristics of the active substance, such as precipitated quality, molecular mass, heat-tolerance were test (Lin et. al. 2013).
Assay of of antifungal activity
B25 was assayed for its antagonistic activity in vitro against Fusarium oxysporum f.sp.cubense, Corynespora cassiicola, Alternaria solani, Botrytis cinerea, Colletotrichum gloeosporioides and Aspergillus niger via duel culture method. A fungal disc of five day old mycelial mat of 1 cm diameter was placed in the centre of the petri plate containing PDA (potato dextrose agar). And the 24 hr old Bacillus B25 culture was single streaked at a distance of approx. 3 cm from the fungal disc. The plate was incubated at 28°C and observed after every 24 hrs for any inhibition of mycelial growth.
The antifungal activity against FOC of precipitated protein and all fractions acquired in the procedure of separation was tested by agar-diffusion method. A fungal disc was placed in the centre of PDA. Plate. Wells of 0.5 cm diameter were made at a distance of approx. 3 cm from the fungal disc. And tested substances were discharged in the wells. The Plate was incubated at 28°C and observed for any inhibition of mycelial growth.
Production and purification of antibiotics
A loop of B25 cells from a slant culture of fresh nutrient agar was inoculated to a 250 ml flask containing 100 ml LB broth (pH 7.0). The flask was incubated on a rotary shaker at 200 rpm for 9 ~ 12 h at 28°C. This fresh culture was inoculated to fermentation broth, each 2 ml. These flasks were incubated under the same conditions as described above for 48 h. Culture supernatants obtained after removed the cells by centrifugation at 6000 r/min for 20 min for further study.
The proteins were precipitated from the supernatant at 70% (w/v) (NH4)2 SO4 saturation. The latter was added in small portions with constant stirring for 30 min. The stirring was continued for 1 h, and the mixture was kept overnight at 4°C. The precipitate was collected by centrifugation at 10000 r/min for 30 min, dissolved in a 1/20 (v/v) phosphate buffer (0.02 mol/L, pH 6.8), and dialyzed for 48 h with 8 changes in the same buffer (500 ml each) to remove ammonium sulfate. The dialysates were condensed to yield precipitated proteins, which were further purified by column chromatography. A part of the precipitated proteins were dissolved in phosphate buffer (0.02 mol/L, pH 6.8), and the antifungal activity of the precipitated proteins was tested against Fusarium oxysporum by agar-diffusion method.
Chromatography was carried out on a DEAE-sepharose fast Flow ion exchange column previously equilibrated with 0.02 mol/L phosphate buffer (pH 6.8). The column was eluted with NaCl in phosphate buffer (0.02 mol/L, pH 6.8) to desorb the absorbed proteins (fractions I, II, III, IV). Each fraction was dialyzed in distilled water to remove NaCl, and then adjusted to the same concentration with phosphate buffer. Anti-fungal activity was tested against Fusarium oxysporum by agar- diffusion method. Fraction III was subsequently chromatographed on SephadexG-100. The column was eluted with 0.02 mol/L phosphate buffer (pH 6.8) to collect one main protein peak (P1) and another small protein peak (P2). The antifungal activity of the two fractions was tested. The protein concentration was determined using the method of Bradford (1976), with bovine serum albumin as a standard.
NanoLC-ESI-MS/MS analysis
The antifungal protein was separated by SDS-PAGE, analyzed by NanoLC-ESI-MS/MS, (ProtTech.Inc).
Results
Spectrum of antifungal activity
The antifungal spectra of B25 against FOC, Corynespora cassiicola, Alternaria solani, Botrytis cinerea, Colletotrichum gloeosporioides and Aspergillus niger, are shown in Table 1. The results indicated that B25 had a wide spectrum of antagonistic activities against plant pathogenic fungi.
Antifungal activity of precipitated proteins
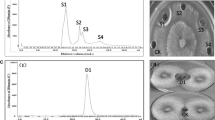
Antifungal factor from B25 was further identified as a kind disease-resistance protein by ammonium sulfate precipitation method. The protein extracted could inhibit the pathogen in PDA (potato dextrose agar) medium and a clear zone of inhibition was observed between the protein and pathogen (Figure 1).
Preliminary function of the antifungal protein
Inhibitory effect of precipitated proteins on FOC as seen under the light microscope demonstrated that it had inhibitory effect on mycelia and spores. The disease-resistance protein caused distortion, tumescence of hyphae and spores (Figure 2).
Isolation and purification of antifungal protein
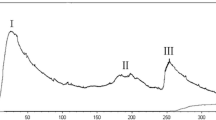
Ion exchange chromatography of B. subtilis B25 culture extract on a DEAE-sepharose. Fast Flow column contained one unabsorbed fraction and 4 adsorbed fractions (I, II, III, and IV) (Figure 3). Of these fractions, only fraction III showed strong antifungal activity (Figure 4), and fraction II showed a less antifungal activity. Fraction III was separated on the SephadexG-100 column into two fractions (P1 and P2) (Figure 5). Only fraction P1 exhibited antifungal activity (Figure 4) and showed a single band in SDS-PAGE (Figure 6). The protein yields at various stages of chromatographic purification are shown in Table 2.
Antifungal activity of fractions from DEAE-sepharose Fast Flow and SephadexG-100 chromatographies toward Fusarium oxysporum. A: control (10 μl 20 mmol/L Na2HPO 4/NaH2PO4 buffer (PB buffer), pH 6.8); B: fraction III from DEAE-sepharose Fast Flow column chromatography; C: 20 μg antifungal protein (fraction P1) in 10 μl PB buffer; D: 12 μg antifungal protein (fraction P1) in 10 μl PB buffer.
Amino acid sequence analysis
17 peptides were obtained by NanoLC-ESI-MS/MS. The amino acid sequences of 17 peptides segments were listed in Table 3. The protein mass was 38708.67 Da, and it’s isoelectric point (pI) was 5.63. After searching the protein database of NCBI for the identity, we found that it was a hypothetical protein, which was derived from the genome of Bacillus amyloliquefaciens FZB42(gi 154685475). Except for the amino sequence, no any other information such as function, location, mass, and isoelectric point had ever been reported. Now, we found that it can be secreted by the bacteria, and acted on the plant fungi in the environment.
Conclusion
The growing awareness of preservation o f the environment and health concerns drive the search for bio-safer and environmental friendly products than chemical pesticides. Increased concerns over the impact of chemicals on the environment have resulted in increase interest in biocontrol strategies. Biological control of Fusarium wilt by utilizing selected antagonistic bacteria might be an alternative approach.
The novel protein may be a potential biocontrol candidate and the report of it can supply additional literature on Bacillus subtilis antifungal proteins which control the fusarium wilt of banana. Further investigations of the nature and the structure, together with development of suitable application, could have great potential for the control of plant pathogenic fungi.
Discussion
The molecular mass of it was not only larger than any other small antibiotic peptides, but also different from those of other reported antifungal proteins from B. subtilis, such as Bacisubin (41.9 kDa), protein from B.s G87 (50.8 kDa), protease (41.38 kDa), bacillomycin D synthetase A (448.21 kDa). Additionally, it was demonstrated neither protease, nor ribonuclease activity. And the protein has wide antifungal spectrum, which include plant pathogens and Aspergillus niger The action of the protein on the bacteria was not tested.
Ethical standards
All participants gave written informed consent. All authors have no dispute on the order of the writers.
References
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72: 248-254. 10.1016/0003-2697(76)90527-3
Carrillo C, Teruel JA, Aranda FJ, Ortiz A: Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 2003, 1611(1–2):91-97. doi:10.1016/S0005 -2736 (03)00029-4
Chen X, Li J, Sun Q, Tong Y, Xu J: Isolation, purification and characterization of antifungal protein from rice endophytic bacterim Bacillus subtilis G87. Acta Microbiol Sinica 2010, 50: 1353.
Delcambe L, Peypoux F, Besson F, Guinand M, Michel G: Structure of iturin and iturin-like substance. Biochem Society Transac 1977, 5(4):1122-1124.
Lin BY: Partial properties and purification of antifungal substance from Bacillus subtilis B25 strain. Master thesis of Hainan University, China; 2013:1-51.
Lin BY, Zhang RY, Tan ZQ: Preliminary study of antimicrobial substance produced by Bacillus subtilis B25. Guangdong Agri Sci 2013, 1: 82-84.
Liu H: Identification of antagonistic bacteria against banana wilt and its preliminary application. Master thesis of Hainan University, China; 2011:1-50.
Liu Y, Chen Z, Ng TB, Zhang J, Zhou M, Song F, Lu F: Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides 2007, 28: 553-559. 10.1016/j.peptides.2006.10.009
Liu H, Tan ZQ, Zhang RY: Identification of a Strain of Antagonistic Bacterium against Fusarium oxy sp orum f. sp. cubense and Control Experiment of Bagged Seedlings in vitro. China Southern Fruit 2011, 40(3):36-39.
Loeffler W, Tschen JSM, Vanittanakom N, Kulger M, Knorpp E, Hsieh TF, Wu TG: Antifungal effects of bacilysin and fengycin from Bacillus subtilis F29-3. A comparison with activities of other Bacillus an-tibiotics. J Phytopathol 1986, 115(3):204-213. doi:10.1111/j.1439-0434. 1986. tb00878.x 10.1111/j.1439-0434.1986.tb00878.x
Luo Y, Sun L, Zhu Z, Ran W, Shen Q: Identification and characterization of an anti-fungi Fusarium oxysporum f. sp. cucumerium Protease from the Bacillus subtilis Strain N7. J Microbiol 2013, 51(3):359-366. 10.1007/s12275-013-2627-6
Peypoux F, Besson F, Michel G: Characterization oa new antibiotic of iturin group bacilloycin D. J Antibiot 1980, 33(10):1146-1149. 10.7164/antibiotics.33.1146
Peypoux F, Pommier MT, Marion D, Ptak M, Das BC, Michel G: Revised structure of mycosubtilin, a lipidolipid antibiotic from B. subtilis . J Antibiot 1986, 39(5):636-641. 10.7164/antibiotics.39.636
Peypoux F, Bonmatin JM, Wallach J: Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 1999, 51(5):553-563. doi:10.1007/s002530051432 10.1007/s002530051432
Sengupta S, Banerjee AB, Bose SK: γ-Glutamyl and D- or L-peptide linkages in mycobacillin, a cyclic peptide antibiotic. Biochem J 1971, 121: 839-846.
Stein T: Bacillus subtilis antibiotics: structure, syntheses and specific functions. Mol Microbiol 2005, 56: 845-857. 10.1111/j.1365-2958.2005.04587.x
Tsuge K, Akiyama T, Shoda M: Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol 2001, 183(21):6265-6273. doi:10.1128/JB.183.21 10.1128/JB.183.21.6265-6273.2001
Acknowledgments
This work was supported by grants from Hainan Natural Science Foundation (80819), Doctoral Science Foundation of Hainan University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
This study was funded by Hainan Natural Science Foundation and Doctoral Science Foundation of Hainan University.
Authors’ contributions
ZQT and RYZ made a substantial contribution to the conception and design of the study. BYL made a substantial contribution to the acquisition and analysis of the data. All authors had a hand in the preparation of this manuscript based on their interpretation of the data, and reviewed, approved and agreed upon the manuscript in its final format.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tan, Z., Lin, B. & Zhang, R. A novel antifungal protein of Bacillus subtilis B25. SpringerPlus 2, 543 (2013). https://doi.org/10.1186/2193-1801-2-543
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-1801-2-543