Abstract
Gastrin-releasing peptide (GRP) has been proposed as a peptidergic molecule for behavioral fear and itching. Immunohistochemistry and in situ hybridization studies have shown that GRP and GRP receptor are widely distributed in forebrain areas. Less information is available for the functional action for GRP in the prefrontal cortex including the anterior cingulate cortex (ACC). Here we used whole-cell patch-clamp recording technique to study the modulation of synaptic transmission by GRP in the ACC. We found that GRP increased the frequency of sIPSCs recorded while had no significant effect on sEPSCs in ACC pyramidal neurons. The facilitatory effect of GRP on sIPSCs was blocked by the GRP receptor antagonist, RC3095. In the presence of TTX, however, GRP had no effect on the mIPSCs. Therefore, activation of GRP receptor may facilitate the excitation of the interneurons and enhanced spontaneous GABAergic, but not glutamatergic neurotransmission. Similar results on GRP modulation of GABAergic transmission were observed in the insular cortex and amygdala, suggesting a general possible effect of GRP on cortical inhibitory transmission. Our results suggest that GRP receptor is an important regulator of inhibitory circuits in forebrain areas.
Similar content being viewed by others
Introduction
Gastrin-releasing peptide (GRP) is a mammalian analogue of bombesin (BB), a 14 amino acid-containing peptide first isolated from the skin of the frog Bombina bombina [1, 2]. Anatomic studies have shown that GRP and its receptors are widely distributed in the central nervous system, in addition to the gastrointestinal (GI) tract [2–10]. GRP has been implicated in many physiological and pathological conditions such as the regulation of the circadian rhythm, exocrine and endocrine secretions, smooth muscle contraction, inflammation, feeding, fear and behavioral itch [11–17].
Recent studies on GRP in sensory systems have triggered new interests on GRP. At the level of the spinal cord, it has been reported that GRP may serve as a selective signaling transmitters for itching sensation [14, 15]. In the amygdala, it has been reported that GRP may contribute to regulation of neuronal excitability, and contribute to behavioral fear [18]. Although it has been known that GRP is distributed in cortical areas, less is known about the possible modulatory effects of GRP on cortical circuits. The anterior cingulate cortex (ACC), a key structure of the prefrontal cortex, plays an established role in learning and memory, drug addiction, and chronic pain [19–22]. In the present study, we have investigated the effects of GRP on both excitatory and inhibitory transmission in the ACC. Our results show that the GRP selectively facilitate GABAergic but not glutamatergic neurotransmission. The facilitation may result from the GRP-induced inward current and firing of GABAergic interneurons in the ACC.
Methods
Animals
Adult male C57BL/6 mice were purchased from Charles River (6-10 weeks old). All mice were maintained on a 12 h light/dark cycle with food and water provided ad libitum. All protocols used were approved by The Animal Care and use Committee at the University of Toronto and conform to NIH guidelines.
Whole-cell Patch Clamp Recordings
Adult male mice were anesthetized with 1-2% halothane and decapitated. Coronal slices (300 μm) containing the ACC, amygdala or insular cortex will be prepared using routine methods used in our laboratory [23, 24]. Slices were then transferred to a submerged recovery chamber with oxygenated (95% O2 and 5% CO2) ACSF at room temperature. After a one-hour recovery period, slices were placed in a recording chamber on the stage of an Axioskop 2FS microscope (Zeiss) equipped with infrared DIC optics for visually-guided whole cell patch clamp recordings. Pyramidal neurons or interneurons in Layer II-III in the ACC were recorded with an Axon 200B amplifier (Molecular device, Union city, CA). Recording electrodes (2-5 M) contained an internal solution composed of (in mM): Kgluconate, 120; NaCl, 5; MgCl2 1; EGTA, 0.5; Mg-ATP, 2; Na3GTP, 0.1; HEPES, 10; pH 7.2; 280-300 mOsmol. The membrane potentials were held at -70 mV throughout all experiments. When recording GABAA receptor-mediated currents, K-gluconate was replaced by Cs-MeSO3 and a holding potential of 10 mV. Spontaneous EPSCs were recorded in the presence of GABAA receptor antagonist, picrotoxin (100 μM) and spontaneous IPSCs were recorded in the presence of a NMDA receptor antagonist, AP5 (100 μM) and a non-NMDA receptor antagonist, CNQX (20 μM). GRP and its receptor antagonist RC3095 were purchased from Sigma. To examine the mIPSCs, TTX (1 μM) was bath-applied in the perfusion solution. The sIPSCs/mIPSCs were analyzed with the Mini Analysis Program v5.2.4 (Synaptosoft Inc., Decatur, GA). Access resistance was 15-30MO and monitored throughout the experiments. Data were discarded if access resistance changed > 15% during an experiment. Signals were filtered at 1 kHz, digitized at 10 kHz.
Passive and Active Membrane Properties
Off-line analysis was performed using Clampfit version 9 (Axon Instruments). Resting membrane potential (RMP) was the low-pass readout of the electrode amplifier and was not corrected for liquid junction potential (~12 mV) after terminating the recording. The membrane potential was measured immediately after establishing the whole-cell configuration. Only neurons that had a resting membrane potential more negative than -60 mV were further investigated. Conductance was determined from the linear slope (between -60 mV to -80 mV) of the current-voltage (I-V; Vhold = -70 mV) relationships. Action potentials (APs) were detected in response to suprathreshold current injections from a holding potential around -70 mV. Depolarizing currents of 5~200 pA (400-ms duration) were delivered in increments of 5 pA until an AP was evoked. The rheobase was defined as the minimum current required to evoke an action potential. The AP voltage threshold (Vthreshold) was defined as the first point on the rising phase of the spike at which the change in voltage exceeded 50 mV/ms. The spike amplitude was quantified as the difference between the Vthreshold and the peak voltage. The spike width was measured at 1/2 of the total spike amplitude (measured from the Vthreshold level). The amplitude of the afterhyperpolarization (AHP) was estimated as the difference between the Vthreshold and the peak of AHP.
Immunohistochemistry
Mice were deeply anesthetized with halothane and perfused transcardially with 50-100 ml saline followed by 150-500 ml of cold 0.1 M phosphate buffer (PB) containing 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde/PBS and then will be placed in 30% sucrose in 0.2 M PBS, embedded in the OCT compound and frozen. Coronal sections (30 μM thickness) were cut using a cryostat. For dual fluorescent immunohistochemistry, sections were incubated overnight with anti-GRPR (1:50; rabbit polycolonal, Santa Cruz Biotecnology) and anti-GAD67 (1:300; mouse monoclonal, Chemicon) antibodies at 4°C. Sections were then be washed 3 times with PBS 0.1 M and incubated for 2 hours with anti Mouse-FITC and rabbit-rhodamine conjugated secondary antibodies (1:200; Chemicon). Images of the ACC areas of at 0.7-μm intervals with 20× lens were obtained with Bio-Rad Laboratories MRC 1000 laser-scanning confocal fluorescent imaging system.
Data Analysis
Results were expressed as mean ± standard error of the mean (S. E. M.). Statistical comparisons were performed with the use of one- or two-way analysis of variance (ANOVA) with the post-hoc Scheffe F-test in immunocytochemical experiments. Analysis of mIPSCs/sEPSCs was performed with cumulative probability plots and was compared using the Kolmogorov-Smirnov (K-S) test for significant differences. In all cases, P < 0.05 was considered statistically significant.
Results
The expression of GRP and GRP receptors in the ACC
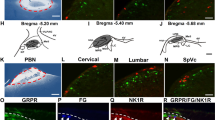
To understand if GRP may be distributed in mouse ACC, we took the advantage of online brain map** data base (the Allen Mouse Brain Atlas). We found that in adult mouse brains the Grp gene is highly enriched in the ACC (Figure 1A); http://www.alleninstitute.org), including the regions of Cg1 and Cg2. Consistent with Grp distribution in the ACC, the Grp receptors (GRPR) mRNA are also highly expressed in the ACC (Figure 1B); taken from the Allen Mouse Brain Atlas). To confirm the distribution of GRP receptor in the ACC, we performed double-label immunohistochemistry using specific antibodies for GRP receptor and GABAergic neurons (see(Figure 2A and 2B). In addition to the ACC, we have also examined the regions of the insular cortex (IC) and basal lateral amygdala (BLA). We found that GRP receptor was highly expressed in these areas. Particularly, GRP receptor was co-localized with GAD67, a marker for GABA in some neurons, suggesting GRP receptor is expressed in a subpopulation of GABAergic interneurons. These results indicate that GRP may play modulatory roles in these brain areas.
Expression of the GRP and GRPR in the ACC (from the Allen Mouse Brain Atlas). (A) In situ hybridization on a coronal section of mouse brain, showing the expression of the Grp gene in the anterior cingulate cortex (ACC) of wild-type mice (rectangled area http://mouse.brain-map.org/viewImage.do?imageId=79611383). (B) In situ hybridization on a sagittal section of mouse brain, showing expression of the Grp receptor gene in ACC of wild-type mice (rectangled area http://mouse.brain-map.org/viewImage.do?imageId=73493204).
GABA immunoreactivity in the GRPR neurons. Double-immunostaining was performed using anti-GRP-R antibody (in red, left column) and an antibody specific for GABAergic neurons (anti-GAD67 antibody, in green center column) in the anterior cingulate cortex (ACC), basolateral amygdale (BLA) and insular cortex (IC). A subpopulation of GRP-R immunoreactivity was detected in GABAergic neurons (arrows), merged in right column. (A) Upper panels show images from low-powered microscopy. Scale bar = 10 μm. (B) Lower panels show parts of the same sections at higher magnification. Scale bar = 50 μm.
Morphological and electrophysiological properties of interneurons and pyramidal neurons in the ACC
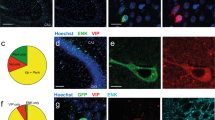
Since GRP receptor is expressed in many neurons in the ACC, we wanted to know whether it may function differently in different types of neurons, i.e pyramidal neurons and inhibitory interneurons. Next we performed whole-cell patch-clamp experiments to examine the effects of GRP on ACC neurons. As previously reported, we distinguished local inhibitory neurons and pyramidal neurons morphologically and electrophysiologically [23, 25]. In some experiments, we also performed whole-cell patch clamp recording combined with biocytin labeling in ACC neurons (see(Figure 3 for examples). Typical layer II/III pyramidal cells had a prominent apical dendrite, which ascended toward the superficial layers, while their basal dendrites were mainly located within the same layer as the soma (Figure 3C). Somata of interneurons were usually ovoid in shape and the soma size was smaller than that of pyramidal cells. In contrast to the pyramidal cells, interneuron was lack of the apical dendrite and displayed 2-5 primary dendrites extending in all directions ((Figure 3D).
Morphological and electrophysiological properties of interneurons and pyramidal neurons in the ACC. (A) Representative coronal section showing the placement of a whole-cell patch recording in a cingulate slice. (B) Diagram representation of the location of the recorded neurons in layer II/III. (C and D) Photomicrograph of a representative biocytin--labeled layer II/III ACC pyramidal neuron (C) interneuron (D) as visualized with confocal laser scanning microscopy. (E) Pyramidal neurons showed different firing properties from those observed in interneurons after current injection (see F). (F) Interneurons were identified by their firing properties. When injected with current step (100 pA within 400 ms), interneurons showed fast spiking properties. (G and H) current-voltage relationship constructed from values taken at the end of pulses (dots in E and F).
Interneurons and pyramidal cells were found to exhibit significantly different input resistances and resting membrane potentials (see Table 1). In response to a continuous depolarizing conditions, pyramidal cells usually fired APs with an rapid initial phase followed by a slow gradual decline, a phenomenon called "spike frequency adaptation"(Figure 3E), whereas interneurons fired a regular train of APs (Figure 3F). We also compared the voltage-current relationship (I-V) of pyramidal neurons and interneurons. The I-V for pyramidal neurons was linear at membrane potentials between -65 and -90 mV, with inward rectification between -90 mV and -120 mV (Figure 3G), while for interneurons was linear between -40 mV and -80mV and showed a slight inward rectification between -100 mV and -120 mV (Figure 3H). Moreover, we found that interneurons exhibited a less Rheobase current (interneurons: 56.8 ± 11.8 pA, n = 11; pyramidal neurons: 95.7 ± 7.3 pA, n = 11, p < 0.01), a lower action potential amplitude (interneurons: 88.2 ± 2.6 mV, n = 11; pyramidal neurons: 93.8 ± 0.8 mV, n = 11, p < 0.01), a narrower action potential half-width (interneurons: 1.00 ± 0.05 ms, n = 11; pyramidal neurons: 1.50 ± 0.03 ms, n = 11, p < 0.001) and a bigger after-hyperpolarization (interneurons: -24.8 ± 2.5 mV, n = 11; pyramidal neurons: -6.9 ± 0.3 mV, n = 11, p < 0.001) compared to the pyramidal cells (see Table 1).
GRP induced inward currents in interneurons but small or undetectable currents in pyramidal neurons
To examine the function of GRP receptor in the ACC, GRP was applied through bath solution and the responses of pyramidal neurons and interneurons were recorded. At holding potential of -70 mV a short application of GRP (300 nM) induced a slowly develo** inward current (peak 16.9 ± 1.8 pA) that recovered slowly over 10 min (n = 8/10 interneurons). However, in pyramidal neurons there was undetectable currents upon GRP application (300 nM, n = 7). In the presence of GRP receptor antagonist, RC3095 (3 μM), the effect of GRP induced inward current in interneurons (n = 7) was completely blocked (data not shown). These results indicate that GRP selectively activated interneuronal GRP receptor in the ACC.
Activation of GRP receptors enhances spontaneous GABAergic, but not glutamatergic transmission
Since GRP can excite interneurons, we speculated that activation of GRP receptor would affect the release of GABA in the ACC. To test this idea, we studied the effect of GRP on GABAergic transmission. At a holding potential of 10 mV and with the blockade of NMDA and AMPA/KA current by using AP-5 (50 μM) and CNQX (20 μM), we recorded spontaneous inhibitory postsynaptic currents (sIPSCs) in pyramidal neurons of ACC slices from adult mice. GRP (300 nM) increased both frequency (from 7.0 ± 0.8 Hz to 10.6 ± 1.1 Hz, n = 7, p < 0.001) and amplitude (from 17.1 ± 0.5 pA to 20.3 ± 0.6 pA, n = 7, p < 0.05) of sIPSCs (Figure 4A-D). This facilitation was reversible after the washout of GRP (Figure 4A-D)). Moreover, the effect of GRP was concentration-dependent. The frequency of sIPSCs was significantly increased to 140.1 ± 7.8% (n = 10, p < 0.001) and 183.4 ± 10.6% (n = 10, p < 0.001) at 300 and 1000 nM GRP, respectively (Figure 4E). No significant effect was observed for 30 nM GRP (100.5 ± 12.4%, n = 5, p = 0.1, Figure 4E). The mean amplitude of sIPSCs was also significantly increased at both 300 nM GRP (110.6 ± 3.9%, n = 5, p < 0.05) and 1000 nM GRP (124.8 ± 9.0%, n = 5, p < 0.01) (Figure 4E). To further test whether the facilitatory effect of sIPSCs by GRP is mediated by GRP receptor, we applied the selective GRP receptor antagonist RC3095 following the GRP application. We found that RC3095 (3 μM, n = 5) completely eliminate the enhancement of either frequency or amplitude of sIPSCs in the ACC neurons (Figure 5A-C). The application of GABAA receptor antagonist picrotoxin (50 μM) abolished all sIPSCs (Figure 5D-F).
Activation of GRP receptor by GRP reversibly increased sIPSCs in pyramidal neurons on the ACC. (A) The representative example of GRP (300 nM) modulation of sIPSCs in a ACC pyramidal neuron. The trace below represents sIPSCs recorded before, during and after GRP application. The bottom 3 traces are presented at an expanded scale. (B and C) Time course for the GRP-induced enhancement of sIPSC frequency and amplitude in the neuron shown in (A). Note the effect of GRP is reversible. (D) Statistical results showed the GRP-induced enhancement of sIPSC frequency and amplitude in the neuron shown in (A). Note the effect of GRP is reversible. *Indicates a significant difference between control and GRP. * < 0.05, ** < 0.01. (E) The facilitatory effect of GRP on sIPSC frequency is concentration dependent (30 nM, n = 6; 300 nM, n = 5; 1000 nM, n = 7). * < 0.05, ** < 0.01, *** < 0.001.
The GRP-induced sIPSC facilitation is blocked by RC3095 and picrotoxin. (A and B) The effect of GRP (300 nM) could be blocked by RC3095 (3 μM, n = 4), suggesting that the GRP's action is mediated by GRPR (upper). Representative sIPSCs recorded in a pyramidal cell from a control mouse at a holding potential of +10 mV under baseline conditions (upper), during GRP application (middle), and after the GRPR antagonist was added (lower). (C) Time course of reversible drug effect of RC3095 (3 μM) to the enhancement of sIPSC induced by GRP treatment. (D) The effect of GRP (300 nM) could be blocked by picrotoxin (100 μM, n = 4). Representative sIPSCs recorded in a pyramidal neuron under baseline conditions (upper), during GRP application (middle), and after picrotoxin was added (lower). (E) Time course of reversible drug effect of picrotoxin (100 μM) to the enhancement of sIPSC induced by GRP treatment.
We next tested if glutamatergic neurotransmission in the ACC was affected by GRP application. At a holding potential of -70 mV and with the blockade of GABA current in the presence of picrotoxin (100 μM), we recorded spontaneous excitatory postsynaptic currents (sEPSCs) from pyramidal neurons of ACC. We found that bath application of GRP (300 nM) had no significant effect on either the frequency or amplitude of sEPSCs in ACC pyramidal neurons (n = 5, Figure 6A-B).
Increase of GABA release by GRP is action potential dependent
To investigate whether GRP receptors are involved in regulating GABA release in the ACC, we conducted experiments where RC3095 (3 μM) was applied to the bath solution after the GRP application. We found that the facilitatory effect of GRP on sIPSCs was completely reversed (Figure 7A-C, n = 5), suggesting that the effect of GRP is mediated by GRPRs. To further confirm these results, we examined miniature inhibitory postsynaptic currents (mIPSCs) in the presence of TTX (1 μM). Bath application of GRP at three different doses (0.03, 0.3 and 3 μM) did not produce any significant effect on either the frequency or amplitude of mIPSCs (Figure 7D-F). Taken together, these results demonstrate that effects of GRP were not due to a presynaptic mechanism, but rather associated with depolarization and triggering action-potentials in GABAergic interneurons.
Increase of GABA release by GRP is action potential dependent. (A) The effect of GRP (300 nM) could be blocked by TTX (1 μM, n = 4). (B) Representative sIPSCs recorded in a pyramidal neuron under baseline conditions (upper), during GRP application (middle), and after TTX was added (lower). (C) Time course of reversible drug effect of TTX (1 μM) to the enhancement of sIPSC induced by GRP treatment. (D and E) Typical examples showing the effect of GRP (300 nM) on mIPSCs in the presence of TTX. Similar results were obtained from an additional 4 neurons. (F) Statistical results showed in the presence of TTX (1 μM), GRP did not affect either amplitude or frequency of mIPSCs.
Activation of GRP receptor also increased the frequency of sIPSCs in pyramidal neurons in the basolateral anygdala (BLA) and insular cortex (IC)
To examine whether the facilitation of GABAergic transmission by GRP is a general phenomenon in the brain, we tested the effect of GRP on inhibitory transmission in the BLA and IC. Similar to the results found in the ACC, bath application of GRP (300 nM) significantly increase the frequency of sIPSCs recorded in pyramidal neurons in the BLA (p < 0.05, n = 8, Figure 8) and IC (p < 0.05, n = 8, Figure 9). Consistent with the notion that the facilitatory effects are mediated by the GRP receptor, these facilitation were completely blocked by GRP receptor antagonist, RC3095 (3 μM, n = 6; Figure 8, 9). In the presence of TTX (1 μM), GRP had no effect on mIPSCs (n = 5; Figure 8 and 9), Moreover, GRP had no effect on sEPSCs in either BLA or IC (n = 5, Figure 8 and 9).
Activation of GRP receptors increased GABA release in BLA neurons. (A) Bath application of GRP (300 nM) increased the frequency of sIPSCs in pyramidal neurons of BLA (n = 11). (B) The effect of GRP was completely blocked by RC 3091 (3 μM) (n = 5). (C) No increase of the frequency of sIPSCs was observed in the presence of TTX (1 μM) (n = 5). (D) There is no effect of GRP on sEPSCs (n = 4). Holding potential for recording sEPSCs was -70 mV.
Activation of GRP receptors increased GABA release in IC neurons. (A and B) Bath application of GRP (300 nM) increased the frequency of sIPSCs in pyramidal neurons of IC (n = 11). (B) The effect of GRP was completely blocked by RC 3091 (3 μM) (n = 5). (C) However, no increase was observed in the presence of TTX (1 μM) (n = 5). (D) There is no effect of GRP on sEPSCs (n = 4). Holding potential for recording sEPSCs was -70 mV.
Discussion
In the present study, we used whole-cell patch-clamp recording to study the actions of GRP on ACC neurons in adult mice. Our results provide strong electrophysiological evidence that GRP receptor facilitates inhibitory GABA release in the ACC. Activation of GRP receptor preferentially modulated GABAergic, but not glutamatergic transmission. Furthermore, somatodendritic GRP receptor mediated action potential-dependent GABA release in the ACC occurred in other regions, such as the BLA and IC, indicating a general role of GRP receptor in the modulation of cortical GABAergic transmission.
How GRP facilitate GABAergic transmission and modulate the neuronal circuits in the ACC? Several lines of evidence in the present study suggest that GRP acts on somatodendritic GRP receptor in GABAergic neurons to induce neuronal firing and GABA release in interneurons, thereby decreasing the excitability of pyramidal neurons. Bath application of GRP facilitated sIPSC frequency in a concentration- dependent manner, but GRP had little effect on either frequency or amplitude of mIPSCs. These results are similar to the modulatory effects of the GRP/GRP receptor system on GABAergic transmission in lateral amygdala and hippocampus [18, 26]. Interestingly, in the present study, although ACC pyramidal neurons are shown to express GRP receptors, we found little direct effect of GRP on these cell bodies nor on the frequency and amplitude of sEPSC. We cannot completely rule out other possible modulation of GRP on excitatory transmission that cannot be revealed in the present studies. Future studies are clearly needed in further investigating the roles of GRP.
ACC neurons are multi-functional and play important roles in a wide variety of behavioral functions, including sensory pain, memory, emotional and cognitive functions [19–22, 27, 28]. Glutamate is the fast excitatory transmitter [29] and GABA is the inhibitory transmitter in the ACC [30]. A balance between excitatory and inhibitory transmission is critical for many brain functions. Previous reports show that the GRP in the amygdala is inovolved in behavioral fear [18, 31–36]. In addition to the amygdala, recent studies show that lesion of the ACC produced an impairment in trace fear conditioning [37] and electrical stimulation of the ACC induced fear memory [38]. The results of the present study show that GRP may facilitate the GABAergic transmission in the ACC synapses, indicating that GRP may contribute to behavioral fear or trace fear memory by affecting inhibitory transmission within the ACC.
GRP has been implicated in mediation the itch sensation in the spinal cord [14, 15]. The possible roles of GRP within the ACC in behavioral itching have not been investigated. Furthermore, a recent work in the spinal dorsal horn suggests that alteration of inhibitory transmission in the spinal cord is important for behavioral itching [39]. It is possible that GRP may also affect spinal inhibitory transmission, in addition to act as a potential transmitter for itch from the periphery. Future studies are clearly needed in the spinal cord. Furthermore, direct evidence for GRP to act as a neurotransmitter for itching is still lacking. In human studies, ACC and IC have been shown to be involved in itch processing [40–45]. Future studies are required to address whether the modulation of GRP in the ACC inhibitory circuit would contribution to itch sensation. In summary, we report here that GRP play an important role in modulating inhibitory transmission within the ACC, IC and amygdala. It is likely that supraspinal GRP may contribute to a wide range of physiological and pathological functions, rather than act as a selective transmitter for itch as reported at the level of spinal cord.
Conflict of interests
The authors declare that they have no competing interests.
References
Anastasi A, Erspamer V, Bucci M: Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 1971,27(2):166–167. 10.1007/BF02145873
Battey J, Wada E: Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci 1991,14(12):524–528. 10.1016/0166-2236(91)90005-F
Kamichi S, Wada E, Aoki S, Sekiguchi M, Kimura I, Wada K: Immunohistochemical localization of gastrin-releasing peptide receptor in the mouse brain. Brain Res 2005,1032(1–2):162–170. 10.1016/j.brainres.2004.10.068
Ladenheim EE, Jensen RT, Mantey SA, Moran TH: Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res 1992,593(2):168–178. 10.1016/0006-8993(92)91305-X
Moody TW, Getz R, O'Donohue TL, Rosenstein JM: Localization of receptors for bombesin-like peptides in the rat brain. Ann N Y Acad Sci 1988, 547: 114–130. 10.1111/j.1749-6632.1988.tb23880.x
Moody TW, O'Donohue TL, Jacobowitz DM: Biochemical localization and characterization of bombesin-like peptides in discrete regions of rat brain. Peptides 1981,2(1):75–79. 10.1016/S0196-9781(81)80014-9
Moran TH, Moody TW, Hostetler AM, Robinson PH, Goldrich M, McHugh PR: Distribution of bombesin binding sites in the rat gastrointestinal tract. Peptides 1988,9(3):643–649. 10.1016/0196-9781(88)90177-5
Panula P, Yang HY, Costa E: Neuronal location of the bombesin-like immunoreactivity in the central nervous system of the rat. Regul Pept 1982,4(5):275–283. 10.1016/0167-0115(82)90120-3
Wada E, Way J, Lebacq-Verheyden AM, Battey JF: Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci 1990,10(9):2917–2930.
Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battery J: cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron 1991,6(3):421–430. 10.1016/0896-6273(91)90250-4
Gamble KL, Allen GC, Zhou T, McMahon DG: Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci 2007,27(44):12078–12087. 10.1523/JNEUROSCI.1109-07.2007
Petronilho F, Roesler R, Schwartsmann G, Dal Pizzol F: Gastrin-releasing peptide receptor as a molecular target for inflammatory diseases. Inflamm Allergy Drug Targets 2007,6(4):197–200. 10.2174/187152807783334319
Patel O, Shulkes A, Baldwin GS: Gastrin-releasing peptide and cancer. Biochim Biophys Acta 2006,1766(1):23–41.
Sun YG, Chen ZF: A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007,448(7154):700–703. 10.1038/nature06029
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF: Cellular basis of itch sensation. Science 2009,325(5947):1531–1534. 10.1126/science.1174868
Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT: Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes 2008,15(1):58–64.
Ohki-Hamazaki H, Iwabuchi M, Maekawa F: Development and function of bombesin-like peptides and their receptors. Int J Dev Biol 2005,49(2–3):293–300. 10.1387/ijdb.041954ho
Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY: Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell 2002,111(6):905–918. 10.1016/S0092-8674(02)01116-9
Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV: An area specialized for spatial working memory in human frontal cortex. Science 1998,279(5355):1347–1351. 10.1126/science.279.5355.1347
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC: Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997,277(5328):968–971. 10.1126/science.277.5328.968
Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, et al.: Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005,47(6):859–872. 10.1016/j.neuron.2005.08.014
Zhuo M: Glutamate receptors and persistent pain: targeting forebrain NR2B subunits. Drug Discov Today 2002,7(4):259–267. 10.1016/S1359-6446(01)02138-9
Cao XY, Xu H, Wu LJ, Li XY, Chen T, Zhuo M: Characterization of intrinsic properties of cingulate pyramidal neurons in adult mice after nerve injury. Mol Pain 2009, 5–73. 10.1186/1744-8069-5-5
Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, Ren M, Knifed E, Shum F, Quan J, et al.: Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS One 2007,2(1):e167. 10.1371/journal.pone.0000167
Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, et al.: Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci 2005,25(48):11107–11116. 10.1523/JNEUROSCI.1678-05.2005
Lee K, Dixon AK, Gonzalez I, Stevens EB, McNulty S, Oles R, Richardson PJ, Pinnock RD, Singh L: Bombesin-like peptides depolarize rat hippocampal interneurones through interaction with subtype 2 bombesin receptors. J Physiol 1999,518(Pt 3):791–802. 10.1111/j.1469-7793.1999.0791p.x
Zhuo M: Cortical excitation and chronic pain. Trends Neurosci 2008,31(4):199–207. 10.1016/j.tins.2008.01.003
Zhuo M: A synaptic model for pain: long-term potentiation in the anterior cingulate cortex. Mol Cells 2007,23(3):259–271.
Wei F, Li P, Zhuo M: Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 1999,19(21):9346–9354.
Wu LJ, Xu H, Ren M, Zhuo M: Genetic and pharmacological studies of GluR5 modulation of inhibitory synaptic transmission in the anterior cingulate cortex of adult mice. Dev Neurobiol 2007,67(2):146–157. 10.1002/dneu.20331
Yamada K, Santo-Yamada Y, Wada E, Wada K: Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry 2002,7(1):113–117. 116 10.1038/sj/mp/4000974
Martins MR, Reinke A, Valvassori SS, Machado RA, Quevedo J, Schwartsmann G, Roesler R: Non-associative learning and anxiety in rats treated with a single systemic administration of the gastrin-releasing peptide receptor antagonist RC-3095. Peptides 2005,26(12):2525–2529. 10.1016/j.peptides.2005.06.006
Roesler R, Kopschina MI, Rosa RM, Henriques JA, Souza DO, Schwartsmann G: RC-3095, a bombesin/gastrin-releasing peptide receptor antagonist, impairs aversive but not recognition memory in rats. Eur J Pharmacol 2004,486(1):35–41. 10.1016/j.ejphar.2003.12.011
Roesler R, Meller CA, Kopschina MI, Souza DO, Henriques JA, Schwartsmann G: Intrahippocampal infusion of the bombesin/gastrin-releasing peptide antagonist RC-3095 impairs inhibitory avoidanceretention. Peptides 2003,24(7):1069–1074. 10.1016/S0196-9781(03)00179-7
Santo-Yamada Y, Yamada K, Wada E, Goto Y, Wada K: Blockade of bombesin-like peptide receptors impairs inhibitory avoidance learning in mice. Neurosci Lett 2003,340(1):65–68. 10.1016/S0304-3940(03)00077-6
Venturella R, Lessa D, Luft T, Roozendaal B, Schwartsmann G, Roesler R: Dexamethasone reverses the memory impairment induced by antagonism of hippocampal gastrin-releasing peptide receptors. Peptides 2005,26(5):821–825. 10.1016/j.peptides.2004.12.010
Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ: Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA 2003,100(22):13087–13092. 10.1073/pnas.2132313100
Tang J, Ko S, Ding HK, Qiu CS, Calejesan AA, Zhuo M: Pavlovian fear memory induced by activation in the anterior cingulate cortex. Mol Pain 2005, 1: 6. 10.1186/1744-8069-1-6
Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al.: Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65(6):886–898. 10.1016/j.neuron.2010.02.025
Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, Schwaiger M, Ring J: Processing of histamine-induced itch in the human cerebral cortex: a correlation analysis with dermal reactions. J Invest Dermatol 2000,115(6):1029–1033. 10.1046/j.1523-1747.2000.00193.x
Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P: Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain 2001,92(1–2):295–305. 10.1016/S0304-3959(01)00271-8
Herde L, Forster C, Strupf M, Handwerker HO: Itch induced by a novel method leads to limbic deactivations a functional MRI study. J Neurophysiol 2007,98(4):2347–2356. 10.1152/jn.00475.2007
Mochizuki H, Sadato N, Saito DN, Toyoda H, Tashiro M, Okamura N, Yanai K: Neural correlates of perceptual difference between itching and pain: a human fMRI study. Neuroimage 2007,36(3):706–717. 10.1016/j.neuroimage.2007.04.003
Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K: Imaging of central itch modulation in the human brain using positron emission tomography. Pain 2003,105(1–2):339–346. 10.1016/S0304-3959(03)00249-5
Valet M, Pfab F, Sprenger T, Woller A, Zimmer C, Behrendt H, Ring J, Darsow U, Tolle TR: Cerebral processing of histamine-induced itch using short-term alternating temperature modulation--an FMRI study. J Invest Dermatol 2008,128(2):426–433.
Acknowledgements
This work was supported by Grants from the EJLB-CIHR Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, NeuroCanada, and CIHR operating grants (CIHR66975 and CIHR81086) (M. Z.). M.Z. is also supported by the World-Class University (WCU) program of the Ministry of Education, Science and Technology in Korea through KOSEF (R32-10142).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
XYC, VM, PL and LJW performed the experiments included in the manuscript. MZ designed the experiments. XYC, LJW and MZ wrote the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cao, X., Mercaldo, V., Li, P. et al. Facilitation of the inhibitory transmission by gastrin-releasing peptide in the anterior cingulate cortex. Mol Pain 6, 52 (2010). https://doi.org/10.1186/1744-8069-6-52
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-6-52