Abstract
The importance of cardiac fibroblasts in the regulation of myocardial remodelling following myocardial infarction (MI) is becoming increasingly recognised. Studies over the last few decades have reinforced the concept that cardiac fibroblasts are much more than simple homeostatic regulators of extracellular matrix turnover, but are integrally involved in all aspects of the repair and remodelling of the heart that occurs following MI. The plasticity of fibroblasts is due in part to their ability to undergo differentiation into myofibroblasts. Myofibroblasts are specialised cells that possess a more contractile and synthetic phenotype than fibroblasts, enabling them to effectively repair and remodel the cardiac interstitium to manage the local devastation caused by MI. However, in addition to their key role in cardiac restoration and healing, persistence of myofibroblast activation can drive pathological fibrosis, resulting in arrhythmias, myocardial stiffness and progression to heart failure. The aim of this review is to give an appreciation of both the beneficial and detrimental roles of the myofibroblast in the remodelling heart, to describe some of the major regulatory mechanisms controlling myofibroblast differentiation including recent advances in the microRNA field, and to consider how this cell type could be exploited therapeutically.
Similar content being viewed by others
Review
Introduction
At the cellular level, heart tissue constitutes cardiomyocytes, cardiac fibroblasts, vascular and neuronal cells, as well as inflammatory cells under certain pathological conditions. In the healthy heart, cardiac fibroblasts are the most prevalent cell type, accounting for up to 70% of cells, depending on the species in question [1, 2]. Although cardiac fibroblasts have been much less well studied than cardiomyocytes, it is becoming increasingly apparent that the fibroblasts (and their differentiated phenotype, myofibroblasts) are integral to the development, normal function and repair of the heart, as well as contributing to adverse myocardial remodelling, fibrosis and heart failure progression [3, 4]. Through physical and biochemical communication with myocytes and other cell types in the heart and the cardiac extracellular matrix (ECM), fibroblasts are well placed to sense and respond to stress or injury to the myocardium.
Fibroblasts are a heterogeneous population of cells, reflecting both their multiple developmental origins and their exposure to differential physical and chemical microenvironments. Fibroblasts derived from different anatomical sites have been proposed to effectively represent distinct differentiated cell types as they exhibit unique transcriptional signatures that probably reflect phenotypic differences [5]. Such diversity has made precise characterisation of fibroblasts challenging, and there remains no truly unique single marker that unequivocally identifies a cell as a fibroblast [6].
Although fibroblasts have the capacity to proliferate, migrate and regulate ECM turnover to maintain cardiac homeostasis, they are also able to undergo differentiation into a more contractile and synthetic myofibroblast phenotype to aid with cardiac repair following myocardial infarction (MI) [7–9]. Myofibroblasts are not normally found in the healthy myocardium, but are the most prevalent cell type in the infarct scar and are the main effectors of fibrogenesis [10]. Myofibroblasts are characterised by increased expression of particular contractile proteins (for example, α-smooth muscle actin, SMemb, vimentin), focal adhesion proteins (for example, paxillin, tensin, αVβ3 integrin), cell surface receptors (for example, transforming growth factor beta (TGF-β) type II receptor, angiotensin AT1 receptor, Frizzled-2), structural ECM proteins (collagen I, collagen III, fibronectin extra domain A splice variant (FN-ED-A)) and matricellular proteins (for example, periostin, osteopontin, tenascin C) [7–9]. Cardiac myofibroblasts are also highly proliferative, and those isolated from infarcted myocardium exhibit a higher rate of proliferation than cardiac fibroblasts from remote areas [11, 12]. Although myofibroblasts are able to actively migrate to the infarcted region of the heart [13], a process regulated by Wnt/Frizzled signalling [14, 15], they also appear to become less migratory as expression levels of contractile proteins increase [11, 16]. Together these phenotypic changes confer increased tensile and ECM-secretory characteristics on the cells, enabling them to effectively facilitate the wound healing process.
Beneficial and detrimental roles of myofibroblasts
Appreciating the dual roles of cardiac myofibroblasts in the myocardial remodelling process is important, as they can be perceived to be both beneficial and detrimental depending on their prevalence and their temporal and spatial location. The infarct scar is not a simple acellular structure comprising structural ECM molecules; on the contrary, it contains myofibroblasts that maintain a viable, dynamic scar important for maintaining myocardial integrity against a background of continuous mechanical forces associated with the pum** of the heart [17]. Myofibroblasts are essential for rapid and robust (that is, strong and flexible) scar formation following MI. Interference with myofibroblast recruitment can result in infarct expansion, ventricular wall thinning, dilatation, systolic dysfunction and propensity to rupture [7] (Figure 1). Conversely, myofibroblast persistence can contribute to fibrosis and adverse myocardial remodelling, particularly if the myofibroblasts remain active in otherwise healthy areas of the heart away from the original site of injury (reactive fibrosis) [7]. Areas of increased ECM protein deposition can disturb the electrical conductance of the myocardium, thus increasing the likelihood of arrhythmias [18]. Moreover, direct coupling of cardiomyocytes to myofibroblasts, as opposed to fibroblasts, may also promote arrhythmias [19, 20]. Fibrosis in the remote myocardium inevitably leads to increased myocardial stiffness, resulting in systolic and diastolic dysfunction, neurohormonal activation and, ultimately, heart failure [21, 22] (Figure 1).
Summary of the influence of myofibroblast density on post-myocardial infarction remodelling. Low myofibroblast density in the infarct area results in a poorly structured, expansive and vulnerable scar that is prone to rupture or leads to systolic dysfunction and subsequent adverse myocardial remodelling. Although high myofibroblast density is important for a robust, contractile scar, excessive myofibroblast numbers (particularly in the remote myocardium away from the original infarct) drives fibrosis and myocardial stiffness, resulting in contractile dysfunction, arrhythmia and heart failure progression.
Origin of myofibroblasts
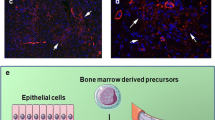
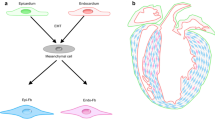
The differential origin of myofibroblasts in the remodelling heart has become a hot topic in recent years [6, 23]. Although once assumed to be solely derived from differentiation of resident fibroblasts, it is now apparent that cardiac myofibroblasts can also be derived from a multitude of alternative cellular precursors. These precursors include epithelial cells (through a process termed epithelial–mesenchymal transition), endothelial cells (through endothelial–mesenchymal transition; EndMT), mesenchymal stem cells, bone marrow-derived circulating progenitor cells (fibrocytes), smooth muscle cells and pericytes [6, 23]. The recruitment of myofibroblasts from such diverse origins underlines their importance in the cardiac repair process, and probably represents optimised responses to different types of stress or injury. However, reports on the precise proportions of cells derived from different sources in different experimental models have varied considerably, so consensus has yet to be reached on the relative importance of myofibroblasts derived from resident cardiac fibroblasts versus extra-cardiac sources [6]. Another important aspect is whether these data are recapitulated in the human scenario. Nevertheless, a picture is now emerging that the source of myofibroblasts in the remodelling heart may depend heavily upon the nature of the initiating stimulus or injury. For example, whereas resident mesenchymal stem cells have been identified as important contributors to the myofibroblast population that drives post-MI scar formation, fibrocyte-derived myofibroblasts may be more important for interstitial fibrosis in the absence of MI [24]. Such knowledge opens up the exciting prospect that selective targeting of distinct myofibroblast populations could be used to protect essential repair mechanisms following MI, whilst reducing remote fibrosis and subsequent adverse myocardial remodelling.
Factors stimulating myofibroblast differentiation
Phenotypic conversion of resident cardiac fibroblasts to myofibroblasts requires integration of both mechanical and biochemical stimuli. Fibroblasts are mechanosensitive and are therefore able to detect the loss of integrity of the ECM that occurs following MI. In response to increased mechanical stress and platelet-derived growth factor, fibroblasts adopt a partially differentiated phenotype known as the proto-myofibroblast [8]. Conversion of the proto-myofibroblast to the fully differentiated myofibroblast occurs in response to additional biochemical signals, particularly increased levels of active TGF-β and FN-ED-A [8], the levels of which are elevated in the damaged region of the heart post MI [25, 26]. Such a phenotypic conversion is also promoted when cardiac fibroblasts are grown in vitro on rigid plastic surfaces; hence studies on cultured cardiac fibroblasts are generally indicative of myofibroblast behaviour [16, 27]. TGF-β is normally present in the interstitium in a latent form, which can be rapidly activated by protease-mediated cleavage of the latency-associated peptide [28]. However, it has also been demonstrated that TGF-β activation can be stimulated directly by mechanical strain without the need for protease activity [29], and this mechanosensitive mechanism probably plays an important role in early myofibroblast conversion.
A number of additional stimuli that promote differentiation to the myofibroblast phenotype have been reported, including specific cytokines, growth factors and ECM molecules; several of which elicit their effects through up regulation of TGF-β activity and/or signalling [30]. There is also emerging evidence for an important role for the transient receptor potential family of ion channels in regulating cardiac myofibroblast differentiation. For example, the TRPM7 channel [89].
Molecular tools for manipulating miR levels (through inhibition or mimicry) have been an area of rapid development and ongoing refinement [88]. As discussed above, several promising miR targets have been identified that appear to regulate myofibroblast differentiation and/or function (Figure 2). Preclinical studies manipulating miR-21 and miR-29 have shown beneficial effects on post-MI cardiac remodelling in rodents. Specifically, a miR-29 mimetic has proven successful in a murine model of cardiac fibrosis [56] and miR-21 inhibition increased survival after MI [55].
Progressive expansion of our knowledge concerning dysregulation of miRs in cardiac (myo)fibroblast phenotype and function will undoubtedly lead to strategies that optimise targeted delivery of miR therapeutics. The ability to deliver therapies directly to selected cell types is indeed a realistic option for future medicine.
Conclusions
Cardiac myofibroblasts represent a unique, yet developmentally diverse, population of cells that play key roles in post-MI infarct healing, but also in adverse cardiac remodelling, fibrosis and progression to heart failure. Improved understanding of not only the origins of myofibroblasts in the post-MI heart, but also the capacity to assign specific roles and regulatory mechanisms to them, creates optimism for the future that this multifunctional cell type can be manipulated therapeutically to optimise infarct scar formation, whilst ameliorating reactive fibrosis.
Abbreviations
- CTGF:
-
Connective tissue growth factor
- ECM:
-
Extracellular matrix
- EndMT:
-
Endothelial–mesenchymal transition
- FN-ED-A:
-
Fibronectin extra domain A splice variant
- IL:
-
Interleukin
- MCP-1:
-
Monocyte chemotactic protein 1
- MI:
-
Myocardial infarction
- miR:
-
microRNA
- MRTF-A:
-
Myocardin-related transcription factor-A
- TNF:
-
Tumour necrosis factor
- TGF-β:
-
Transforming growth factor beta
References
Jugdutt BI: Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough?. Circulation. 2003, 108: 1395-1403. 10.1161/01.CIR.0000085658.98621.49.
Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA: Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007, 293: H1883-H1891. 10.1152/ajpheart.00514.2007.
Porter KE, Turner NA: Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009, 123: 255-278. 10.1016/j.pharmthera.2009.05.002.
Turner NA: The Cardiac Fibroblast. 2011, Kerala: Research Signpost
Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO: Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002, 99: 12877-12882. 10.1073/pnas.162488599.
Krenning G, Zeisberg EM, Kalluri R: The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010, 225: 631-637. 10.1002/jcp.22322.
van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J: Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010, 7: 30-37. 10.1038/nrcardio.2009.199.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002, 3: 349-363. 10.1038/nrm809.
Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De WO, Mareel M, Gabbiani G: Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012, 180: 1340-1355. 10.1016/j.ajpath.2012.02.004.
Peterson DJ, Ju H, Hao J, Panagia M, Chapman DC, Dixon IM: Expression of Gi-2 alpha and Gs alpha in myofibroblasts localized to the infarct scar in heart failure due to myocardial infarction. Cardiovasc Res. 1999, 41: 575-585. 10.1016/S0008-6363(98)00264-8.
Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML: Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005, 39: 699-707. 10.1016/j.yjmcc.2005.07.008.
Beguin PC, Gosselin H, Mamarbachi M, Calderone A: Nestin expression is lost in ventricular fibroblasts during postnatal development of the rat heart and re-expressed in scar myofibroblasts. J Cell Physiol. 2012, 227: 813-820. 10.1002/jcp.22794.
El-Helou V, Gosselin H, Villeneuve L, Calderone A: The plating of rat scar myofibroblasts on matrigel unmasks a novel phenotype; the self assembly of lumen-like structures. J Cell Biochem. 2012, 113: 2442-2450. 10.1002/jcb.24117.
Laeremans H, Rensen SS, Ottenheijm HC, Smits JF, Blankesteijn WM: Wnt/frizzled signalling modulates the migration and differentiation of immortalized cardiac fibroblasts. Cardiovasc Res. 2010, 87: 514-523. 10.1093/cvr/cvq067.
Laeremans H, Hackeng TM, van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, Smits JF, Blankesteijn WM: Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011, 124: 1626-1635. 10.1161/CIRCULATIONAHA.110.976969.
Santiago JJ, Dangerfield AL, Rattan SG, Bathe KL, Cunnington RH, Raizman JE, Bedosky KM, Freed DH, Kardami E, Dixon IM: Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010, 239: 1573-1584. 10.1002/dvdy.22280.
Sun Y, Kiani MF, Postlethwaite AE, Weber KT: Infarct scar as living tissue. Basic Res Cardiol. 2002, 97: 343-347. 10.1007/s00395-002-0365-8.
Rohr S: Myofibroblasts in diseased hearts: new players in cardiac arrhythmias?. Heart Rhythm. 2009, 6: 848-856. 10.1016/j.hrthm.2009.02.038.
Thompson SA, Copeland CR, Reich DH, Tung L: Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation. 2011, 123: 2083-2093. 10.1161/CIRCULATIONAHA.110.015057.
Rosker C, Salvarani N, Schmutz S, Grand T, Rohr S: Abolishing myofibroblast arrhythmogeneicity by pharmacological ablation of alpha-smooth muscle actin containing stress fibers. Circ Res. 2011, 109: 1120-1131. 10.1161/CIRCRESAHA.111.244798.
Pfeffer MA, Braunwald E: Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990, 81: 1161-1172. 10.1161/01.CIR.81.4.1161.
Swynghedauw B: Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999, 79: 215-262.
Zeisberg EM, Kalluri R: Origins of cardiac fibroblasts. Circ Res. 2010, 107: 1304-1312. 10.1161/CIRCRESAHA.110.231910.
Crawford JR, Haudek SB, Cieslik KA, Trial J, Entman ML: Origin of developmental precursors dictates the pathophysiologic role of cardiac fibroblasts. J Cardiovasc Transl Res. 2012, 5: 749-759. 10.1007/s12265-012-9402-7.
Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP: Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011, 108: 582-592. 10.1161/CIRCRESAHA.110.224428.
Deten A, Holzl A, Leicht M, Barth W, Zimmer HG: Changes in extracellular matrix and in transforming growth factor beta isoforms after coronary artery ligation in rats. J Mol Cell Cardiol. 2001, 33: 1191-1207. 10.1006/jmcc.2001.1383.
Mughal RS, Warburton P, O'Regan DJ, Ball SG, Turner NA, Porter KE: Peroxisome proliferator-activated receptor γ-independent effects of thiazolidinediones on human cardiac myofibroblast function. Clin Exp Pharmacol Physiol. 2009, 36: 478-486. 10.1111/j.1440-1681.2008.05088.x.
Dobaczewski M, Chen W, Frangogiannis NG: Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011, 51: 600-606. 10.1016/j.yjmcc.2010.10.033.
Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B: The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol. 2011, 21: 2046-2054. 10.1016/j.cub.2011.11.037.
Kis K, Liu X, Hagood JS: Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011, 13: e27.
Du J, **e J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L: TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010, 106: 992-1003. 10.1161/CIRCRESAHA.109.206771.
Adapala RK, Thoppil R, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK: TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013, 54: 45-52.
Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD: A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012, 23: 705-715. 10.1016/j.devcel.2012.08.017.
Van Nieuwenhoven FA, Turner NA: The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vasc Pharmacol. 2013, 58: 185-190.
Fedak PW, Bai L, Turnbull J, Ngu J, Narine K, Duff HJ: Cell therapy limits myofibroblast differentiation and structural cardiac remodeling: basic fibroblast growth factor-mediated paracrine mechanism. Circ Heart Fail. 2012, 5: 349-356. 10.1161/CIRCHEARTFAILURE.111.965889.
Desmouliere A, Redard M, Darby I, Gabbiani G: Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995, 146: 56-66.
Takemura G, Ohno M, Hayakawa Y, Misao J, Kanoh M, Ohno A, Uno Y, Minatoguchi S, Fujiwara T, Fujiwara H: Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res. 1998, 82: 1130-1138. 10.1161/01.RES.82.11.1130.
Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG: Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006, 324: 475-488. 10.1007/s00441-005-0144-6.
Amerongen MJ, Bou-Gharios G, Popa E, Van AJ, Petersen AH, Van Dam GM, Van Luyn MJ, Harmsen MC: Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008, 214: 377-386. 10.1002/path.2281.
Frangogiannis NG, Michael LH, Entman ML: Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovasc Res. 2000, 48: 89-100. 10.1016/S0008-6363(00)00158-9.
Willems IE, Havenith MG, De Mey JG, Daemen MJ: The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994, 145: 868-875.
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG: The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010, 48: 504-511. 10.1016/j.yjmcc.2009.07.015.
Li Y, Takemura G, Kosai K, Takahashi T, Okada H, Miyata S, Yuge K, Nagano S, Esaki M, Khai NC, Goto K, Mikami A, Maruyama R, Minatoguchi S, Fujiwara T, Fujiwara H: Critical roles for the Fas/Fas ligand system in postinfarction ventricular remodeling and heart failure. Circ Res. 2004, 95: 627-636. 10.1161/01.RES.0000141528.54850.bd.
Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS: Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One. 2012, 7: e39969-10.1371/journal.pone.0039969.
Cunnington RH, Wang B, Ghavami S, Bathe KL, Rattan SG, Dixon IM: Antifibrotic properties of c-Ski and its regulation of cardiac myofibroblast phenotype and contractility. Am J Physiol Cell Physiol. 2011, 300: C176-C186. 10.1152/ajpcell.00050.2010.
Rosenkranz S: TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004, 63: 423-432. 10.1016/j.cardiores.2004.04.030.
Brown RD, Ambler SK, Mitchell MD, Long CS: The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005, 45: 657-687. 10.1146/annurev.pharmtox.45.120403.095802.
Udali S, Guarini P, Moruzzi S, Choi SW, Friso S: Cardiovascular epigenetics: From DNA methylation to microRNAs. Mol Aspects Med. 2013, 10.1016/j.mam.2012.08.001. Epub ahead of print
Bartel DP: MicroRNAs: target recognition and regulatory functions. Cell. 2009, 136: 215-233. 10.1016/j.cell.2009.01.002.
Mann J, Mann DA: Epigenetic regulation of wound healing and fibrosis. Curr Opin Rheumatol. 2013, 25: 101-107. 10.1097/BOR.0b013e32835b13e1.
Orenes-Pinero E, Montoro-Garcia S, Patel JV, Valdes M, Marin F, Lip GY: Role of microRNAs in cardiac remodelling: new insights and future perspectives. Int J Cardiol. 2013, 10.1016/j.ijcard.2012.09.120. Epub ahead of print
Ghosh AK, Nagpal V, Covington JW, Michaels MA, Vaughan DE: Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): differential expression of microRNAs during EndMT. Cell Signal. 2012, 24: 1031-1036. 10.1016/j.cellsig.2011.12.024.
Kumarswamy R, Volkmann I, Jazbutyte V, Dangwal S, Park DH, Thum T: Transforming growth factor-beta-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012, 32: 361-369. 10.1161/ATVBAHA.111.234286.
Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S: MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008, 456: 980-984. 10.1038/nature07511.
Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK: MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009, 82: 21-29. 10.1093/cvr/cvp015.
van Rooij E, Sutherland LB, Thatcher JE, Dimaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN: Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008, 105: 13027-13032. 10.1073/pnas.0805038105.
Park SY, Lee JH, Ha M, Nam JW, Kim VN: miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009, 16: 23-29. 10.1038/nsmb.1533.
Wang J, Huang W, Xu R, Nie Y, Cao X, Meng J, Xu X, Hu S, Zheng Z: MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med. 2012, 16: 2150-2160. 10.1111/j.1582-4934.2012.01523.x.
Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G: MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007, 13: 613-618. 10.1038/nm1582.
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, VandM I, Herias V, Van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE: miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009, 104: 170-178. 10.1161/CIRCRESAHA.108.182535.
Porter KE, Turner NA: Statins and myocardial remodelling: cell and molecular pathways. Expert Rev Mol Med. 2011, 13: e22.
Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SK, Lonning S, Ling H, Ertl G, Bauersachs J: Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008, 103: 485-492. 10.1007/s00395-008-0739-7.
Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, Kubota T, Takeshita A: Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004, 64: 526-535. 10.1016/j.cardiores.2004.07.017.
Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG: Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010, 107: 418-428. 10.1161/CIRCRESAHA.109.216101.
Cunnington RH, Nazari M, Dixon IM: c-Ski, Smurf2, and Arkadia as regulators of TGF-beta signaling: new targets for managing myofibroblast function and cardiac fibrosis. Can J Physiol Pharmacol. 2009, 87: 764-772. 10.1139/Y09-076.
Czubryt MP: Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair. 2012, 5: 19-10.1186/1755-1536-5-19.
Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T: Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005, 167: 71-80. 10.1016/S0002-9440(10)62954-9.
Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K: Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2010, 298: H1072-H1078. 10.1152/ajpheart.00255.2009.
Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A: Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008, 205: 295-303. 10.1084/jem.20071297.
Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, Conway SJ, Aronow BJ, Robbins J, Molkentin JD: Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007, 101: 313-321. 10.1161/CIRCRESAHA.107.149047.
Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML: Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005, 111: 2935-2942. 10.1161/CIRCULATIONAHA.104.510354.
**a Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG: Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011, 58: 902-911. 10.1161/HYPERTENSIONAHA.111.175323.
Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, Doi M, Murakami T, Ninomiya Y, Takigawa M, Tsuji T: Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998, 30: 2411-2422. 10.1006/jmcc.1998.0799.
Daniels A, Van Bilsen M, Goldschmeding R, van Der Vusse GJ, van Nieuwenhoven FA: Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf). 2009, 195: 321-338. 10.1111/j.1748-1716.2008.01936.x.
Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, Van Leeuwen RE, D'Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S: Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009, 206: 113-123.
McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, Nguyen N, ** YF, Bradshaw AD, Lindsey ML: SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011, 301: H497-H505. 10.1152/ajpheart.01070.2010.
Hermans KC, Daskalopoulos EP, Blankesteijn WM: Interventions in Wnt signaling as a novel therapeutic approach to improve myocardial infarct healing. Fibrogenesis Tissue Repair. 2012, 5: 16-10.1186/1755-1536-5-16.
Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF: The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001, 276: 10229-10233. 10.1074/jbc.M100099200.
Kuwahara K, Kinoshita H, Kuwabara Y, Nakagawa Y, Usami S, Minami T, Yamada Y, Fujiwara M, Nakao K: Myocardin-related transcription factor A is a common mediator of mechanical stress- and neurohumoral stimulation-induced cardiac hypertrophic signaling leading to activation of brain natriuretic peptide gene expression. Mol Cell Biol. 2010, 30: 4134-4148. 10.1128/MCB.00154-10.
Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN: Myocardin-related transcription factor-α controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010, 107: 294-304. 10.1161/CIRCRESAHA.110.223172.
Ekert JE, Murray LA, Das AM, Sheng H, Giles-Komar J, Rycyzyn MA: Chemokine (C-C motif) ligand 2 mediates direct and indirect fibrotic responses in human and murine cultured fibrocytes. Fibrogenesis Tissue Repair. 2011, 4: 23-10.1186/1755-1536-4-23.
Morimoto H, Takahashi M, Izawa A, Ise H, Hongo M, Kolattukudy PE, Ikeda U: Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res. 2006, 99: 891-899. 10.1161/01.RES.0000246113.82111.2d.
Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML: Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010, 49: 499-507. 10.1016/j.yjmcc.2010.05.005.
Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, Entman ML: Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009, 83: 511-518. 10.1093/cvr/cvp135.
Frangogiannis NG, Dewald O, **a Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML: Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007, 115: 584-592. 10.1161/CIRCULATIONAHA.106.646091.
Boyle AJ, Yeghiazarians Y, Shih H, Hwang J, Ye J, Sievers R, Zheng D, Palasubramaniam J, Palasubramaniam D, Karschimkus C, Whitbourn R, Jenkins A, Wilson AM: Myocardial production and release of MCP-1 and SDF-1 following myocardial infarction: differences between mice and man. J Transl Med. 2011, 9: 150-10.1186/1479-5876-9-150.
Jessup M, Brozena S: Heart failure. N Engl J Med. 2003, 348: 2007-2018. 10.1056/NEJMra021498.
Montgomery RL, van Rooij E: Therapeutic advances in MicroRNA targeting. J Cardiovasc Pharmacol. 2011, 57: 1-7. 10.1097/FJC.0b013e3181f603d0.
van Rooij E, Purcell AL, Levin AA: Develo** microRNA therapeutics. Circ Res. 2012, 110: 496-507. 10.1161/CIRCRESAHA.111.247916.
Acknowledgements
Research on cardiac fibroblasts in the authors’ laboratories is funded by the British Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Both NAT and KEP contributed to the writing of the manuscript and approved its final submission.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Turner, N.A., Porter, K.E. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair 6, 5 (2013). https://doi.org/10.1186/1755-1536-6-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1755-1536-6-5