Abstract
Background
Marinesco-Sjögren syndrome (MSS) is an autosomal recessive multisystem disorder characterized by the tetralogy of cerebellar ataxia, congenital cataracts, intellectual disability, and progressive muscle weakness due to myopathy. MSS is extremely rare, and its clinical, pathological, and genetic features are not yet fully understood.
Methods
We conducted a nationwide, questionnaire-based survey on MSS in Japan and carefully reviewed the medical records of 36 patients suspected of having this disease. In addition, pathological examinations of muscles, sequence and haplotype analysis in SIL1 were performed.
Results
The patients had been examined between the ages of 2 and 52 years. Delayed psychomotor development and cataracts from early childhood were observed in all patients, whereas no life-threatening events were observed. Mutations in SIL1 were identified in 24 of the 27 patients tested, and 43 of the 48 chromosomes possessed the SIL1 c.936dupG (p.Leu313fs) mutation. The haplotype analysis revealed that 31 of the 32 chromosomes (96.9%) with the c.936dupG mutation had the same haplotype.
Conclusions
The results of haplotype analysis suggested the presence of a founder effect. The clinical features of patients without SIL1 mutations were indistinguishable from those with SIL1 mutations, suggesting the genetic heterogeneity of MSS.
Similar content being viewed by others
Background
Marinesco-Sjögren syndrome (MSS; OMIM 248800) is an autosomal recessive multisystem disorder clinically characterized by the tetralogy of cerebellar ataxia, congenital cataracts, intellectual disability, and progressive muscle weakness due to myopathy [1–3]. Additional clinical features, including short stature, hypergonadotropic hypogonadism [4], and strabismus [5], are also observed. Mutations in SIL1 (Gene ID: 64374) were reported to be causative for MSS [6, 7]. This gene encodes SIL1, also known as BiP-associated protein (BAP), which is an endoplasmic reticulum (ER)-resident protein. Bip is an HSP70 chaperone family member located in the ER, and plays a key role in protein quality control. SIL1 regulates the ATPase cycle of BiP for proper protein folding [8, 9]. SIL1-deficient woozy mutant mice exhibit progressive ataxia caused by the loss of Purkinje cells via ER stress [10].
MSS is an extremely rare disease, and very few cases have been reported. In this study, we performed a nationwide, questionnaire-based survey on MSS with the aim of characterizing its prevalence, clinical features, natural history, muscle pathological findings, and mutation status.
Methods
All clinical materials used in this study were obtained for diagnostic purposes with written informed consent. All surveys and experiments performed in this study were approved by the Ethical Committee of the National Center of Neurology and Psychiatry.
The nationwide, questionnaire-based survey
To elucidate the clinical characteristics of MSS, we conducted a nationwide, questionnaire-based survey in Japan. The first set of questionnaires, which focused on the experience of treating patients suspected of having MSS, was sent to a total of 5,452 Japanese specialists for neurology or pediatric neurology. The second set of questionnaires, which focused on the clinical information of patients with suspected MSS, was sent to their attending physicians. These patients’ medical records were carefully reviewed by 2 of our specialists (M.G., H.K.).
Histochemistry
Biopsied muscle specimens were flash-frozen in isopentane cooled in liquid nitrogen. Transverse serial frozen sections of 10 μm in thickness were subjected to various types of histochemical staining, including hematoxylin and eosin (H&E), modified Gomori-trichrome (mGT), and ATPases. We obtained biopsied skeletal muscles from a total of 17 unrelated patients clinically suspected of having MSS.
Sequence analysis of SIL1
Genomic DNA was extracted from either frozen muscle or peripheral blood lymphocytes using standard protocols. The PCR primers were designed to amplify all the exons of SIL1 together with their flanking intronic regions. The primer sequences are available upon request. Direct sequencing was performed using the BigDye Terminator v3.1 Cycle Sequencing system and an ABI3100 automated Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequence data obtained were analyzed using the SeqScape (Applied Biosystems) program and compared with the genomic sequence of SIL1 in the database (NM_022464). Genetic analysis was performed on DNA from 27 unrelated patients and the parents of 3 of them. Two hundred control chromosomes from healthy individuals were examined for each novel mutation in SIL1 by direct sequencing.
Haplotype analysis of SIL1
For the haplotype analysis of the SIL1 genomic region, we performed direct sequencing of the following 11 common single nucleotide variants (SNVs) in the Japanese population: rs11748097, rs929775, rs10045761, rs1433008, rs11958050, rs7717375, rs7722413, rs3763016, rs6596456, rs700629, and rs12653845 (http://www.ncbi.nlm.nih.gov/SNP/). Samples from 21 patients homozygous for the common c.936dupG mutation, the parents of 3 patients, and 92 control Japanese individuals were analyzed.
Results
Patients
A total of 1,875 responses (34.4% response rate) were received to the first set of questionnaires. The second set of questionnaires was sent to a total of 37 attending physicians (2.0%) who had treated patients suspected of having MSS. The detailed clinical records of a total of 36 patients were carefully reviewed. The shortfall in the number of patients was due to an overlap of 1 case.
Sequence analysis of SIL1
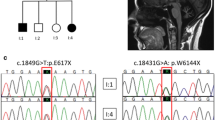
Frozen muscle or peripheral blood lymphocytes were obtained from 27 of the 36 patients suspected of having MSS, for genetic analysis. Mutations in SIL1 were identified in 24 out of the 27 patients. Twenty-one patients were homozygous for the previously reported c.936dupG (p.Leu313fs) mutation in exon 9. Patient 4 was homozygous for the previously reported c.603_607del5 (p.Glu201fs) mutation in exon 6 [11]. Patient 12 was homozygous for the previously reported c.331C > T (p.Arg111X) mutation in exon 4 [6, 7]. Patient 17 was a compound heterozygote for the novel c.617_618TC > AA (p.Leu206Glu) mutation in exon 6 and the c.936dupG (p.Leu313fs) mutation in exon 9 [12]. All mutations except for p.Leu206Glu are predicted to induce premature termination. Mutation of Leu206, which is highly conserved among species, was predicted to exert a deleterious impact on protein function by the Polymorphism Phenoty** v2 (PolyPhen-2; http://genetics.bwh.harvard.edu/pph2/) and the Sorting Intolerant From Tolerant software (SIFT: http://sift.bii.a-star.edu.sg/). None of these nucleic acid changes were found in the 200 chromosomes from healthy Japanese controls or in the Japanese Single Nucleotide Polymorphisms database (http://snp.ims.u-tokyo.ac.jp/index_ja.html).
Haplotype analysis of SIL1
The SIL1 c.936dupG mutation was identified in 43 of the 48 chromosomes (89.6%) in our cohort. The patients carrying this mutation were reported from different areas in Japan. To determine whether this was a result of a founder effect, we performed a haplotype analysis using 11 SNVs within or close to SIL1. The results revealed that 31 of the 32 chromosomes (96.9%) with the c.936dupG mutation had the same haplotype (P1-P24, Table 1). This haplotype was only found in 18 of the 184 chromosomes (9.8%) from the control group, suggesting a founder effect, although 1 chromosome from Patient 1 had a different haplotype.
Clinical features
Table 2 and Additional file 1: Table S1 show a clinical summary of the patients in our series.
The age at which the examination was performed in the 24 patients (10 men and 14 women) with SIL1 mutations varied from 2 to 52 (mean = 20.1 ± 18.1) years. Bilateral cataracts requiring prompt surgical intervention had appeared and rapidly progressed in all 24 patients at the mean age of 3.5 ± 1.2 years. Strabismus was also observed in 55.6% (10/18) of the patients. Cerebellar signs included hypotonia (21/24; 88%), ataxia (16/24; 67%), nystagmus (11/24; 46%), and dysarthria (8/24; 33%) were seen. Brain MRI demonstrated marked atrophy of the cerebellum, particularly the vermis, in all the patients examined (19/19). Mild to moderate intellectual disability, diagnosed by intelligence quotient/developmental quotient between 35 and 70, was seen in 91% (20/22) of the patients. Acquisition of meaningful words occurred at the age of 2.0 ± 0.8 years, and most of the patients had required special-needs education. Muscle weakness was observed in 95% (21/22) of the patients, with delays in motor milestones. Head control was first seen in all the patients at a certain time point between 4 and 18 (mean = 7.8 ± 3.7) months, and sitting at a certain time point between 10 and 36 (mean = 20.0 ± 8.7) months. Eighty percent (16/20) of the patients could stand with support at a certain point between the ages of 1 and 4 years, and walk with support at a certain point between the ages of 2 and 22 (mean = 5.8 ± 2.6) years; however, none of the patients acquired the ability to walk independently. Muscle weakness was slowly progressive and predominantly in the proximal muscles, with the patients becoming wheelchair-bound at a certain time point between the ages of 13 and 28 (mean = 17.4 ± 6.3) years. Serum creatine kinase levels were normal to moderately elevated (28–2000, mean = 389 ± 464; normal < 200 IU/L). Short stature (< -2 SD) was seen in 67% (12/18, mean = -3.6 SD) of the patients, and spinal deformity (8/22; 36%), flat foot (7/22; 32%), and short fingers (5/22; 23%) were also reported. Hypogonadotropic hypogonadism was seen in 3 of 8 (38%) patients (1 with microtestis, 2 with amenorrhea). No marked clinical differences were observed among patients with different SIL1 mutations. No patient had cardiac and respiratory problems.
The 3 patients with no SIL1 mutation (Patients 25, 26, and 27) and the 9 genetically unexamined patients showed clinical features indistinguishable from the patients with SIL1 mutations, including cerebellar signs with cerebellar atrophy on brain images, intellectual disability, congenital cataracts, and muscle weakness. Elevation of serum CK levels was also seen in 2 patients (Table 2).
Pathological findings of skeletal muscles
Biopsied skeletal muscles were obtained from 16 patients with SIL1 mutations and one patient without (Patient 27). All muscle specimens showed myopathic changes of variation in fiber size and endomysial fibrosis. A few necrotic and regenerating fibers were seen in some patients with SIL1 mutations. No neurogenic changes, including fiber type grou** and grouped atrophy, were observed in any of the patients. Importantly, scattered rimmed vacuoles (RVs) were seen in all 16 patients with SIL1 mutations, but not in the patient without (Figure 1).
Discussion
We conducted a nationwide, questionnaire-based survey to clarify the prevalence, clinical and pathological characteristics, and long-term course of MSS in Japanese patients. The total number of patients with MSS was only 36.
From a clinical point of view, it is important to carry out careful ophthalmological examination of MSS patients at a young age if visual acuity is to be preserved, as the cataracts characteristic of MSS usually appear abruptly and develop rapidly from an early age [13, 14]. Indeed, all of the patients in our present series required early and prompt surgical intervention. Marked cerebellar atrophy on brain MRI is another characteristic of this disease, however cerebellar ataxia can be difficult to identify, especially in younger patients with muscle weakness. Skeletal muscle weakness is also a prominent characteristic. Almost all the MSS patients with SIL1 mutations in this series had muscle weakness initially noticed as a delayed motor milestone, which was detected at an earlier age than cataracts, as reported previously [3, 15, 16]. Regarding muscle biopsy, myopathic changes, including RV formation are a characteristic of patients with SIL1 mutations. RVs are not disease-specific, and are often seen in adult-onset chronic myopathies such as inclusion body myositis, distal myopathy with RVs, oculopharyngeal muscular dystrophy, and myofibrillar myopathies. They are rarely observed, however, in childhood-onset myopathies. The presence of RVs in muscle biopsy tissue can be helpful in formulating an early diagnosis of MSS, allowing the ophthalmologist to perform surgery for cataracts to prevent total visual loss. The life prognosis of MSS appears to be comparatively good, as respiratory, cardiac, and swallowing functions are well preserved, even in the patients who are over 50 years of age.
Most of the reported SIL1 gene mutations have been predicted to induce premature termination and loss of function of SIL1. Based on a putative model of SIL1-BiP interaction, the C-terminal 5 amino acids of SIL1 are thought to play a key role in its association with BiP [17]. This concept is further strengthened by the fact that the p.Arg111X, p.Glu201fs, and p.Leu313fs mutations cause the generation of SIL1 proteins lacking the C-terminal region. Complete loss of function due to nonsense-mediated mRNA decay should also be considered. On the other hand, Leu206 in exon 6 is well preserved among species, and the novel nonsynonymous mutation p.Leu206Glu is predicted to exert a deleterious impact on protein function by both SIFT and PolyPhen2. The c.936dupG (p.Leu313fs) mutation in SIL1, which was first reported from Japan [12], is highly common in Japanese MSS patients. Haplotype analysis revealed that whereas 96.9% of chromosomes from MSS patients possessing the c.936dupG mutation had the same haplotype, less than 10% of the chromosomes of the controls did so, suggesting a founder effect.
The results of this study also strongly suggest the genetic heterogeneity of MSS. Three of the 27 patients (11.1%) had no SIL1 mutation, but demonstrated the cardinal features of MSS, including congenital cataracts, ataxia, intellectual disability, and myopathy. We could not exclude the possibility of the mutation occurred in the promoter or other non-coding region of SIL1 in these 3 patients. Previous reports also showed that approximately one-half of the MSS patients were genetically diagnosed as MSS from mutations in SIL1[7, 16]. The absence of RVs in the muscle biopsy tissue of one patient with no SIL1 mutation suggests the existence of a different disease mechanism(s) in such patients. Further analysis is required to identify the other causative genes for MSS.
Conclusions
MSS is an extremely rare disease, but a possible founder effect was present in Japan. The life prognosis of MSS is comparatively good, and early diagnosis is important for prevention of a total visual loss. Other causative genes for MSS can cause indistinguishable clinical features via different disease mechanisms.
References
Sjogren T: Hereditary congenital spinocerebellar ataxia accompanied by congenital cataract and oligophrenia; a genetic and clinical investigation. Confin Neurol. 1950, 10: 293-308.
Herva R, von Wendt L, von Wendt G, Saukkonen AL, Leisti J, Dubowitz V: A syndrome with juvenile cataract, cerebellar atrophy, mental retardation and myopathy. Neuropediatrics. 1987, 18: 164-169. 10.1055/s-2008-1052473.
Superneau DW, Wertelecki W, Zellweger H, Bastian F: Myopathy in marinesco-sjogren syndrome. Eur Neurol. 1987, 26: 8-16. 10.1159/000116305.
Skre H, Berg K: Linkage studies on marinesco-sjogren syndrome and hypergonadotropic hypogonadism. Clin Genet. 1977, 11: 57-66.
Dotti MT, Bardelli AM, De Stefano N, Federico A, Malandrini A, Vanni M, Guazzi GC: Optic atrophy in marinesco-sjogren syndrome: an additional ocular feature: report of three cases in two families. Ophthalmic Paediatr Genet. 1993, 14: 5-7. 10.3109/13816819309087616.
Anttonen AK, Mahjneh I, Hamalainen RH, Lagier-Tourenne C, Kopra O, Waris L, Anttonen M, Joensuu T, Kalimo H, Paetau A, Tranebjaerg L, Chaigne D, Koenig M, Eeg-Olofsson O, Udd B, Somer M, Somer H, Lehesjoki AE: The gene disrupted in marinesco-sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat Genet. 2005, 37: 1309-1311. 10.1038/ng1677.
Senderek J, Krieger M, Stendel C, Bergmann C, Moser M, Breitbach-Faller N, Rudnik-Schöneborn S, Blaschek A, Wolf NI, Harting I, North K, Smith J, Muntoni F, Brockington M, Quijano-Roy S, Renault F, Herrmann R, Hendershot LM, Schröder JM, Lochmüller H, Topaloglu H, Voit T, Weis J, Ebinger F, Zerres K: Mutations in SIL1 cause marinesco-sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat Genet. 2005, 37: 1312-1314. 10.1038/ng1678.
Zoghbi HY: SILencing misbehaving proteins. Nat Genet. 2005, 37: 1302-1303. 10.1038/ng1205-1302.
Shomura Y, Dragovic Z, Chang HC, Tzvetkov N, Young JC, Brodsky JL, Guerriero V, Hartl FU, Bracher A: Regulation of Hsp70 function by HspBP1: structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol Cell. 2005, 17: 367-379.
Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL: Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005, 37: 974-979. 10.1038/ng1620.
Takahata T, Yamada K, Yamada Y, Ono S, Kinoshita A, Matsuzaka T, Yoshiura K, Kitaoka T: Novel mutations in the SIL1 gene in a Japanese pedigree with the marinesco-sjögren syndrome. J Hum Genet. 2010, 55: 142-146. 10.1038/jhg.2009.141.
Eriguchi M, Mizuta H, Kurohara K, Fujitake J, Kuroda Y: Identification of a new homozygous frameshift insertion mutation in the SIL1 gene in 3 Japanese patients with marinesco-sjogren syndrome. J Neurol Sci. 2008, 270: 197-200. 10.1016/j.jns.2008.02.012.
Ishikawa T, Kitoh H, Awaya A, Nonaka I: Rapid cataract formation in marinesco-sjogren syndrome. Pediatr Neurol. 1993, 9: 407-408. 10.1016/0887-8994(93)90114-R.
Shimizu T, Matsuishi T, Yamashita Y, Koga Y, Ohtaki E, Kato H, Goto Y, Nonaka I: Marinesco-sjogren syndrome: can the diagnosis be made prior to cataract formation?. Muscle Nerve. 1997, 20: 909-910.
Horvers M, Anttonen AK, Lehesjoki AE, Morava E, Wortmann S, Vermeer S, van de Warrenburg BP, Willemsen MA: Marinesco-sjogren syndrome due to SIL1 mutations with a comment on the clinical phenotype. Eur J Paediatr Neurol. 2013, 17: 199-203. 10.1016/j.ejpn.2012.09.007.
Krieger M, Roos A, Stendel C, Claeys KG, Sonmez FM, Baudis M, Bauer P, Bornemann A, de Goede C, Dufke A, Finkel RS, Goebel HH, Häussler M, Kingston H, Kirschner J, Medne L, Muschke P, Rivier F, Rudnik-Schöneborn S, Spengler S, Inzana F, Stanzial F, Benedicenti F, Synofzik M, Lia Taratuto A, Pirra L, Tay SK, Topaloglu H, Uyanik G, et al: SIL1 mutations and clinical spectrum in patients with marinesco-sjogren syndrome. Brain. 2013, 136: 3634-3644. 10.1093/brain/awt283.
Howes J, Shimizu Y, Feige MJ, Hendershot LM: C-terminal mutations destabilize SIL1/BAP and can cause marinesco-sjogren syndrome. J Biol Chem. 2012, 287: 8552-8560. 10.1074/jbc.M111.333286.
Acknowledgements
The authors would like to thank Kanako Goto and Maki Takami for their invaluable technical support and assistance with the genetic analysis. The authors also thank Jeremy Williams (MA, Professor), Dr. Helena Popiel (MS, PhD, Lecturer), and Dr. Edward Barroga (DVM, PhD, Associate Professor) of the Department of International Medical Communications of Tokyo Medical University for editing and reviewing the manuscript.
Funding
This study was supported partly by grants for Research on Intractable Diseases, Comprehensive Research on Disability Health and Welfare, and Applying Health Technology, from the Ministry of Health, Labour and Welfare, Japan; partly by Intramural Research Grants 23–4, 23–5, 22–5 for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry; and partly by JSPS KAKENHI Grant Numbers 23390236 and 24390227.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing of interest.
Authors’ contributions
MG had full access to all the data in the study and wrote the manuscript; MO performed the mutation analysis; HK participated in analyzing all the clinical data; KS, MS, SN, I Nonaka, and I Nishino were involved in data interpretation and also supervised manuscript preparation. YKH supervised all aspects of the study, including study design, data interpretation, and manuscript preparation. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Goto, M., Okada, M., Komaki, H. et al. A nationwide survey on Marinesco-Sjögren syndrome in Japan. Orphanet J Rare Dis 9, 58 (2014). https://doi.org/10.1186/1750-1172-9-58
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1172-9-58