Abstract
Background
Non-coding RNA molecules, such as microRNAs, may play an important role in carcinogenesis. Recent studies have indicated that microRNAs are involved in initiation and progression of various malignancies. However, little work has been done to compare the microRNA expression patterns in oral cancer. In this study, we constructed an animal model of oral squamous cell carcinoma to investigate expression profiles of microRNAs in oral carcinogenesis.
Methods
The animal model of oral squamous cell carcinoma was conducted by tri-weekly (Monday, Wednesday, and Friday) painting with 5% DMBA in acetone. Six Syrian hamsters, including three from the treated group and three from the control group, were used as a training group for microRNA microarray analysis. All microarray data were analyzed by Significance Analysis of Microarrays (SAM) and CLUSTER 3.0 software, and this result was further confirmed by qRT-PCR assay.
Results
Seventeen microRNAs were differentially expressed in oral squamous cell carcinoma. Five microRNAs (hsa-miR-21, hsa-miR-200b, hsa-miR-221, hsa-miR-338, and mmu-miR-762) were significantly upregulated and twelve microRNAs (hsa-miR-16, hsa-miR-26a, hsa-miR-29a, hsa-miR-124a, hsa-miR-125b, mmu-miR-126-5p, hsa-miR-143, hsa-miR-145, hsa-miR-148b, hsa-miR-155, hsa-miR-199a, and hsa-miR-203) were down-regulated in cancer tissues. The expression levels of hsa-miR-21 and hsa-miR-16 seen with Stem-loop qRT-PCR were also seen in microarray analysis in all samples.
Conclusion
Our findings identified specific microRNA expression in oral squamous cell carcinoma and suggested that microRNAs have a role in oral carcinogenesis.
Similar content being viewed by others
Background
MicroRNAs (miRNAs) are small, noncoding RNAs (~20–22 nucleotides) that have critical functions in various biological processes [1]. These naturally occurring miRNAs function by binding to target mRNAs, resulting in the degradation or translational inhibition of the mRNA, based upon the degree of complementarity with it. First described in 1993 in the nematode Caenorhabditis elegans [2], to date, thousands of miRNAs have been cloned in higher eukaryotes and a number have been shown to play a role in cell proliferation, apoptosis, growth and morphogenesis [3–5]. At present, dysregulation of miRNAs has been shown to be involved in tumor initiation and progression.
The explosion of data on miRNAs and cancer has put them in the spotlight over the past few years. Numerous studies have highlighted the suspected role of miRNAs in tumorigenesis and have established that profiling of these miRNAs represents an informative method for determining developmental lineage and the differentiation state of various malignancies. The initial connection of miRNAs and cancer was elucidated in leukemia and hematological malignancies, later spurring interest in solid malignancies. For example, one of the first lines of evidence for direct involvement of miRNAs in cancer was the finding that miR-15 and miR-16 are located within a 30 kb deletion in chronic lymphocytic leukemia (CLL), and that both genes were deleted or underexpressed in most cases of this cancer [6]. Abnormal expression of microRNAs has been found in a variety of solid tumors, including colon, breast, lung, thyroid, glioblastomas, prostate, lymphomas, ovarian, hepatocellular, cervical, and pancreatic carcinomas [7–17].
Comparatively, oral cancer has received very little attention in this area of genome profiling. It is identified as a significant public health threat worldwide because its treatment often produces dysfunction and distortions in speech, mastication and swallowing, dental health, and even in the ability to interact socially. It is one of the 10 most frequent cancers in human males worldwide, with about two thirds of all cases occurring in develo** countries [18]. The most common type of oral cancer is squamous cell carcinoma. At present, the management of oral squamous cell carcinoma (OSCC) includes combinations of surgery, radiotherapy, and chemotherapy [19]. Despite improvements in these therapies, the 5-year survival rate has not improved significantly and remains at about 50% [20]. In clinical practice, treatment planning and prognosis for patients with OSCC are mainly based on the TNM classification. TNM classification provides significant diagnostic information concerning the tumor, but does not give the clinician sufficient therapeutic biological information, such as the metastatic potential or the sensitivity or resistance of the tumor to radiotherapy and chemotherapy [21]. There is an urgent need for diagnostic methods for distinguishing high-risk patients from other patients in order that optimal managements can be applied.
As such, some of the urgent priorities in this field are the need to identify and elucidate novel genes or pathways that may choreograph this disease. In the present study, by using the miRNA microarray technique, we have measured the relative expression of microRNAs in 7,12-dimethyl-benz- [a]-anthrance (DMBA)-induced OSCC in Syrian hamster. We hope that it can contribute to early diagnosis and treatment of this malignancy.
Methods
Animals
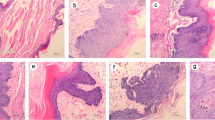
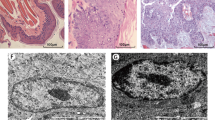
The construction of the animal model was conducted at West China College of Stomatology, Sichuan University. Twenty-four adult male (150 to 250 g) Syrian hamsters (6 weeks old; sydw, Sichuan, China) were randomly divided into two experimental groups (Group A and B) and one control group (Group C) (n = 8 for each group). After one week of acclimatization, both cheek pouches of each animal in the experimental groups were treated with 5% DMBA (Sigma, St Louis, MO, USA) in acetone. DMBA was applied tri-weekly (Monday, Wednesday and Friday) with a paintbrush. The animals from group A received carcinogen for about 12 weeks. Group B received carcinogen about 12 weeks, with an additional 6-week period of observation. Group C received no treatment and served as blank control. The animal grou**s and protocol of carcinogen application are summarized in Table 1.
All tissue samples were collected under pentobarbital anesthesia for histopathological examination and miRNA microarray. Six Syrian hamsters, including three from group A and B (12 wk, 18 wk, and 18 wk, respectively) and three from group C (blank control group), were used as a training group for miRNA microarray analysis. All of the handling measures used with the Syrian hamsters were in accordance with approved guidelines (Guidelines for the Care and Use of Laboratory Animals) established by the Chinese Council on Animal Care.
Fabrication of the miRNA microarray
The miRNA microarrays were obtained from CapitalBio Corporation (Bei**g, China), corresponding to the current release of the Sanger miRNA database (http://microrna.sanger.ac.uk; August 2007). The individual oligonucleotide probe was printed in triplicate on chemically modified glass slides in a 21 × 21 spot configuration of each subarray. The spot diameter was 130 mm, and distance from center to center was 185 mm. A total of 924 mature miRNA sequences were assembled and integrated into our miRNA microarray design. These microarray probes included 677 human miRNAs (including 122 predicted miRNA sequences) [ In summary, the specific miRNA expression levels identified by our study were similar with those reported in other studies, and suggested that a number of miRNAs could be significant in OSCC development. The next step will be to perform functional research of the three microRNAs (hsa-miR-338, mmu-miR-762, and mmu-miR-126-5p) that were not found to have been altered in any malignancies.Conclusion
Abbreviations
- DMBA:
-
7,12-dimethyl-benz [a]-anthrance
- CLL:
-
chronic lymphocytic leukemia
- OSCC:
-
oral squamous cell carcinoma
- SAM:
-
Significance Analysis of Microarrays.
References
Bartel DP: MicroRNAs: Genomics biogenesis, mechanism, and function. Cell. 2004, 116: 281-297. 10.1016/S0092-8674(04)00045-5.
Lee RC, Feinbaum RL, Ambrose V: The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993, 75: 843-854. 10.1016/0092-8674(93)90529-Y.
Carleton M, Cleary MA, Linsley PS: MicroRNAs and cell cycle regulation. Cell Cycle. 2007, 6: 2127-2132.
Miska EA: How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005, 15: 563-568. 10.1016/j.gde.2005.08.005.
Callis TE, Chen JF, Wang DZ: MicroRNAs in skeletal and cardiac muscle development. DNA Cell Biol. 2007, 26: 219-225. 10.1089/dna.2006.0556.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM: Frequent deletions and down-regulation of micro-RNA genes miR-15 and miR-16 at 13q14 in chronic lymphocytic leukemia. PNAS. 2002, 99: 15524-15529. 10.1073/pnas.242606799.
Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N: MicroRNA Expression Profiles Associated With Prognosis and Therapeutic Outcome in Colon Adenocarcinoma. JAMA. 2008, 299: 425-436. 10.1001/jama.299.4.425.
Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM: MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65: 7065-7070. 10.1158/0008-5472.CAN-05-1783.
Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M: Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006, 9: 189-198. 10.1016/j.ccr.2006.01.025.
He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A: The role of microRNA genes in papillary thyroid carcinoma. PNAS. 2005, 102: 19075-19080. 10.1073/pnas.0509603102.
Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G: Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005, 334: 1351-1358. 10.1016/j.bbrc.2005.07.030.
Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T: MicroRNA Expression Profiling in Prostate Cancer. Cancer Res. 2007, 67: 6130-6135. 10.1158/0008-5472.CAN-07-0533.
Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A: High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer. 2004, 39: 167-169. 10.1002/gcc.10316.
Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T: Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006, 25: 2537-2545. 10.1038/sj.onc.1209283.
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH: MicroRNA Expression Profiles in Serous Ovarian Carcinoma. Clin Cancer Res. 2008, 14: 2690-2695. 10.1158/1078-0432.CCR-07-1731.
Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O'brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G: MicroRNAs exhibit high frequency genomic alterations in human cancer. PNAS. 2006, 103: 9136-9141. 10.1073/pnas.0508889103.
Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP: MicroRNA Expression Patterns to Differentiate Pancreatic Adenocarcinoma From Normal Pancreas and Chronic Pancreatitis. JAMA. 2007, 297: 1901-1908. 10.1001/jama.297.17.1901.
Ferlay J, Bray F, Pisani P, Parkin DM: GLOBOCAN 2002 Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No.5, version 2.0. 2004, Lyon, France: IARC Press
Carvalho AL, Ikeda MK, Magrin J, Kowalski LP: Trends of oral and oropharyngeal cancer survival over five decades in 3267 patients treated in a single institution. Oral Oncol. 2004, 40: 71-76. 10.1016/S1368-8375(03)00138-6.
Kessler P, Grabenbauer G, Leher A, Bloch-Birkholz A, Vairaktaris E, Neukam FW: Neoadjuvant and adjuvant therapy in patients with oral squamous cell carcinoma Long-term survival in a prospective, non-randomized study. Br J Oral Maxillofac Surg. 2008, 46: 1-5. 10.1016/j.bjoms.2007.08.006.
de Aguiar AF, Kowalski LP, de Almeida OP: Clinicopathological and immunohistochemical evaluation of oral squamous cell carcinoma in patients with early local recurrence. Oral Oncol. 2007, 43: 593-601. 10.1016/j.oraloncology.2006.07.003.
**e X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K: Systematic discovery of regulatory motifs in human promoters and 3'UTRs by comparison of several mammals. Nature. 2005, 434: 338-345. 10.1038/nature03441.
Watanabe T, Takeda A, Mise K, Okuno T, Suzuki T, Minami N: Stage-specific expression of microRNAs during Xenopus development. FEBS Lett. 2005, 579: 318-324. 10.1016/j.febslet.2004.11.067.
Thomson JM, Parker J, Perou CM, Hammond SM: A custom microarray platform for analysis of microRNA gene expression. Nat Methods. 2004, 1: 47-53. 10.1038/nmeth704.
Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. PNAS. 2001, 98: 5116-5121. 10.1073/pnas.091062498.
Eisen MB, Spellman PT, Brown PO, Botstein D: Cluster analysis and display of genome-wide expression patterns. PNAS. 1998, 95: 14863-14868. 10.1073/pnas.95.25.14863.
Schmittgen TD, Jiang J, Liu Q, Yang L: A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Research. 2004, 32: e43-10.1093/nar/gnh040.
Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC: The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006, 24: 1151-1161. 10.1038/nbt1239.
Salley JJ: Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954, 33: 253-262.
Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, Dell'Aquila ML, Alder H, Rassenti L, Kipps TJ, Bullrich F, Negrini M, Croce CM: MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. PNAS. 2004, 101: 11755-11760. 10.1073/pnas.0404432101.
Patterson TA, Lobenhofer EK, Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, Fang H, Kawasaki ES, Hager J, Tikhonova IR, Walker SJ, Zhang L, Hurban P, de Longueville F, Fuscoe JC, Tong W, Shi L, Wolfinger RD: Performance comparison of one-color and twocolor platforms within the MicroArray Quality Control (MAQC) project. Nat Biotechnol. 2006, 24: 1140-1150. 10.1038/nbt1242.
Luo MY, Tian ZG, Xu Z: Construction and application of a microarray for profiling microRNA expression. Prog Biochem Biophys. 2007, 34: 31-41.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR: MicroRNA expression profiles classify human cancers. Nature. 2005, 435: 834-838. 10.1038/nature03702.
Tong AW, Nemunaitis J: Modulation of miRNA activity in human cancer: a new paradigm for cancer gene therapy?. Cancer Gene Ther. 2008, 15: 341-355. 10.1038/cgt.2008.8.
Chang SS, Jiang WW, Smith I, Poeta LM, Begum S, Glazer C, Shan S, Westra W, Sidransky D, Califano JA: MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008, 123: 2791-2797. 10.1002/ijc.23831.
Tran N, McLean T, Zhang XY, Zhao CJ, Thomson JM, O'Brien C, Rose B: MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Comm. 2007, 358: 12-17. 10.1016/j.bbrc.2007.03.201.
Yang N, Coukos G, Zhang L: MicroRNA epigenetic alterations in human cancer: One step forward in diagnosis and treatment. Int J Cancer. 2008, 122: 963-968. 10.1002/ijc.23325.
Chan JA, Krichevsky AM, Kosik KS: MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65: 6029-6033. 10.1158/0008-5472.CAN-05-0137.
Weiler J, Hunziker J, Hall J: Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease?. Gene Ther. 2006, 13: 496-502. 10.1038/sj.gt.3302654.
Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T: Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64: 3753-3756. 10.1158/0008-5472.CAN-04-0637.
Acknowledgements
We thank Liang Zhang, Jianqing Zhao, and Hongwei Liu (CapitalBio) for their technical assistance. This project was supported by National Natural Science Foundation of China (Grant 30550002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
In our study, all authors have contributed significantly, and that all authors are in agreement with the content of the manuscript. Each author's contribution to the paper:TY: First author, background literature search, data analysis, development of final manuscript
XYW: Corresponding author, research instruction, data analysis, development of final manuscript. RGG: background literature search, data analysis. AL: research instruction, development of final manuscript. SY: research instruction, background literature search. YTC: data analysis, background literature search. YMW: research instruction, development of final manuscript. CMW: research instruction, data analysis. XZY: background literature search, data analysis.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Yu, T., Wang, Xy., Gong, Rg. et al. The expression profile of microRNAs in a model of 7,12-dimethyl-benz[a]anthrance-induced oral carcinogenesis in Syrian hamster. J Exp Clin Cancer Res 28, 64 (2009). https://doi.org/10.1186/1756-9966-28-64
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-9966-28-64