Abstract
Over the last years, evidence has accumulated suggesting that by systematically reducing the amount of dietary carbohydrates (CHOs) one could suppress, or at least delay, the emergence of cancer, and that proliferation of already existing tumor cells could be slowed down. This hypothesis is supported by the association between modern chronic diseases like the metabolic syndrome and the risk of develo** or dying from cancer. CHOs or glucose, to which more complex carbohydrates are ultimately digested, can have direct and indirect effects on tumor cell proliferation: first, contrary to normal cells, most malignant cells depend on steady glucose availability in the blood for their energy and biomass generating demands and are not able to metabolize significant amounts of fatty acids or ketone bodies due to mitochondrial dysfunction. Second, high insulin and insulin-like growth factor (IGF)-1 levels resulting from chronic ingestion of CHO-rich Western diet meals, can directly promote tumor cell proliferation via the insulin/IGF1 signaling pathway. Third, ketone bodies that are elevated when insulin and blood glucose levels are low, have been found to negatively affect proliferation of different malignant cells in vitro or not to be usable by tumor cells for metabolic demands, and a multitude of mouse models have shown anti-tumorigenic properties of very low CHO ketogenic diets. In addition, many cancer patients exhibit an altered glucose metabolism characterized by insulin resistance and may profit from an increased protein and fat intake.
In this review, we address the possible beneficial effects of low CHO diets on cancer prevention and treatment. Emphasis will be placed on the role of insulin and IGF1 signaling in tumorigenesis as well as altered dietary needs of cancer patients.
Similar content being viewed by others
Introduction
When defining the factors of a healthy lifestyle that aims at preventing a disease like cancer, a logical approach is to compare individuals that get the disease with those that don't. Cancer, which might be considered a disease of civilization, has consistently been reported to be very rare among uncivilized hunter-gatherer societies [1–4]. This observation makes sense from an evolutionary perspective from which it is reasonable to assume that the lifestyle factors that protect our genome against tumorigenesis have been selected for early in the history of the genus homo when humans lived as hunter-gatherers [5]. In particular, the time since the neolithic revolution, which meant the transition from foraging and nomadism to agriculture and settlement, spans a fraction less than 1% of human history. Thus, the switch from the "caveman's diet" consisting of fat, meat and only occasionally roots, berries and other sources of carbohydrate (CHO) to a nutrition dominated by easily digestible CHOs derived mainly from grains as staple food would have occurred too recently to induce major adoptions in our genes encoding the metabolic pathways. This is even more the case for the changes that occurred over the past 100 years, in particular the switch from labor in the field to a sedentary lifestyle and an increase in the consumption of easily digestible CHOs with high glycemic indices (GIs), leading to diseases of civilization that are strongly associated with the so-called Western way of life [6]. Despite a large heterogeneity in regional occupation, modern hunter-gatherers share certain lifestyle factors that are not frequently met in Westernized societies, including regular physical activity, sun exposure, sufficient sleep, low chronic stress and the lack of foods that would also not have been available to our pre-neolithic ancestors. While there is already compelling evidence for the beneficial roles of regular physical activity and sufficient vitamin D in the prevention and treatment of cancer, the influence of the altered nutritional patterns in the Western diet is less clearly defined.
Modern hunter-gatherers' diet
Data from 229 hunter-gatherer societies included in the revised Ethnographic Atlas indicate that hunter-gatherer diets differ from typical Western ones in basically two aspects: first, a strong reliance on animal foods (45-65% of energy or E%) and second, the consumption of low-GI plant foods such as vegetables, fruits, seeds and nuts [7]. This is consistent with stable isotope studies of human fossils [8, 9]. As a consequence, the amount and type of carbohydrates in the typical western diet differ markedly from the ones that our genes adapted to. In particular, Cordain and colleagues estimated that modern hunter-gatherers derived about 22-40 E% from CHOs and 19-30 E% from protein, which is lower and higher, respectively, than recommended by Western food agencies. Recently, Ströhle & Hahn confirmed that the energy derived from CHOs - despite being dependent upon geographic latitude and ecological environment - in modern hunter-gatherers is markedly lower than in Westernized societies [10]. High CHO intake, in particular in the form of sugar and other high GI foods, has been linked to modern diseases like metabolic syndrome [11], Alzheimer's disease [12, 13], cataract and macula degeneration [14–16] and gout [17]. Intriguingly, with the possible exception of Alzheimer's disease [25, 89, 90].
Indirect effects of glucose availability
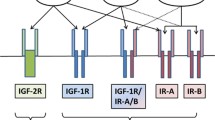
Besides delivering more glucose to the tumor tissue, hyperglycemia has two other important negative effects for the host: First, as pointed out by Ely and Krone, even modest blood glucose elevations as they typically occur after a Western diet meal competitively impair the transport of ascorbic acid into immune cells [88, 91]. Ascorbic acid is needed for effective phagocytosis and mitosis, so that the immune response to malignant cells is diminished. Second, it has been shown in vitro and in vivo that hyperglycemia activates monocytes and macrophages to produce inflammatory cytokines that play an important role also for the progression of cancer [92–94] (see below). Third, high plasma glucose concentrations elevate the levels of circulating insulin and free IGF1, two potent anti-apoptotic and growth factors for most cancer cells [60]. Free IGF1 is elevated due to a decreased transcription of IGF binding protein (IGFBP)-1 in the liver mediated by insulin [95]. Due to expression of GLUT2, the β-cells of the pancreas are very sensitive to blood glucose concentration and steeply increase their insulin secretion when the latter exceeds the normal level of ~5 mM. In the typical Western diet consisting of three meals a day (plus the occasional CHO-rich snacks and drinks), this implies that insulin levels are elevated above the fasting baseline over most of the day. Both insulin and IGF1 activate the PI3K/Akt/mTOR/HIF-1α pathway by binding to the IGF1 receptor (IGF1R) and insulin receptor (IR), respectively (Figure 2). In addition, insulin stimulates the release of the pro-inflammatory cytokine interleukin (IL)-6 from human adipocytes [96]. Thus, it could be hypothesized that a diet which repeatedly elevates blood glucose levels due to a high GL provides additional growth stimuli for neoplastic cells. In this respect, Venkateswaran et al. have shown in a xenograft model of human prostate cancer that a diet high in CHO stimulated the expression of IRs and phosphorylation of Akt in tumor tissue compared to a low CHO diet [97]. In colorectal [27], prostate [24] and early stage breast cancer patients [23, 98] high insulin and low IGFBP-1 levels have been associated with poor prognosis. These findings again underline the importance of controlling blood sugar and hence insulin levels in cancer patients. Dietary restriction and/or a reduced CHO intake are straightforward strategies to achieve this goal.
Altered nutritional needs of cancer patients
Cancer patients and those with metabolic syndrome share common pathological abnormalities. Since 1885, when Ernst Freund described signs of hyperglycemia in 70 out of 70 cancer patients [99], it has been repeatedly reported that glucose tolerance and insulin sensitivity are diminished in cancer patients even before signs of cachexia (weight loss) become evident [100–102]. Both diabetes and cancer are characterized by a common pathophysiological state of chronic inflammatory signalling and associated insulin resistance. In cancer patients, insulin resistance is thought to be mediated by an acute phase response that is triggered by pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α [101] and IL-6 [103]. In animal and human studies, removal of the tumor resulted in improved glucose clearance, suggesting that these cytokines are secreted, at least in part, from the tumor tissue itself [104, 105]. The impact on the metabolism of the host is illustrated in Figure 3. In the liver, the inflammatory process leads to increased gluconeogenesis that is fuelled by lactate secreted from the tumor as well as glycerol from fatty acid breakdown and the amino acid alanine [106] from muscle proteolysis. Gluconeogenesis is an energy-consuming process and might contribute to cancer cachexia by increasing total energy expenditure. Despite increased lipolysis, hepatic production of ketone bodies is usually not enhanced in cancer patients [107, 108]. This is in contrast to starvation, where the ketone bodies acetoacetate and β-hydroxybutyrate counteract proteolysis by providing energy for the brain and muscles [109]. In muscle, glucose uptake and glycogen synthesis are inhibited already at early stages of tumor progression, while fatty acid oxidation remains at normal levels or is increased [110, 111]. In the latter case, more fat has to be provided from lipolysis in the adipose tissue. In addition, muscles progressively lose protein to provide amino acids for hepatic synthesis of acute-phase proteins and as precursors for gluconeogenesis. Thus, insulin resistance contributes to fat loss and muscle wasting, the two hallmarks of cancer cachexia. At the same time, it makes more glucose in the blood available for tumor cells.
Fat and ketone bodies: anti-cachectic effects
It therefore seems reasonable to assume that dietary carbohydrates mainly fuel malignant cells which express the insulin-independent glucose transporters GLUT1 and GLUT3, while muscle cells are more likely to benefit from an increased fat and protein intake. This was summarized as early as in 1977 by C. Young, who stated that lipid sources predominate the fuel utilization of peripheral tissue of patients with neoplastic disease compared to healthy subjects [112]. In addition, most malignant cells lack key mitochondrial enzymes necessary for conversion of ketone bodies and fatty acids to ATP [40, 113, 114], while myocytes retain this ability even in the cachectic state [107]. This led some authors to propose a high-fat, ketogenic diet (KD) as a strategy to selectively improve body composition of the host at the expense of the tumor [113, 115, 116]. The traditional KDs, which recommended protein and CHO to account, in combination, for roughly 20 E% (in the incorrect assumption that they were equivalent due to gluconeogenesis) and fat for the remaining 80 E%, have been widely used to treat childhood epilepsia since the 1920s [117]. KDs are also used to treat adiposity [118] and currently adult epilepsy [119]. In the 1980s, Tisdale and colleagues investigated the effects of a ketogenic diet consisting mainly of medium chain triglycerides (MCTs) on two aggressive animal tumor models that were known to lack the ability to utilize ketone bodies. While the diet had no effect on rats bearing the Walker 256 sarcoma [120], it decreased the cachectic weight loss in proportion to its fat content in mice bearing the mouse-specific colon carcinoma MAC16 [121]. For the latter, they further proved an anti-cachectic effect of a ketogenic diet in which the MCTs were replaced with long chain triglycerides (LCTs), although to a somewhat lesser extent [122]. Contrary to LCTs, MCTs do not require transport in chylomicrones, but readily reach the liver where they are metabolized to yield high amounts of ketone bodies. Interestingly, administration of insulin was able to reduce the weight loss similar to the ketogenic MCT diet, but at the expense of a 50% increase in tumor size, which could be counteracted by addition of β-hydroxybutyrate in the drinking water [123]. The supporting effect of insulin on tumor growth has been known since 1924, when Händel and Tadenuma described the nourishing effect of insulin on tumor tissue in an animal model [124], showing evidence that reducing insulin might reduce tumor growth.
Clinical studies on fat and cachexia
Clinical studies investigating the anti-cachectic effects of high-fat diets are, however, rare. Fearon et al. administered a 70% MCT diet supplemented with β-hydroxybutyrate parenterally to five late-stage cachectic patients. After seven days on the diet, mean body weight had increased by 2 kg and their physical performance status had improved [125]. Nebeling et al. investigated the effects of a MCT-based ketogenic diet taken ad libitum (60% MCT oil, 20% protein, 10% CHO, 10% other fats) on body weight and glucose metabolism in two pediatric patients with advanced-stage astrocytoma. Within 7 days on the diet, blood glucose levels had decreased to normal, while glucose uptake by the tumor estimated from FDG-PET scans had decreased by an average value of 21.8%. Notably, body weight remained stable throughout the study period of 8 weeks. In a randomized controlled study, Breitkreuz et al. showed that by supplementing the normal diet of 11 under-nourished, non-diabetic patients suffering from metastatic gastro-intestinal cancers with a fat-enriched liquid supplementation for 8 weeks, it was possible to reverse the loss of body weight and lean tissue mass and to improve several quality-of-life parameters in the treatment group, while the control group continued to lose body and lean tissue weight [126]. The supplement contained 66% energy from fat, of which 45% were monounsaturated, 27% saturated (both LCT and MCT) and 28% polyunsaturated; mean energy intake ranged between 1000 and 2000 kcal/day and tended to be higher in patients receiving the additional fat drink.
The benefits of mild ketosis
The study of Breitkreuz et al. shows that ketosis might not be necessary to improve the cachectic state of cancer patients. In recent years, however, more evidence has emerged from both animal and laboratory studies indicating that cancer patients could benefit further from a very low CHO KD. In their mouse models, Tisdale et al. already noted that the KD not only attenuated the cachectic effects of the tumor, but also that the tumors grew more slowly (although they did not attribute this to a direct anti-tumor effect of β-hydroxybutyrate). Tumor growth inhibition through a KD has now been established in many animal models, is supported by a few clinical case reports, and laboratory studies have begun to reveal the underlying molecular mechanisms.
In vitro studies
More than 30 years ago, Magee et al. were the first to show that treating transformed cells with various, albeit supra-physiological, concentrations of β-hydroxybutyrate causes a dose-dependent and reversible inhibition of cell proliferation [116]. Their interpretation of the results that ''...ketone bodies interfere with either glucose entry or glucose metabolism...'' has been confirmed and further specified by Fine et al., who connected the inhibition of glycolysis in the presence of abundant ketone bodies to the overexpression of uncoupling protein-2 (UCP-2), a mitochondrial defect occurring in many tumor cells [127]. In normal cells, abundant acetyl-CoA and citrate from the breakdown of fatty acids and ketone bodies would inhibit key enzymes of glycolysis to ensure stable ATP levels; in tumor cells, however, the same phenomenon would imply a decrease in ATP production if the compensatory ATP production in the mitochondria was impaired. For several colon and breast cancer cell lines, Fine et al. showed that the amount of ATP loss under treatment with acetoacetate was related to the level of UCP-2 expression.
Very recently, Maurer et al. demonstrated that glioma cells - although not negatively influenced by β-hydroxybutyrate - are not able to use this ketone body as a substitute for glucose when starved of the latter, contrary to benign neuronal cells [128]. This supports the hypothesis that under low glucose concentrations, ketone bodies could serve benign cells as a substitute for metabolic demands while offering no such benefit to malign cells.
Animal studies
To our knowledge, the first and - with a total of 303 rats and nine experiments - most extensive study of a KD in animals was conducted by van Ness van Alstyne and Beebe in 1913 [129]. Experiments were divided into two classes: in the first class, rats in the treatment arm were fed a CHO-free diet consisting of casein and lard for several weeks before plantation of a Buffalo sarcoma, while the control arm received either bread only or casein, lard and lactose. Rats on the CHO-free diet not only gained more weight than the controls, but also exhibited much less tumor growth and mortality rates, the differences being "... so striking as to leave no room for doubt that the diet was an important factor in enabling the rats to resist the tumor after growth had started." In a second class of experiments using either the slow-growing Jensen sarcoma or the aggressive Buffalo sarcoma, the rats were put on the CHO-free diet on the same day that the tumor was planted. This time, differences between the treatment and control groups were "... so slight that ... one is left in no doubt of the ineffectiveness of non-carbohydrate feeding begun at the time of tumor implantation." Interestingly, this parallels the observation of Fearon et al. that rats who started to receive a KD at the same day as tumor transplantation did not differ from controls in either body or tumor weight after 14 d [120]. In these rats, it was noted that despite persistent ketosis, blood glucose levels were not significantly lower than in controls which were also fed ad libitum. This stability of blood glucose, independent of ketosis, was subsequently confirmed in studies in which mice were fed ad libitum on a KD [84, 114, 121–123, 130] although two studies reported a drop in blood glucose concentrations compared with the control group [116, 131]. In the study of Magee et al., however, diet was presented as a liquid vegetable oil and energy intake was not monitored, allowing for the possibility that the animals underate voluntarily, in this way consuming a "caloric restricted KD" used in several experimental settings from the Seyfried lab [84, 114, 132], which was shown therein to be superior to the unrestricted KD in tumor growth control. That "caloric restriction" per se can hamper tumor growth has been impressively demonstrated already in 1942 by A. Tannenbaum in a series of comprehensive mouse models with different mouse strains and tumor induction types [133]. Throughout all experimental series, a strict restriction of food intake (impeding weight gain) several weeks before inducing tumorigenesis by application of 3,4 benzpyrene decreased the appearance rate and appearance time of tumors in the diet mice compared to the ad libitum controls. Notably, the calorie-restricted diet was composed of 53% CHOs compared to 69% in the control group. Despite a lack of data on blood glucose and ketone body levels, it could be speculated that the strict restriction of food per se (to 50-60% of the control group) induced a ketotic state and thus the ketones were - at least to some extend - responsible for the effects observed.
In Table 1, we summarize the main results of various mouse studies that determined the effects of KDs on tumor growth and host survival. The results seem to indicate an anti-tumor effect of ketosis. Freedland et al. indeed reported that the mice with the highest levels of ketone bodies had the longest survival times in a human prostate cancer xenograft model [134]. But other studies suggest that there are further possible factors to consider. Seyfried et al. used linear regression to show that plasma glucose and IGF1 levels are a better predictor of tumor growth than ketone bodies in a murine astrocytoma model [84]. Tumor growth in this as well as in a follow-up [114] study was only retarded when the KD had been restricted to induce body weight loss, again underlining the effect of caloric restriction per se. This contrasts with other studies showing growth-inhibitory effects of unrestricted or higher-caloric KDs despite neither decreases in blood glucose concentration nor body weight loss compared with a control group [130, 134, 135]. According to Otto et al., whose diet had been enriched in MCT and omega-3 fatty acids, fat quality might play a role in explaining these results [130]. The situation in humans might be different as well, as for example Fine et al. found no correlation between calorie intake or weight loss and disease progression in ten patients on an unrestricted KD [136] (see also below).
Concerning fat quality, Freedland et al. observed that a diet rich in corn oil might stimulate prostate cancer growth to a greater extent than one rich in saturated fat [134]. A recent study suggests, however, that tumor growth inhibition neither depends on fat quality nor ketone body levels [131]. In this case, mice injected with either murine squamous cell carcinoma or human colorectal carcinoma cells received a low CHO, high-protein diet in which ~ 60 E% was derived from protein, 10-15 E% from CHO and ~ 25 E% from fat. No systemic ketosis was measured, yet tumors grew significantly less compared with a standard diet containing 55 E% from CHO and 22 E% from the same fat source. IGF1 levels and body weight remained stable, so these findings could not be attributed to one of these factors. There was, however, a significant drop in blood glucose, insulin and lactate levels, and a positive correlation between blood lactate as well as insulin levels and tumor growth was found. The study of Venkateskwaran et al. indicates that in prostate cancer insulin and/or IGF1 play major roles in driving tumor cell proliferation [97].
The diversity of these findings should not be surprising, given the variety of mice strains, tumor cell lines, diet composition and time of diet initiation relative to tumor planting. Instead, it seems remarkable that the same basic treatment, namely drastic restriction of CHOs, apparently induces anti-tumoral effects via different pathways. Thus, it may depend on the circumstances which variables - including blood glucose, insulin, lactate, IGF1, fat quality and ketone bodies - are the best predictors of and responsible for the anti-tumor effects of very low CHO diets.
Human studies
Until now, no randomized controlled trials have been conducted to evaluate the effects of a KD on tumor growth and patient survival. It has to be noted in general, however, that any dietary intervention requiring a dramatic change of life style makes randomized studies nearly impossible - however, even prospective cohort studies are missing. There is only anecdotal evidence that such a diet might be effective as a supportive treatment. One study investigated whether a high-fat diet (80% non-nitrogenous calories from fat) would inhibit tumor cell replication compared to a high-dextrose diet (100% non-nitrogenous calories from dextrose) in 27 patients with gastro-intestinal cancers [137]. Diets were administered parenterally and cell proliferation assessed using thymidine labeling index on tumor samples. After 14 days, the authors found a non-significant trend for impaired proliferation in the high-fat group. Whether ketosis was achieved with this regime was not evaluated, but blood glucose levels were comparable in both trial groups. A very recent pilot trial demonstrated the feasibility of a low CHO up to a ketogenic regimen implemented for 12 weeks in very advanced outpatient cancer patients. Notably, severe side effects were not observed, nearly all standard blood parameters improved and some measures of quality of life changed for the better [138]. The first attempt to treat cancer patients with a long-term controlled KD was reported by L. Nebeling in 1995 for two pediatric patients with astrocytoma [139]. The results of those two cases were very encouraging and the diet was described in detail in another publication [140]. Implementing a KD with additional calorie restriction in a female patient with glioblastoma multiforme clearly demonstrated that this intervention was able to stop tumor growth [132]. This was achieved, however, on the expense of a dramatic weigh loss of 20% over the intervention period, which is no option for the majority of metastatic cancer patients being in a catabolic state. A first clinical study applying a non-restricted KD for patients with glioblastoma (ERGO-study, NHI registration number NCT00575146), which was presented at the 2010 ASCO meeting [141], showed good feasibility and suggested some anti-tumor activity. The protocol of another clinical interventional trial (RECHARGE trial, NCT00444054) treating patients with metastatic cancer by a very low CHO diet was published in 2008 [142], and preliminary data from this study presented at the 2011 ASCO-meeting showed a clear correlation between disease stability or partial remission and high ketosis, independent of weight loss and unconscious caloric restriction of the patients [136]. While a randomized study for the treatment of prostate cancer patents applying the Atkins diet (NCT00932672) is currently recruiting patients at the Duke University, another trial posted at the clinical trials database (ClinicalTrials.gov) is not yet open for recruitment (NCT01092247). Very recently, two Phase I studies applying a ketogenic diet based on KetoCal® 4:1 started recruitment at the University of Iowa, intended to treat prostate cancer patients (KETOPAN, NCT01419483) and non-small cell lung cancer (KETOLUNG, NCT01419587). Thus, in the future, several data should be available to judge whether this kind of nutrition is useful as either a supportive or even therapeutic treatment option for cancer patients.
Is there a role for carbohydrate restriction in the prevention of cancer?
"Prevention of cancer" can refer to either the inhibition of carcinogenesis per se or - once that cells made the transition to malignancy - the sufficient delay of tumor growth, so that it remains undetected and asymptomatic during a subject's lifespan. There is evidence that even modest CHO restriction may influence both of these mechanisms positively through various pathways. The IGF1R-IR pathway has already been discussed: once a potentially carcinogenic somatic mutation has occurred, the probability for carcinogenesis of a cell that is borderline between apoptosis and malignancy might be raised by high levels of insulin and IGF1 in the micro-environment. Once a cell became malignant, high insulin and IGF1 levels might accelerate proliferation and progression towards a more aggressive, glycolytic phenotype. In rats treated with the carcinogen N-methyl-N-nitrosourea, it has been shown that lowering the CHO content of the diet from 60 E% to 40 E% with a simultaneous increase in protein was sufficient to lower postprandial insulin levels as well as decrease the appearance rate of tumors from (18.2 ± 1.3)%/wk to (12.9 ± 1.4)%/wk (p < 0.05), however with no statistically significant effect on tumor latency and weight measured after 10 wk [143]. Similarly, a recent study reported that NOP mice, which normally have a 70 - 80% chance of develo** breast cancer over their lifetime due to genetic mutations, stayed tumor-free at 1 year of age when their calories from CHO were limited to 15%, while almost half of those on a 55% CHO diet developed tumors [131]. Notably, only 3 out of 11 mice in the 15% CHO group died with having a tumor compared to 7 out of 10 in the 55% CHO group; at death, significantly lower plasma insulin levels had been measured for the low CHO group. These results support the epidemiological [25, 29, 31, 32] and in vitro[81, 144] findings that high CHO diets, in particular those including high GI foods, promote mammary tumorigenesis via the sustained action of insulin.
Lower insulin levels may further increase the chance of intermittent ketosis, in particular if CHO restriction is combined with exercise, calorie restriction or intermittent fasting. Seyfried and Shelton [40] pointed out the possibility of ketone bodies to help in cancer prevention through their ability to protect the mitochondria from inflammation and ROS. Being more satiating than low-fat diets [145, 146], a low CHO diet would make it easier to avoid caloric overconsumption or to implement intermittent fasting as an additional lifestyle change [147].
Avoidance of chronic inflammation
Another potential benefit of low CHO diets might lie in their influence upon inflammatory processes that take place within various tissues. Inflammation is a well-established driver of early tumorigenesis and accompanies most, if not all cancers [148]. Chronic, 'smouldering' inflammation can both cause and develop along with neoplasia. There is evidence that chronic intake of easily digestible CHOs is able to promote such an inflammatory state in leukocytes and endothelial cells [94]. In obese individuals [149] and healthy subjects who underwent eccentric exercise training [150], the inflammatory state was further augmented postprandially through a high CHO intake, but not through high-fat, low CHO meals in the latter study. Maybe more importantly, even moderate CHO restriction has been shown to effectively target several important markers of atherosclerosis and type II diabetes, both of which are associated with chronic inflammation [151–157]. Forsythe et al. showed that in overweight individuals with dyslipidemia a very low CHO diet had a more favorable effect than a low fat diet in reducing several markers of inflammation [158]. Given these findings, it can be hypothesized that a diet with a low GL positively affects cancer risk through reducing postprandial hyperglycemia and the associated inflammatory response.
In this context, it is important to note that a low CHO diet offers further possibilities to target inflammation through omission or inclusion of certain foods. Usually, CHO restriction is not only limited to avoiding sugar and other high-GI foods, but also to a reduced intake of grains. Grains can induce inflammation in susceptible individuals due to their content of omega-6 fatty acids, lectins and gluten [159, 160]. In particular gluten might play a key role in the pathogenesis of auto-immune and inflammatory disorders and some malignant diseases. In the small intestine, gluten triggers the release of zonulin, a protein that regulates the tight junctions between epithelial cells and therefore intestinal, but also blood-brain barrier function. Recent evidence suggests that overstimulation of zonulin in susceptible individuals could dysregulate intercellular communication promoting tumorigenesis at specific organ sites [161].
Paleolithic-type diets, that by definition exclude grain products, have been shown to improve glycemic control and cardiovascular risk factors more effectively than typically recommended low-fat diets rich in whole grains [162]. These diets are not necessarily very low CHO diets, but focus on replacing high-GI modern foods with fruits and vegetables, in this way reducing the total GL. This brings us back to our initial perception of cancer as a disease of civilization that has been rare among hunter-gatherer societies until they adopted the Western lifestyle. Although there are certainly many factors contributing to this phenomenon, the evidence presented in this review suggests that reduction of the high CHO intake that accounts for typically > 50 E% in the Western diet may play its own important role in cancer prevention and outcome.
Conclusions
(i) We summarize our main findings from the literature regarding the role of dietary CHO restriction in cancer development and outcome.
Most, if not all, tumor cells have a high demand on glucose compared to benign cells of the same tissue and conduct glycolysis even in the presence of oxygen (the Warburg effect). In addition, many cancer cells express insulin receptors (IRs) and show hyperactivation of the IGF1R-IR pathway. Evidence exists that chronically elevated blood glucose, insulin and IGF1 levels facilitate tumorigenesis and worsen the outcome in cancer patients.
(ii) The involvement of the glucose-insulin axis may also explain the association of the metabolic syndrome with an increased risk for several cancers. CHO restriction has already been shown to exert favorable effects in patients with the metabolic syndrome. Epidemiological and anthropological studies indicate that restricting dietary CHOs could be beneficial in decreasing cancer risk.
(iii) Many cancer patients, in particular those with advanced stages of the disease, exhibit altered whole-body metabolism marked by increased plasma levels of inflammatory molecules, impaired glycogen synthesis, increased proteolysis and increased fat utilization in muscle tissue, increased lipolysis in adipose tissue and increased gluconeogenesis by the liver. High fat, low CHO diets aim at accounting for these metabolic alterations. Studies conducted so far have shown that such diets are safe and likely beneficial, in particular for advanced stage cancer patients.
(iv)CHO restriction mimics the metabolic state of calorie restriction or - in the case of KDs - fasting. The beneficial effects of calorie restriction and fasting on cancer risk and progression are well established. CHO restriction thus opens the possibility to target the same underlying mechanisms without the side-effects of hunger and weight loss.
(v) Some laboratory studies indicate a direct anti-tumor potential of ketone bodies. During the past years, a multitude of mouse studies indeed proved anti-tumor effects of KDs for various tumor types, and a few case reports and pre-clinical studies obtained promising results in cancer patients as well. Several registered clinical trials are going to investigate the case for a KD as a supportive therapeutic option in oncology.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- CHO:
-
carbohydrate
- CT:
-
computed tomography
- E%:
-
percentage of energy
- FDG:
-
18F-fluoro-2-deoxyD-glucose
- GI:
-
glycemic index
- GL:
-
glycemic load
- HIF-1α:
-
hypoxia inducible factor-1α
- IGF:
-
insulin like growth factor
- IR:
-
insulin receptor
- KD:
-
ketogenic diet
- LCT:
-
long chain triglycerides
- MMP:
-
matrix metalloproteinase
- MCT:
-
medium chain triglycerides
- mTOR:
-
mammalian target of rapamycin
- PET:
-
positron emission tomography
- PI13K:
-
Phosphoinositide 3-kinase
- ROS:
-
reactive oxygen species.
References
Levine I: Cancer among the American Indians and its bearing upon the ethnologicaI distribution of the disease. J Cancer Res Clin Oncol 1910, 9: 422-435.
Orenstein AJ: Freedom Of Negro Races From Cancer. Br Med J 1923, 2: 342.
Prentice G: Cancer Among Negroes. Br Med J 1923, 2: 1181.
Brown GM, Cronk LB, Boag TJ: The occurrence of cancer in an Eskimo. Cancer 1952, 5: 142-143. 10.1002/1097-0142(195201)5:1<142::AID-CNCR2820050119>3.0.CO;2-Q
Eaton SB, Konner M, Shostak M: Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. Am J Med 1988, 84: 739-749. 10.1016/0002-9343(88)90113-1
Carrera-Bastos P, Fontes-Villalba M, O'Keefe JH, Lindeberg S, Cordain L: The western diet and lifestyle and diseases of civilization. Research Reports in Clinical Cardiology 2011, 2: 15-35.
Cordain L, Miller JB, Eaton SB, Mann N: Macronutrient estimations in hunter-gatherer diets. Am J Clin Nutr 2000, 72: 1589-1592.
Hu Y, Shang H, Tong H, Nehlich O, Liu W, Zhao C, Yu J, Wang C, Trinkaus E, Richards MP: Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc Natl Acad Sci USA 2009, 106: 10971-10974. 10.1073/pnas.0904826106
Richards MP: A brief review of the archaeological evidence for Palaeolithic and Neolithic subsistence. Eur J Clin Nutr 2002, 56: 16. p following 1262 10.1038/sj.ejcn.1601676
Ströhle A, Hahn A: Diets of modern hunter-gatherers vary substantially in their carbohydrate content depending on ecoenvironments: results from an ethnographic analysis. Nutrition Research 2011, 31: 429-435. 10.1016/j.nutres.2011.05.003
Weinberg SL: The diet-heart hypothesis: a critique. J Am Coll Cardiol 2004, 43: 731-733. 10.1016/j.jacc.2003.10.034
Henderson ST: High carbohydrate diets and Alzheimer's disease. Med Hypotheses 2004, 62: 689-700. 10.1016/j.mehy.2003.11.028
Seneff S, Wainwright G, Mascitelli L: Nutrition and Alzheimer's disease: the detrimental role of a high carbohydrate diet. Eur J Intern Med 2011, 22: 134-140. 10.1016/j.ejim.2010.12.017
Chiu CJ, Milton RC, Gensler G, Taylor A: Dietary carbohydrate intake and glycemic index in relation to cortical and nuclear lens opacities in the Age-Related Eye Disease Study. Am J Clin Nutr 2006, 83: 1177-1184.
Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack LT Jr, Hankinson SE, Willett WC, Taylor A: Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am J Clin Nutr 2006, 83: 880-886.
Kaushik S, Wang JJ, Flood V, Tan JS, Barclay AW, Wong TY, Brand-Miller J, Mitchell P: Dietary glycemic index and the risk of age-related macular degeneration. Am J Clin Nutr 2008, 88: 1104-1110.
Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J: Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000, 59: 539-543. 10.1136/ard.59.7.539
Roe CM, Fitzpatrick AL, **ong C, Sieh W, Kuller L, Miller JP, Williams MM, Kopan R, Behrens MI, Morris JC: Cancer linked to Alzheimer disease but not vascular dementia. Neurology 2010, 74: 106-112. 10.1212/WNL.0b013e3181c91873
Boffetta P, Nordenvall C, Nyren O, Ye W: A prospective study of gout and cancer. Eur J Cancer Prev 2009, 18: 127-132. 10.1097/CEJ.0b013e328313631a
Braun S, Bitton-Worms K, Leroith D: The Link between the Metabolic Syndrome and Cancer. Int J Biol Sci 2011, 7: 1003-1015.
Cheung N, Shankar A, Klein R, Folsom AR, Couper DJ, Wong TY: Age-related macular degeneration and cancer mortality in the atherosclerosis risk in communities study. Arch Ophthalmol 2007, 125: 1241-1247. 10.1001/archopht.125.9.1241
Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA: Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 2009, 27: 1082-1086. 10.1200/JCO.2008.19.1098
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N: Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol 2002, 20: 42-51. 10.1200/JCO.20.1.42
Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ: Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008, 9: 1039-1047. 10.1016/S1470-2045(08)70235-3
Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R: Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007, 30: 561-567. 10.2337/dc06-0922
Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, Kantarjian HM, O'Brien SM: Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer 2004, 100: 1179-1185. 10.1002/cncr.20071
Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS: Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol 2009, 27: 176-185. 10.1200/JCO.2008.17.9945
Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA: Is Diabetes Mellitus an Independent Risk Factor for Colon Cancer and Rectal Cancer? Am J Gastroenterol 2011.
Augustin LS, Dal Maso L, La Vecchia C, Parpinel M, Negri E, Vaccarella S, Kendall CW, Jenkins DJ, Francesch S: Dietary glycemic index and glycemic load, and breast cancer risk: a case-control study. Ann Oncol 2001, 12: 1533-1538. 10.1023/A:1013176129380
Melnik BC, John SM, Schmitz G: Over-stimulation of insulin/IGF1 signaling by Western diet may promote diseases of civilization: lessons learnt from Laron syndrome. Nutr Metab (Lond) 2011, 8: 41. 10.1186/1743-7075-8-41
Sieri S, Pala V, Brighenti F, Pellegrini N, Muti P, Micheli A, Evangelista A, Grioni S, Contiero P, Berrino F, Krogh V: Dietary glycemic index, glycemic load, and the risk of breast cancer in an Italian prospective cohort study. Am J Clin Nutr 2007, 86: 1160-1166.
Wen W, Shu XO, Li H, Yang G, Ji BT, Cai H, Gao YT, Zheng W: Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. Am J Clin Nutr 2009, 89: 283-289.
Braunstein A: Wratschebnaje obosrnije. 1921, 7: 291.
Bierich R: Über die Beteiligung des Bindegewebes an der experimentellen Krebsbildung. Virchows Archiv f Pathol Anatom und Physiol 1922, 23: 1-19.
Bierich R: Über die Vorgänge Beim Einwuchern der Krebszellen. Wien Klin Wochenschr 1927, 6: 1599-1603.
Warburg O: Über den Stoffwechsel der Carzinomzelle. Klinische Wochenschrift 1925, 534-536.
Warburg O, Posener K, Negelein E: Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr 1924, 309-344.
Warburg O, Wind F, Negelein E: Über den Stoffwechsel der Tumoren im Körper. Klinische Wochenschrift 1926, 828-832.
Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011, 144: 646-674. 10.1016/j.cell.2011.02.013
Seyfried TN, Shelton LM: Cancer as a metabolic disease. Nutr Metab (Lond) 2010, 7: 7. 10.1186/1743-7075-7-7
Warburg O: On respiratory impairment in cancer cells. Science 1956, 124: 269-270.
Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, et al.: Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol 2006, 175: 913-923. 10.1083/jcb.200512100
Robey RB, Hay N: Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 2006, 25: 4683-4696. 10.1038/sj.onc.1209595
Robey RB, Hay N: Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 2009, 19: 25-31. 10.1016/j.semcancer.2008.11.010
Young CD, Anderson SM: Sugar and fat - that's where it's at: metabolic changes in tumors. Breast Cancer Res 2008, 10: 202. 10.1186/bcr1852
Deberardinis RJ, Lum JJ, Thompson CB: Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem 2006, 281: 37372-37380. 10.1074/jbc.M608372200
Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM: The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem 2002, 277: 33895-33900. 10.1074/jbc.M204681200
Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM: Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res 2003, 44: 1100-1112. 10.1194/jlr.M300045-JLR200
Laplante M, Sabatini DM: mTOR signaling at a glance. J Cell Sci 2009, 122: 3589-3594. 10.1242/jcs.051011
Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N: mTOR, translation initiation and cancer. Oncogene 2006, 25: 6416-6422. 10.1038/sj.onc.1209888
Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, Wang Y, **g Y, Yang H, Chen R, et al.: Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA 2011, 108: 4129-4134. 10.1073/pnas.1014769108
Zha X, Sun Q, Zhang H: mTOR upregulation of glycolytic enzymes promotes tumor development. Cell Cycle 2011, 10: 1015-1016. 10.4161/cc.10.7.15063
Koppenol WH, Bounds PL, Dang CV: Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011, 11: 325-337. 10.1038/nrc3038
Cully M, You H, Levine AJ, Mak TW: Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 2006, 6: 184-192. 10.1038/nrc1819
Choi NC, Fischman AJ, Niemierko A, Ryu JS, Lynch T, Wain J, Wright C, Fidias P, Mathisen D: Dose-response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002, 54: 1024-1035. 10.1016/S0360-3016(02)03038-9
Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL: Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 2003, 97: 1015-1024. 10.1002/cncr.11159
Bentzen SM, Gregoire V: Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol 2011, 21: 101-110. 10.1016/j.semradonc.2010.10.001
LeRoith D: Can endogenous hyperinsulinaemia explain the increased risk of cancer development and mortality in type 2 diabetes: evidence from mouse models. Diabetes Metab Res Rev 2010, 26: 599-601. 10.1002/dmrr.1139
Huang XF, Chen JZ: Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009, 10: 610-616. 10.1111/j.1467-789X.2009.00607.x
Pollak M: Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 2008, 8: 915-928. 10.1038/nrc2536
Fontana L, Partridge L, Longo VD: Extending healthy life span--from yeast to humans. Science 2010, 328: 321-326. 10.1126/science.1172539
Lee C, Longo VD: Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene 2011, 30: 3305-3316. 10.1038/onc.2011.91
Bloom WL, Azar GJ: Similarities Of Carbohydrate Deficiency And Fasting. I. Weight Loss, Electrolyte Excretion, And Fatigue. Arch Intern Med 1963, 112: 333-337. 10.1001/archinte.1963.03860030087006
Fery F, Bourdoux P, Christophe J, Balasse EO: Hormonal and metabolic changes induced by an isocaloric isoproteinic ketogenic diet in healthy subjects. Diabete Metab 1982, 8: 299-305.
Klein S, Wolfe RR: Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol 1992, 262: E631-636.
Azar GJ, Bloom WL: Similarities Of Carbohydrate Deficiency And Fasting. Ii. Ketones, Nonesterified Fatty Acids And Nitrogen Excretion. Arch Intern Med 1963, 112: 338-343. 10.1001/archinte.1963.03860030092007
Jiang W, Zhu Z, Thompson HJ: Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res 2008, 68: 5492-5499. 10.1158/0008-5472.CAN-07-6721
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W: High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000, 60: 916-921.
Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ: Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer 2007, 97: 646-653. 10.1038/sj.bjc.6603922
Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, et al.: Ketones and lactate "fuel" tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 2010, 9: 3506-3514. 10.4161/cc.9.17.12731
Semenza GL: Tumor metabolism: cancer cells give and take lactate. J Clin Invest 2008, 118: 3835-3837.
Baumann F, Leukel P, Doerfelt A, Beier CP, Dettmer K, Oefner PJ, Kastenberger M, Kreutz M, Nickl-Jockschat T, Bogdahn U, et al.: Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol 2009, 11: 368-380. 10.1215/15228517-2008-106
Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S: The role of matrix metalloproteinases (MMPs) in human caries. J Dent Res 2006, 85: 22-32. 10.1177/154405910608500104
Williams AC, Collard TJ, Paraskeva C: An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene 1999, 18: 3199-3204. 10.1038/sj.onc.1202660
Park HJ, Lyons JC, Ohtsubo T, Song CW: Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer 1999, 80: 1892-1897. 10.1038/sj.bjc.6690617
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ: Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res 2006, 66: 5216-5223. 10.1158/0008-5472.CAN-05-4193
Fang JS, Gillies RD, Gatenby RA: Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol 2008, 18: 330-337. 10.1016/j.semcancer.2008.03.011
Demetrakopoulos GE, Linn B, Amos H: Rapid loss of ATP by tumor cells deprived of glucose: contrast to normal cells. Biochem Biophys Res Commun 1978, 82: 787-794. 10.1016/0006-291X(78)90851-3
Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R, Opipari A, Liu JR: Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol 2011, 122: 389-95. 10.1016/j.ygyno.2011.04.024
Shim H, Chun YS, Lewis BC, Dang CV: A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci USA 1998, 95: 1511-1516. 10.1073/pnas.95.4.1511
Masur K, Vetter C, Hinz A, Tomas N, Henrich H, Niggemann B, Zanker KS: Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br J Cancer 2011, 104: 345-352. 10.1038/sj.bjc.6606050
Gatenby RA, Gillies RJ: Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004, 4: 891-899. 10.1038/nrc1478
Santisteban GA, Ely JT, Hamel EE, Read DH, Kozawa SM: Glycemic modulation of tumor tolerance in a mouse model of breast cancer. Biochem Biophys Res Commun 1985, 132: 1174-1179. 10.1016/0006-291X(85)91930-8
Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P: Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 2003, 89: 1375-1382. 10.1038/sj.bjc.6601269
Koroljow S: Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q 1962, 36: 261-270. 10.1007/BF01586115
McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, Olivi A, Quinones-Hinojosa A: Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery 2008, 63: 286-291. discussion 291 10.1227/01.NEU.0000315282.61035.48
Maestu I, Pastor M, Gomez-Codina J, Aparicio J, Oltra A, Herranz C, Montalar J, Munarriz B, Reynes G: Pretreatment prognostic factors for survival in small-cell lung cancer: a new prognostic index and validation of three known prognostic indices on 341 patients. Ann Oncol 1997, 8: 547-553. 10.1023/A:1008212826956
Krone CA, Ely JT: Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther 2005, 4: 25-31. 10.1177/1534735404274167
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM: Fasting serum glucose level and cancer risk in Korean men and women. Jama 2005, 293: 194-202. 10.1001/jama.293.2.194
Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, Shikata K, Hata J, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y: Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology 2009, 136: 1234-1241. 10.1053/j.gastro.2008.12.045
Ely JT, Krone CA: Glucose and cancer. N Z Med J 2002, 115: U123.
Shanmugam N, Reddy MA, Guha M, Natarajan R: High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003, 52: 1256-1264. 10.2337/diabetes.52.5.1256
Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL: Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology 2006, 147: 2518-2525. 10.1210/en.2005-0519
Dandona P, Chaudhuri A, Ghanim H, Mohanty P: Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol 2007, 99: 15B-26B.
Rajaram S, Baylink DJ, Mohan S: Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev 1997, 18: 801-831. 10.1210/er.18.6.801
LaPensee CR, Hugo ER, Ben-Jonathan N: Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways. Endocrinology 2008, 149: 5415-5422. 10.1210/en.2008-0549
Venkateswaran V, Haddad AQ, Fleshner NE, Fan R, Sugar LM, Nam R, Klotz LH, Pollak M: Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst 2007, 99: 1793-1800. 10.1093/jnci/djm231
Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Hartwick W, Hoffma B, Hood N: Insulin-like growth factor binding proteins 1 and 3 and breast cancer outcomes. Breast Cancer Res Treat 2002, 74: 65-76. 10.1023/A:1016075709022
Freund E: Zur Diagnose des Carcinoms. Wien med Bl 1885, 1: 268-269.
Lundholm K, Holm G, Schersten T: Insulin resistance in patients with cancer. Cancer Res 1978, 38: 4665-4670.
McCall JL, Tuckey JA, Parry BR: Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br J Surg 1992, 79: 1361-1363. 10.1002/bjs.1800791240
Marat D, Noguchi Y, Yoshikawa T, Tsuburaya A, Ito T, Kondo J: Insulin resistance and tissue glycogen content in the tumor-bearing state. Hepatogastroenterology 1999, 46: 3159-3165.
Makino T, Noguchi Y, Yoshikawa T, Doi C, Nomura K: Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br J Surg 1998, 85: 1658-1662. 10.1046/j.1365-2168.1998.00938.x
Yoshikawa T, Noguchi Y, Matsumoto A: Effects of tumor removal and body weight loss on insulin resistance in patients with cancer. Surgery 1994, 116: 62-66.
Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnquist HJ, Larsson J: Improved glucose metabolism after subtotal pancreatectomy for pancreatic cancer. Br J Surg 1993, 80: 1047-1050. 10.1002/bjs.1800800841
Waterhouse C, Jeanpretre N, Keilson J: Gluconeogenesis from alanine in patients with progressive malignant disease. Cancer Res 1979, 39: 1968-1972.
Rich AJ, Wright PD: Ketosis and nitrogen excretion in undernourished surgical patients. JPEN J Parenter Enteral Nutr 1979, 3: 350-354. 10.1177/0148607179003005350
Conyers RA, Need AG, Rofe AM, Potezny N, Kimber RJ: Nutrition and cancer. Br Med J 1979, 1: 1146.
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr: Brain metabolism during fasting. J Clin Invest 1967, 46: 1589-1595. 10.1172/JCI105650
Gambardella A, Paolisso G, D'Amore A, Granato M, Verza M, Varricchio M: Different contribution of substrates oxidation to insulin resistance in malnourished elderly patients with cancer. Cancer 1993, 72: 3106-3113. 10.1002/1097-0142(19931115)72:10<3106::AID-CNCR2820721036>3.0.CO;2-G
Korber J, Pricelius S, Heidrich M, Muller MJ: Increased lipid utilization in weight losing and weight stable cancer patients with normal body weight. Eur J Clin Nutr 1999, 53: 740-745. 10.1038/sj.ejcn.1600843
Young VR: Energy metabolism and requirements in the cancer patient. Cancer Res 1977, 37: 2336-2347.
Tisdale MJ, Brennan RA: Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer 1983, 47: 293-297. 10.1038/bjc.1983.38
Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN: The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007, 4: 5. 10.1186/1743-7075-4-5
Conyers RA, Need AG, Durbridge T, Harvey ND, Potezny N, Rofe AM: Cancer, ketosis and parenteral nutrition. Med J Aust 1979, 1: 398-399.
Magee BA, Potezny N, Rofe AM, Conyers RA: The inhibition of malignant cell growth by ketone bodies. Aust J Exp Biol Med Sci 1979, 57: 529-539. 10.1038/icb.1979.54
Freeman JM, Kossoff EH: Ketosis and the ketogenic diet, 2010: advances in treating epilepsy and other disorders. Adv Pediatr 2010, 57: 315-329. 10.1016/j.yapd.2010.08.003
Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, Yancy WS, Phinney SD: Low-carbohydrate nutrition and metabolism. Am J Clin Nutr 2007, 86: 276-284.
Kossoff EH, Dorward JL: The modified Atkins diet. Epilepsia 2008,49(Suppl 8):37-41.
Fearon KC, Tisdale MJ, Preston T, Plumb JA, Calman KC: Failure of systemic ketosis to control cachexia and the growth rate of the Walker 256 carcinosarcoma in rats. Br J Cancer 1985, 52: 87-92. 10.1038/bjc.1985.153
Tisdale MJ, Brennan RA, Fearon KC: Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer 1987, 56: 39-43. 10.1038/bjc.1987.149
Tisdale MJ, Brennan RA: A comparison of long-chain triglycerides and medium-chain triglycerides on weight loss and tumour size in a cachexia model. Br J Cancer 1988, 58: 580-583. 10.1038/bjc.1988.263
Beck SA, Tisdale MJ: Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer 1989, 59: 677-681. 10.1038/bjc.1989.140
Händel M, Tadeuma K: Über die Beziehung des Geschwulstwachstums zur Ernährung und zum Stoffwechsel. II. Mitteilung. Versuche zur Frage der Bedeutung der Kohlenhydrate für das Wachstum des Rattencarcinoms. Klin Wochenschr 1924, 288-293.
Fearon KC, Borland W, Preston T, Tisdale MJ, Shenkin A, Calman KC: Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr 1988, 47: 42-48.
Breitkreutz R, Tesdal K, Jentschura D, Haas O, Leweling H, Holm E: Effects of a high-fat diet on body composition in cancer patients receiving chemotherapy: a randomized controlled study. Wien Klin Wochenschr 2005, 117: 685-692. 10.1007/s00508-005-0455-3
Fine EJ MA, Quadros EV, Sequeira JM, Feinman RD: Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell international 2009, 9:14: 11.
Maurer GD, Brucker DP, Baehr O, Harter PN, Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP, Rieger J: Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer 2011, 11: 315. 10.1186/1471-2407-11-315
van Ness van Alstyne E, Beebe SP: Diet studies in transplantable tumors. I. The effect of non-carbohydrate diet upon the growth of transplantable sarcoma in rats. J Med Res 1913, 217-232.
Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, Voelker HU, Thiede A, Coy JF: Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008, 8: 122. 10.1186/1471-2407-8-122
Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, et al.: A Low Carbohydrate, High Protein Diet Slows Tumor Growth and Prevents Cancer Initiation. Cancer Res 2011.
Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN: Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond) 2010, 7: 33. 10.1186/1743-7075-7-33
Tannenbaum A: The Genesis and Growth of Tumors. II. Effects of Caloric Restriction per se. Cancer Res 1942, 2: 460-467.
Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, Cohen P, Hwang D, Peterson B, Fields T, et al.: Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008, 68: 11-19. 10.1002/pros.20683
Masko EM, Thomas JA, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH, Dewhirst MW, Pizzo SV, Freedland SJ: Low-carbohydrate diets and prostate cancer: how low is "low enough"? Cancer Prev Res (Phila) 2010, 3: 1124-1131. 10.1158/1940-6207.CAPR-10-0071
Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano M, Tomuta N, Bontempo A, Sparano JA: A pilot safety and feasibility trial of a reduced carbohydrate diet in patients with advanced cancer. J Clin Oncol 2011.,29(suppl; abstr e13573):
Rossi-Fanelli F, Franchi F, Mulieri M, Cangiano C, Cascino A, Ceci F, Muscaritoli M, Seminara P, Bonomo L: Effect of energy substrate manipulation on tumour cell proliferation in parenterally fed cancer patients. Clin Nutr 1991, 10: 228-232. 10.1016/0261-5614(91)90043-C
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U: Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 2011, 8: 54. 10.1186/1743-7075-8-54
Nebeling LC, Miraldi F, Shurin SB, Lerner E: Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr 1995, 14: 202-208.
Nebeling LC, Lerner E: Implementing a ketogenic diet based on medium-chain triglyceride oil in pediatric patients with cancer. J Am Diet Assoc 1995, 95: 693-697. 10.1016/S0002-8223(95)00189-1
Rieger J, Baehr O, Hattingen E, Maurer G, Coy J, Weller M, Steinbach J: The ERGO trial: A pilot study of a ketogenic diet in patients with recurrent glioblastoma. J Clin Oncol (Meeting Abstracts) 2010, 28: e12532.
Fine EJ, Segal-Isaacson CJ, Feinman R, Sparano J: Carbohydrate restriction in patients with advanced cancer: a protocol to assess safety and feasibility with an accompanying hypothesis. Commun Oncol 2008, 5: 22-26.
Moulton CJ, Valentine RJ, Layman DK, Devkota S, Singletary KW, Wallig MA, Donovan SM: A high protein moderate carbohydrate diet fed at discrete meals reduces early progression of N-methyl-N-nitrosourea-induced breast tumorigenesis in rats. Nutr Metab (Lond) 2010, 7: 1. 10.1186/1743-7075-7-1
Osborne CK, Bolan G, Monaco ME, Lippman ME: Hormone responsive human breast cancer in long-term tissue culture: effect of insulin. Proc Natl Acad Sci USA 1976, 73: 4536-4540. 10.1073/pnas.73.12.4536
Jonsson T, Granfeldt Y, Erlanson-Albertsson C, Ahren B, Lindeberg S: A paleolithic diet is more satiating per calorie than a mediterranean-like diet in individuals with ischemic heart disease. Nutr Metab (Lond) 2010, 7: 85. 10.1186/1743-7075-7-85
Nickols-Richardson SM, Coleman MD, Volpe JJ, Hosig KW: Perceived hunger is lower and weight loss is greater in overweight premenopausal women consuming a low-carbohydrate/high-protein vs high-carbohydrate/low-fat diet. J Am Diet Assoc 2005, 105: 1433-1437. 10.1016/j.jada.2005.06.025
Mavropoulos JC, Isaacs WB, Pizzo SV, Freedland SJ: Is there a role for a low-carbohydrate ketogenic diet in the management of prostate cancer? Urology 2006, 68: 15-18.
Mantovani A, Allavena P, Sica A, Balkwill F: Cancer-related inflammation. Nature 2008, 454: 436-444. 10.1038/nature07205
Gonzalez F, Minium J, Rote NS, Kirwan JP: Altered tumor necrosis factor alpha release from mononuclear cells of obese reproductive-age women during hyperglycemia. Metabolism 2006, 55: 271-276. 10.1016/j.metabol.2005.08.022
Depner CM, Kirwan RD, Frederickson SJ, Miles MP: Enhanced inflammation with high carbohydrate intake during recovery from eccentric exercise. Eur J Appl Physiol 2010, 109: 1067-1076. 10.1007/s00421-010-1448-0
Rudnick PA, Taylor KW: Effect Of Prolonged Carbohydrate Restriction On Serum-Insulin Levels In Mild Diabetes. Br Med J 1965, 1: 1225-1228. 10.1136/bmj.1.5444.1225
Garg A, Bantle JP, Henry RR, Coulston AM, Griver KA, Raatz SK, Brinkley L, Chen YD, Grundy SM, Huet BA, et al.: Effects of varying carbohydrate content of diet in patients with non-insulin-dependent diabetes mellitus. Jama 1994, 271: 1421-1428. 10.1001/jama.271.18.1421
Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, Gleed A, Jacobs DB, Larson G, Lustig RH, et al.: Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond) 2008, 5: 9. 10.1186/1743-7075-5-9
Perez-Guisado J, Munoz-Serrano A, Alonso-Moraga A: Spanish Ketogenic Mediterranean Diet: a healthy cardiovascular diet for weight loss. Nutr J 2008, 7: 30. 10.1186/1475-2891-7-30
Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD: Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44: 297-309. 10.1007/s11745-008-3274-2
Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, Hansson A, Soderstrom M, Lindeberg S: Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol 2009, 8: 35. 10.1186/1475-2840-8-35
Elhayany A, Lustman A, Abel R, Attal-Singer J, Vinker S: A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: a 1-year prospective randomized intervention study. Diabetes Obes Metab 2010, 12: 204-209. 10.1111/j.1463-1326.2009.01151.x
Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS: Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008, 43: 65-77. 10.1007/s11745-007-3132-7
Cordain L: Cereal grains: humanity's double-edged sword. World Rev Nutr Diet 1999, 84: 19-73.
Cordain L, Toohey L, Smith MJ, Hickey MS: Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr 2000, 83: 207-217.
Fasano A: Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011, 91: 151-175. 10.1152/physrev.00003.2008
Klonoff DC: The beneficial effects of a Paleolithic diet on type 2 diabetes and other risk factors for cardiovascular disease. J Diabetes Sci Technol 2009, 3: 1229-1232.
Acknowledgements
We are grateful to the two anonymous referees for their suggestions that helped to improve this paper. We also would like to thank Bill Lemke and Sebastian Baier for fruitful discussions and comments on a previous version of this paper. UK appreciates a research grant from the "Deutsche Gesellschaft für Ernährungsmedizin (DGEM)". This publication was funded by the German Research Foundation (DFG) and the University of Würzburg in the funding program Open Access Publishing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RJK drafted the manuscript, UK drafted figures and parts of the manuscript, both authors finalized the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Klement, R.J., Kämmerer, U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer?. Nutr Metab (Lond) 8, 75 (2011). https://doi.org/10.1186/1743-7075-8-75
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1743-7075-8-75