Abstract
Glycogen storage disease type I (GSDI), an inborn error of carbohydrate metabolism, is caused by defects in the glucose-6-transporter/glucose-6-phosphatase complex, which is essential in glucose homeostasis. Two types exist, GSDIa and GSDIb, each caused by different defects in the complex. GSDIa is characterized by fasting intolerance and subsequent metabolic derangements. In addition to these clinical manifestations, patients with GSDIb suffer from neutropenia with neutrophil dysfunction and inflammatory bowel disease.
With the feasibility of novel cell-based therapies, including hepatocyte transplantations and liver stem cell transplantations, it is essential to consider long term outcomes of liver replacement therapy. We reviewed all GSDI patients with liver transplantation identified in literature and through personal communication with treating physicians. Our review shows that all 80 GSDI patients showed improved metabolic control and normal fasting tolerance after liver transplantation. Although some complications might be caused by disease progression, most complications seemed related to the liver transplantation procedure and subsequent immune suppression. These results highlight the potential of other therapeutic strategies, like cell-based therapies for liver replacement, which are expected to normalize liver function with a lower risk of complications of the procedure and immune suppression.
Similar content being viewed by others
Introduction
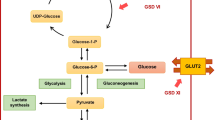
Glycogen storage disease type I (GSDI) is an autosomal recessive inborn error of carbohydrate metabolism caused by defects in the glucose-6-phosphate transporter (G6PT)/glucose-6-phosphatase (G6Pase) complex [1, 2]. G6PT/G6Pase complex plays a crucial role in interprandial glucose homeostasis and consists of a catalytic subunit, glucose-6-phosphatase-α (G6Pase-α) encoded by the G6PC gene and a glucose-6-phosphatase transporter (G6PT), encoded by the SLC37A4 gene. Deficient activity of G6Pase-α causes GSDIa [3] and deficient activity of G6PT causes GSDIb [4]. GSDI is a relatively rare disorder with an incidence of 1:100.000, represented in 80% of the patients by GSDIa and in 20% by GSDIb [5].
G6Pase catalyzes the final step in glycogenolysis and in gluconeogenesis in the lumen of the endoplasmic reticulum in primarily liver, but also kidney and intestine, by hydrolyzation of glucose-6-phosphate (G6P) to glucose and inorganic phosphate. Because G6Pase affects both glycogenolysis and gluconeogenesis, inactivating mutations in the G6PC or the SLC37A4 gene result in severely reduced fasting tolerance. Clinical complications in patients include hepatomegaly, nephromegaly, hypoglycemia, hyperlipidemia, hyperuricemia, lactic acidemia, and growth retardation [5]. In addition to the clinical manifestations in GSDIa, patients with GSDIb generally also suffer from neutropenia, impaired neutrophil function and inflammatory bowel disease.
Prevention of hypoglycemia is crucial in the treatment of GSDI [5]. This is achieved by frequent feedings during day and night or nocturnal gastric drip feeding. Any feeding problem can result in a hypoglycemic event, with risk of cognitive impairment, seizures and finally death. This represents a constant threat for patients and their parents, severely affecting quality of life.
Despite progress in the treatment of GSDI, metabolic control remains challenging and hepatic, renal and/or immunologic complications may arise. Because of the prominent hepatic manifestations in GSDI, orthotopic liver transplantations have been performed [5]. Short-term outcome of liver transplantation for GSDI is encouraging, but very few papers report long-term follow-up. With the advent of less invasive cell-based therapies, including hepatocyte transplantations and liver stem cell transplantations, which might become a therapeutic option for all patients with GSDI, it is eminent to know the long-term outcomes of liver specific therapies. We therefore reviewed short-term and long-term outcomes of liver transplantations in GSDIa and GSDIb patients.
Methodology
English-language literature was systematically reviewed through searches in PubMed and in the references of relevant publications to find all GSDIa and GSDIb liver transplantations published in literature. Through personal communication with treating physicians, we completed information and identified additional cases.
Results
We identified 58 patients with GSDIa who underwent a liver transplantation between 1982 and 2012 (Table 1, Additional file 1: Table S1); 3 of these patients received a second liver transplantation. The average age at transplantation was 20 years (range: 4.3-50 years). A living-related transplantation was performed in 16 cases. 6 Patients underwent a combined liver-kidney transplantation (Table 2). The immunosuppressive regime consisted of steroids, combined with cyclosporine in 12 patients, with cyclosporine and azathioprine in 17 patients and with tacrolimus in 9 patients. The specific immunosuppressant medication was not reported in 22 cases (Table 1).
The indication for liver transplantation varied and included hepatic adenomas/liver abnormalities/focal nodular hyperplasia (29 patients), poor metabolic control (27 patients), growth retardation (13 patients, some with delayed puberty and sexual maturation), renal failure (5 patients, 3 of whom received a combined liver-kidney transplantation), bleeding complications leading to anemia (1 patient) and acute pancreatitis due to severe hypertriglyceridemia (1 patient).
4 Patients with GSDIa died; 1 due to rejection related liver failure (15 years after 1st liver transplantation, 1 year after 2nd liver transplantation with combined kidney transplantation), 1 committed suicide 3 years post-transplantation, 1 because of metastatic hepatocellular carcinoma 4 months after transplantation, and 1 because of pancreatitis and sepsis 2 months post-transplantation. All other patients were alive at time of follow-up (range several months to 11.3 years post-transplantation). In all cases, liver function was good and metabolic control normalized, without a specific dietary regime. In 13 cases, catch-up growth was mentioned (Table 3). The patients with catch-up growth reported were all children or teenagers and for 2 of these, sexual maturation was also reported. In addition, one patient of 27 years old showed an increase of 5.4 cm in height 2 years after transplantation.
The complication reported most frequently was acute or chronic renal failure. Acute renal failure occurred in 8 patients, including 1 patient with pre-transplantation renal failure. One of these 8 patients required temporary dialysis. Chronic renal failure was seen in 6 patients after transplantation and 2 of these patients required dialysis. In addition, 1 case of gouty arthritis was reported 4 years post-transplantation. It is unclear whether this was due to renal failure. None of the patients who had received a combined liver-kidney transplantation developed renal failure.
Transplantation associated complications were seen in 18/58 patients and included hepatic artery thrombosis (2 patients), late portal vein thrombosis (2 patients), hepatic vein obstruction (2 patients), prolonged drainage (2 patients), acute (steroid responsive) rejection (5 patients), chronic rejection (3 patients) and a never functioning liver transplant (2 patients). Complications that might have been caused by immune suppression included hypertension (4 patients), starting one month after transplantation and reversible insulin-dependent diabetes (3 patients), starting within the first week after transplantation (Table 4). In addition, various infections were reported (5 with cytomegalovirus 1 month after transplantation and 1 patient with hepatitis 4 years after transplantation).
Liver transplantation was performed in 22 patients with GSDIb between 1991 and 2012, at an average age of 10 years (range 1–44 years) (Table 1, Additional file 1: Table S2). One patient had a kidney transplantation 2 years prior to the liver transplantation, with good function of both grafts reported 8 months after liver transplantation (Table 2). Indications for liver transplantation in GSDIb patients included poor metabolic control (21 patients) and/or recurrent infections (10 patients), growth retardation (3 patients), and oral and anal ulcera (1 patient). Immune suppression after transplantation involved cyclosporine in 1 patient and tacrolimus in 16 patients. The immune suppressive medication wat not reported in the other 6 patients.
At follow-up, 1 patient had died 1.4 months after transplantation, due to systemic candidiasis. In all patients, metabolic abnormalities were corrected by transplantation. Catch-up growth was reported in 2 cases (Table 3). In 14 patients, neutropenia improved, while in 7 patients neutropenia persisted. One report mentioned prolonged bleeding time and bruises after several years. 7 Patients had transplantation associated complications, including anemia in the first days after the transplantation (1 patient) and various infectious diseases shortly after transplantation (6 patients). Complications potentially associated with immune suppressive therapy included 1 patient with hepatitis B, seven months after transplantation, and 1 patient with cytomegalovirus infection several years after transplantation (Table 4). 1 patient developed tacrolimus encephalopathy soon after transplantation, which was resolved upon withdrawal of tacrolimus. None of the reports described renal complications.
Discussion
In this review, we show that liver transplantations sustainably corrected fasting tolerance and the induced metabolic abnormalities associated with GSDIa and GSDIb, thereby immensely improving quality of life for patients. In addition, catch-up growth was seen in most patients with growth retardation (13/13 (100%) and 2/3 (67%) in GSDIa and GSDIb patients, respectively).
The extra-hepatic symptoms of the disease might however persist after liver transplantation. In GSDIa patients, renal failure was the most common complication (14/58 (24%) of patients) and 3/14 (21%) required dialysis. The natural course of renal function in GSDI patients shows a biphasic pattern [41]. We identified only 1 patient with both pre- and post-transplantation renal failure; renal function was restored in the other 4 patients with pre-transplantation renal dysfunction. Notably, 3 of these 4 patients had received a combined liver-kidney transplantation. In the other patients, renal dysfunction developed after transplantation. It is yet unclear whether post-transplantation renal failure in GSDIa represents progression of the disease, a secondary reaction to poor metabolic control, toxicity from immune suppressive medication after liver transplantation, or a combination. Strikingly, none of the GSDIb patients developed renal failure. We are unaware of a pathophysiological mechanism to explain why GSDIa patients would be more prone to develop renal failure than GSDIb patients, nor has this been observed in our experience with GSDI patients. Potentially, this is a coincidental finding due to the relatively small number of patients evaluated.
In GSDIb patients, persistent neutropenia was the most important complication (7/22 (32%) of patients). Neutropenia in GSDIb has recently been attributed to a second G6P hydrolase, called G6Pase-β [42]. The G6PT/G6Pase-β complex maintains glucose homeostasis and function in neutrophils. Deficiency of the G6PT/G6Pase-β complex in neutrophils leads to impaired endogenous glucose production and enhanced endoplasmic reticulum stress, oxidative stress and apoptosis, leading to neutropenia [42]. Migration of neutrophils from the blood to inflamed tissues (e.g. intestines, liver adenoma) might further contribute to the neutropenia [43]. It remains unclear why neutropenia improves after liver replacement in some patients and persists in others. It is possible that improved metabolic control and general well-being result in decreased inflammation, leading to higher blood neutrophil concentrations. Second, immediate increase in neutrophil count after liver transplantation might be related to the neutrophilic effect of steroid therapy. However, recurrence of neutropenia after steroid tapering has not been reported. Finally, host/donor bone marrow chimerism has been observed after liver transplantation [44]. Donor-derived leukocytes, co-transplanted with the liver graft, can migrate into the recipient’s immune system and bone marrow. This phenomenon may result in both long-term tolerance induction and induction of enzymatic activity in extra-hepatic tissue. Clearly, although the molecular mechanism causing congenital neutropenia in GSDIb has now been elucidated, many aspects of the phenotype remain poorly understood.
Our review shows that there are still many complications related to the liver transplantation procedure (18/58 (31%) in GSDIa and 8/22 (36%) in GSDIb patients), as well as complications suspected to be related to immune suppressive therapy (13/58 (22%) in GSDIa and 3/22 (14%) in GSDIb patients). In the light of these complications, novel therapeutic strategies, like hepatocyte and liver stem cell transplantations might represent attractive alternatives for liver transplantation in GSDI patients. Liver (stem) cells can be infused through the portal vein, which is considerably less invasive than liver transplantation and hence not associated with surgery related complications. We identified 3 GSDIa and GSDIb patients, treated with hepatocyte transplantations (Additional file 1: Table S3, Additional file 1: Table S4). Normalization of metabolic parameters was observed in all patients after transplantation and no therapy-related complications were mentioned. This concurs with previously reported beneficial effects from human hepatocyte transplantations for different hepatic indications (metabolic, acute and chronic liver failure) [45]. However, these beneficial effects were short-lived and effects subsided within months. Similarly, metabolic improvement decreased in one of the GSDI patients treated with hepatocytes after 3 years and eventually subsided completely [46]. Nevertheless, these case reports show that cell-based therapies can restore liver function for at least a limited period, which might be beneficial in acute situations awaiting a liver transplantation.
Liver stem cell transplantations might provide additional advantages. Stem cells are highly proliferative and have the potential to bypass the current shortage of donor livers by expansion in vitro or in vivo upon engraftment. Furthermore, liver stem cell transplantations might require less immune suppression. For allograft survival after solid organ transplantation, all patients require life-long immune suppression, with serious associated side effects, including toxicity, malignancy development and infectious complications. Human fetal-liver derived hepatocytes have been given for end-stage decompensated liver cirrhosis without immune suppression, based on the concept that fetal cells do not express HLA yet [47]. Short term outcomes were promising, but long term follow up has not been reported. This illustrates that progenitor cell-based therapies might be given without or with reduced immune suppression. In this context, autologous transplantation with genetically corrected stem cells [48, 49] and hepatocyte-like cells generated from autologous induced pluripotent stem cells [50] have exciting potential for the future.
Despite promising, some complications might still occur after stem cell based therapies, including renal complications. New-born G6PC knock-out mice treated with bone marrow-derived myelomonocytic cells displayed restored G6Pase activity and improved liver functional parameters, without amelioration of renal involvement [51]. Similarly, there is concern that hepatic adenoma and carcinoma might develop in the cells with a defect G6PT/G6Pase complex with the use of cell-based therapies that do not replace all patient cells. However, a recent study has demonstrated that the occurrence of hepatocellular adenoma was prevented by gene therapy in G6pc-/- mice, despite partial and variable G6Pase activity, but with normalized blood metabolite profiles and glucose tolerance [52]. This concurs with the observation that development of hepatocellular adenoma and carcinoma appears to be related to the degree of steatosis and has been shown to regress with improved metabolic control [53], as is expected from cell-based therapies.
In conclusion, all GSDI patients reviewed in this article showed improved metabolic control and normal fasting tolerance after liver transplantation. This dramatically improved the quality of life of these patients, but a substantial number of patients experienced complications. Although some complications might be caused by disease progression, most seemed related to the liver transplantation procedure and subsequent immune suppression. These complications underscore the need for improvement of therapeutic strategies and emphasize the potential of novel (stem) cell-based treatments.
References
Chou JY, Jun HS, Mansfield BC: Glycogen storage disease type I and G6Pase-beta deficiency: etiology and therapy. Nat Rev Endocrinol. 2010, 6: 676-688. 10.1038/nrendo.2010.189.
Froissart R, Piraud M, Boudjemline AM, Vianey-Saban C, Petit F, Hubert-Buron A, Eberschweiler PT, Gajdos V, Labrune P: Glucose-6-phosphatase deficiency. Orphanet J Rare Dis. 2011, 6: 27-1172-6-27-
Lei KJ, Shelly LL, Pan CJ, Sidbury JB, Chou JY: Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993, 262: 580-583. 10.1126/science.8211187.
Hiraiwa H, Pan CJ, Lin B, Moses SW, Chou JY: Inactivation of the glucose 6-phosphate transporter causes glycogen storage disease type 1b. J BiolChem. 1999, 274: 5532-5536.
Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP: Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002, 161 (Suppl 1): S20-S34.
Malatack JJ, Finegold DN, Iwatsuki S, Shaw BW, Gartner JC, Zitelli BJ, Roe T, Starzl TE: Liver transplantation for type I glycogen storage disease. Lancet. 1983, 1: 1073-1075.
Coire CI, Qizilbash AH, Castelli MF: Hepatic adenomata in type Ia glycogen storage disease. Arch Pathol Lab Med. 1987, 111: 166-169.
Martinez Ibanez V, Margarit C, Tormo R, Infante D, Iglesias J, Allende H, Lloret J, Jimenez A, Boix-Ochoa J: Liver transplantation in metabolic diseases. Report of five pediatric cases. Transplant Proc. 1987, 19: 3803-3804.
Poe R, Snover DC: Adenomas in glycogen storage disease type 1. Two cases with unusual histologic features. Am J Surg Pathol. 1988, 12: 477-483. 10.1097/00000478-198806000-00008.
Kirschner BS, Baker AL, Thorp FK: Growth in adulthood after liver transplantation for glycogen storage disease type I. Gastroenterology. 1991, 101: 238-241.
Kay RM, Eckardt JJ, Goldstein LI, Busuttil RW: Metastatic hepatocellular carcinoma to bone in a liver transplant patient. A case report. Clin Orthop Relat Res. 1994, 303: 237-241.
Reid CJ, Hebert D: Acute renal failure complicating liver transplantation in twin sisters with glycogen storage disease type Ia. Transplant Proc. 1996, 28: 3629-3631.
Matern D, Starzl TE, Arnaout W, Barnard J, Bynon JS, Dhawan A, Emond J, Haagsma EB, Hug G, Lachaux A, Smit GP, Chen YT: Liver transplantation for glycogen storage disease types I, III, and IV. Eur J Pediatr. 1999, 158 (Suppl 2): S43-S48.
Faivre L, Houssin D, Valayer J, Brouard J, Hadchouel M, Bernard O: Long-term outcome of liver transplantation in patients with glycogen storage disease type Ia. J Inherit Metab Dis. 1999, 22: 723-732. 10.1023/A:1005544117285.
Koestinger A, Gillet M, Chiolero R, Mosimann F, Tappy L: Effect of liver transplantation on hepatic glucose metabolism in a patient with type I glycogen storage disease. Transplantation. 2000, 69: 2205-2207. 10.1097/00007890-200005270-00045.
Lerut JP, Ciccarelli O, Sempoux C, Danse E, de Flandre J, Horsmans Y, Sokal E, Otte JB: Glycogenosis storage type I diseases and evolutiveadenomatosis: an indication for liver transplantation. TransplInt. 2003, 16: 879-884.
Panaro F, Andorno E, Basile G, Morelli N, Bottino G, Fontana I, Bertocchi M, DiDomenico S, Miggino M, Saltalamacchia L, Ghinolfi D, Bonifazio L, Jarzembowski TM, Valente U: Simultaneous liver-kidney transplantation for glycogen storage disease type IA (von Gierke's disease). Transplant Proc. 2004, 36: 1483-1484. 10.1016/j.transproceed.2004.05.070.
Arikan C, Kilic M, Nart D, Ozgenc F, Ozkan T, Tokat Y, Yagci RV, Aydogdu S: Hepatocellular carcinoma in children and effect of living-donor liver transplantation on outcome. Pediatr Transplant. 2006, 10: 42-47. 10.1111/j.1399-3046.2005.00395.x.
Carreiro G, Villela-Nogueira CA, Coelho H, Basto S, Pannain VL, Caroli-Bottino A, Ribeiro Filho J: Orthotopic liver transplantation in glucose-6-phosphatase deficiency–Von Gierke disease–with multiple hepatic adenomas and concomitant focal nodular hyperplasia. J Pediatr Endocrinol Metab. 2007, 20: 545-549.
Davis MK, Weinstein DA: Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008, 12: 137-145. 10.1111/j.1399-3046.2007.00803.x.
Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS: Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009, 51: 483-490. 10.1016/j.jhep.2009.05.026.
Carvalho PM, Silva NJ, Dias PG, Porto JF, Santos LC, Costa JM: Glycogen Storage Disease type 1a - a secondary cause for hyperlipidemia: report of five cases. J Diabetes Metab Disord. 2013, 12: 25-6581-12-25-
Sokal EM, Lopez-Silvarrey A, Buts JP, Otte JB: Orthotopic liver transplantation for type I glycogenosis unresponsive to medical therapy. J Pediatr Gastroenterol Nutr. 1993, 16: 465-467. 10.1097/00005176-199305000-00021.
Labrune P: Glycogen storage disease type I: indications for liver and/or kidney transplantation. Eur J Pediatr. 2002, 161 (Suppl 1): S53-S55.
Iyer SG, Chen CL, Wang CC, Wang SH, Concejero AM, Liu YW, Yang CH, Yong CC, Jawan B, Cheng YF, Eng HL: Long-term results of living donor liver transplantation for glycogen storage disorders in children. Liver Transpl. 2007, 13: 848-852. 10.1002/lt.21151.
Belingheri M, Ghio L, Sala A, Menni F, Trespidi L, Ferraresso M, Berardinelli L, Rossi G, Edefonti A, Parini R: Combined liver-kidney transplantation in glycogen storage disease Ia: a case beyond the guidelines. Liver Transpl. 2007, 13: 762-764. 10.1002/lt.21147.
Kaihara S, Ushigome H, Sakai K, Yoshizawa A, Nobori S, Suzuki T, Okamoto M, Ochiai T, Yoshimura N: Preemptive living donor liver transplantation in glycogen storage disease Ia: case report. Transplant Proc. 2008, 40: 2815-2817. 10.1016/j.transproceed.2008.07.026.
Lachaux A, Boillot O, Stamm D, Canterino I, Dumontet C, Regnier F, Floret D, Hermier M: Treatment with lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) and orthotopic liver transplantation for glycogen storage disease type Ib. J Pediatr. 1993, 123: 1005-1008. 10.1016/S0022-3476(05)80403-2.
Tanaka A, Tanaka K, Kitai T, Yanabu N, Tokuka A, Sato B, Mori S, Inomoto T, Shinohara H, Uemoto S, Tokunaga Y, Inomata Y, Yamaoka Y: Living related liver transplantation across ABO blood groups. Transplantation. 1994, 58: 548-553. 10.1097/00007890-199409150-00004.
Martinez-Olmos MA, López-Sanromán A, Martín-Vaquero P, Molina-Pérez E, Bárcena R, Vicente E, Candela A, Pallardo-Sánchez LF: Liver transplantation for type Ibglycogenosis with reversal of cyclic neutropenia. Clin Nutr. 2001, 20: 375-377. 10.1054/clnu.2001.0432.
Adachi M, Shinkai M, Ohhama Y, Tachibana K, Kuratsuji T, Saji H, Maruya E: Improved neutrophil function in a glycogen storage disease type 1b patient after liver transplantation. Eur J Pediatr. 2004, 163: 202-206. 10.1007/s00431-004-1405-1.
Bhattacharya N, Heaton N, Rela M, Walter JH, Lee PJ: The benefits of liver transplantation in glycogenosis type Ib. J Inherit Metab Dis. 2004, 27: 539-540.
Martin AP, Bartels M, Schreiber S, Buehrdel P, Hauss J, Fangmann J: Successful staged kidney and liver transplantation for glycogen storage disease type Ib: a case report. Transplant Proc. 2006, 38: 3615-3619. 10.1016/j.transproceed.2006.10.160.
Karaki C, Kasahara M, Sakamoto S, Shigeta T, Uchida H, Kanazawa H, Kakiuchi T, Fukuda A, Nakazawa A, Horikawa R, Suzuki Y: Glycemic management in living donor liver transplantation for patients with glycogen storage disease type 1b. Pediatr Transplant. 2012, 16: 465-470. 10.1111/j.1399-3046.2012.01705.x.
Kasahara M, Horikawa R, Sakamoto S, Shigeta T, Tanaka H, Fukuda A, Abe K, Yoshii K, Naiki Y, Kosaki R, Nakagawa A: Living donor liver transplantation for glycogen storage disease type Ib. Liver Transpl. 2009, 15: 1867-1871. 10.1002/lt.21929.
Marega A, Fregonese C, Tulissi P, Vallone C, Gropuzzo M, Toniutto PL, Baccarani U, Bresadola F, Toso F, Montanaro D: Preemptive liver-kidney transplantation in von Gierke disease: a case report. Transplant Proc. 2011, 43: 1196-1197. 10.1016/j.transproceed.2011.03.003.
Demirci G, Becker T, Nyibata M, Lueck R, Bektas H, Lehner F, Tusch G, Strassburg C, Schwarz A, Klempnauer J, Nashan B: Results of combined and sequential liver-kidney transplantation. Liver Transpl. 2003, 9: 1067-1078. 10.1053/jlts.2003.50210.
Chen CL, Chen YS, Liu PP, Chiang YC, Cheng YF, Huang TL, Eng HL: Living related donor liver transplantation. J Gastroenterol Hepatol. 1997, 12: S342-S345. 10.1111/j.1440-1746.1997.tb00519.x.
Liu PP, de Villa VH, Chen YS, Wang CC, Wang SH, Chiang YC, Jawan B, Cheung HK, Cheng YF, Huang TL, Eng HL, Chuang FR, Chen CL: Outcome of living donor liver transplantation for glycogen storage disease. Transplant Proc. 2003, 35: 366-368. 10.1016/S0041-1345(02)03951-9.
Morioka D, Kasahara M, Takada Y, Corrales JP, Yoshizawa A, Sakamoto S, Taira K, Yoshitoshi EY, Egawa H, Shimada H, Tanaka K: Living donor liver transplantation for pediatric patients with inheritable metabolic disorders. Am J Transplant. 2005, 5: 2754-2763. 10.1111/j.1600-6143.2005.01084.x.
Martens DH, Rake JP, Navis G, Fidler V, van Dael CM, Smit GP: Renal function in glycogen storage disease type I, natural course, and renopreservative effects of ACE inhibition. Clin J Am Soc Nephrol. 2009, 4: 1741-1746. 10.2215/CJN.00050109.
Chou JY, Jun HS, Mansfield BC: Neutropenia in type Ib glycogen storage disease. Curr Opin Hematol. 2010, 17: 36-42. 10.1097/MOH.0b013e328331df85.
Visser G, de Jager W, Verhagen LP, Smit GP, Wijburg FA, Prakken BJ, Coffer PJ, Buitenhuis M: Survival, but not maturation, is affected in neutrophil progenitors from GSD-1b patients. J Inherit Metab Dis. 2012, 35: 287-300. 10.1007/s10545-011-9379-4.
Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, Murase N, Kendall RS, Kocova M, Rudert WA, Zeevi A, Van Thiel D: Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher's disease. N Engl J Med. 1993, 328: 745-749. 10.1056/NEJM199303183281101.
Fisher RA, Strom SC: Human hepatocyte transplantation: worldwide results. Transplantation. 2006, 82: 441-449.
Muraca M, Burlina AB: Liver and liver cell transplantation for glycogen storage disease type IA. Acta Gastroenterol Belg. 2005, 68: 469-472.
Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K, Venkateswarlu J, Pande G, Habibullah CM: Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010, 19: 409-418.
Yiu WH, Lee YM, Peng WT, Pan CJ, Mead PA, Mansfield BC, Chou JY: Complete normalization of hepatic G6PC deficiency in murine glycogen storage disease type Ia using gene therapy. MolTher. 2010, 18: 1076-1084.
Yiu WH, Pan CJ, Allamarvdasht M, Kim SY, Chou JY: Glucose-6-phosphate transporter gene therapy corrects metabolic and myeloid abnormalities in glycogen storage disease type Ib mice. Gene Ther. 2007, 14: 219-226. 10.1038/sj.gt.3302869.
Unternaehrer JJ, Daley GQ: Induced pluripotent stem cells for modelling human diseases. Philos Trans R Soc Lond B Biol Sci. 2011, 366: 2274-2285. 10.1098/rstb.2011.0017.
Resaz R, Emionite L, Vanni C, Astigiano S, Puppo M, Lavieri R, Segalerba D, Pezzolo A, Bosco MC, Oberto A, Eva C, Chou JY, Varesio L, Barbieri O, Eva A: Treatment of newborn G6pc(-/-) mice with bone marrow-derived myelomonocytes induces liver repair. J Hepatol. 2011, 55: 1263-1271. 10.1016/j.jhep.2011.02.033.
Lee YM, Jun HS, Pan CJ, Lin SR, Wilson LH, Mansfield BC, Chou JY: Prevention of hepatocellular adenoma and correction of metabolic abnormalities in murine glycogen storage disease type Ia by gene therapy. Hepatology. 2012, 56: 1719-1729. 10.1002/hep.25717.
Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, Kishnani PS: Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005, 28: 153-162. 10.1007/s10545-005-7500-2.
Acknowledgements
We gratefully acknowledge JP Rake, P Labrune, JJ Malatack, and DA Weinstein for their contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SB identified all the cases to be included, analyzed and interpreted the data and drafted the manuscript. PS reviewed the manuscript. PS, GV and SF contacted treating physicians and reviewed the manuscript. In addition, GV and SF analyzed and interpreted the data. All authors read and approved the final manuscript.
Electronic supplementary material
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Boers, S.J., Visser, G., Smit, P.G. et al. Liver transplantation in glycogen storage disease type I. Orphanet J Rare Dis 9, 47 (2014). https://doi.org/10.1186/1750-1172-9-47
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1172-9-47