Abstract
Background
BMP-induced chemotaxis of mesenchymal progenitors is fundamental for vertebrate development, disease and tissue repair. BMP2 induces Smad and non-Smad signalling. Whereas signal transduction via Smads lead to transcriptional responses, non-Smad signalling induces both, transcriptional and immediate/early non-transcriptional responses. However, the molecular mechanisms by which BMP2 facilitates planar cell polarity, cortical actin rearrangements, lamellipodia formation and chemotaxis of mesenchymal progenitors are poorly understood. Our aim was to uncover the molecular mechanism by which BMP2 facilitates chemotaxis via the BMP2-dependent activation of PI3K and spatiotemporal control of PIP3 production important for actin rearrangements at the mesenchymal cell cytocortex.
Results
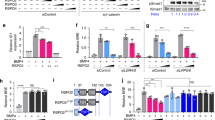
We unveiled the molecular mechanism by which BMP2 induces non-Smad signalling by PI3K and the role of the second messenger PIP3 in BMP2-induced planar cell polarity, cortical actin reorganisation and lamellipodia formation. By using protein interaction studies, we identified the class Ia PI3K regulatory subunit p55γ to act as a specific and non-redundant binding partner for BMP receptor type II (BMPRII) in concert with the catalytic subunit p110α. We mapped the PI3K interaction to a region within the BMPRII kinase. Either BMP2 stimulation or increasing amounts of BMPRI facilitated p55γ association with BMPRII, but BMPRII kinase activity was not required for the interaction. We visualised BMP2-dependent PIP3 production via PI3K p55γ/p110α and were able to localise PIP3 to the leading edge of intact cells during the process of BMP2-induced planar cell polarity and actin dependent lamellipodia formation. Using mass spectrometry, we found the highly PIP3-sensitive PH-domain protein LL5β to act as a novel BMP2 effector in orchestrating cortical actin rearrangements. By use of live cell imaging we found that knock-down of p55γ or LL5β or pharmacological inhibition of PI3K impaired BMP2-induced migratory responses.
Conclusions
Our results provide evidence for an important contribution of the BMP2-PI3K (p55γ/p110α)- PIP3-LL5β signalling axis in mesenchymal progenitor cell chemotaxis. We demonstrate molecular insights into BMP2-induced PI3K signalling on the level of actin reorganisation at the leading edge cytocortex. These findings are important to better understand BMP2–induced cytoskeletal reorganisation and chemotaxis of mesenchymal progenitors in different physiological or pathophysiological contexts.
Similar content being viewed by others
Background
Gradients of bone morphogenetic proteins (BMPs) act as mesenchymal guidance cues during development, disease and tissue repair by molecular mechanisms that remain poorly defined [1]. In particular, the directional migration (chemotaxis) of neural crest cells, bone marrow stromal cells and endothelial cells along gradients of BMP2 has been reported [2–5]. BMPs signal through binding to cell surface hetero-oligomeric receptor complexes comprising type I (BMPRI) and type II (BMPRII) receptors [6]. Activated BMP receptor complexes induce canonical-Smad and non-Smad signalling cascades [7]. Activation of the type I receptor kinase by the type II receptor kinase induces phosphorylation and thus nuclear translocation of Smad1/5/8, leading to transcription of Smad-dependent target genes [8].
Whereas the molecular basis of canonical Smad signalling and its role in gene transcription is well explored, the molecular activation mechanism and the cellular functions of the non-Smad pathways, which rather act directly and independently of gene transcription, are poorly understood. In particular, the molecular mechanism of BMP-induced phosphatidylinositol 3-kinase (PI3K) activation, its signalling route and cellular function are poorly characterised. In recent years, several studies unveiled a requirement of PI3K for BMP2-induced migration of various cell types with mesenchymal origin by yet unknown mechanisms [9–11].
Here, for the first time, we addressed the molecular activation mechanism of BMP2-induced PI3K signalling in undifferentiated mesenchymal progenitor cells and the role of the lipid-product of PI3K, the membrane-bound second messenger PtdIns-3, 4, 5-triphosphate (PI (3, 4, 5) P3; hereafter referred to as PIP3) in BMP2-induced actin reorganisation.
Class Ia PI3Ks are dimeric lipid kinases composed of one out of five possible regulatory subunits encoded by Pik3r1 (encoding splice isoforms p85α, p55α and p50α), Pik3r2 (p85β) or Pik3r3 (p55γ) [12, 13]. The regulatory subunit is bound by one of three catalytic subunits, termed p110, encoded by Pik3ca (p110α), Pik3cb (p110β) or Pik3cd (p110δ) [14]. Catalytic activity is initiated upon regulatory subunit Src homology 2 (SH2) domain binding to phospho-tyrosine (pTyr) residues within a specific peptide context [15]. Thereafter, activated PI3K phosphorylates the 3-hydroxyl group of PtdIns-4, 5-bisphosphate (PIP2) to produce the second messenger PIP3. PIP3 recruits Pleckstrin homology (PH) domain-containing regulators to the inner plasma membrane. One main PI3K effector is protein kinase B (PKB/Akt) [16]. Besides Akt, PH-domain-containing cytoskeletal regulators sense PIP3 and mediate cortical actin dynamics at the so-called leading edge cytocortex. As such, the PH-like domain family B member 2 (Phldb2, hereafter referred to as LL5β) acts as a sensitive PIP3 effector during the establishment of planar cell polarity (PCP), lamellipodia formation, protrusion and subsequent chemotaxis [46–48], suggests that the p55γ/p110α complex positively regulates BMP2-induced motility, chemotaxis, and invasion of endothelial and cancer cells [9, 49, 50]. Whether the PI3K p55γ/p110α dimer indeed represents an attractive molecular target to interfere with BMP2-related cancers will require intense investigations in future.
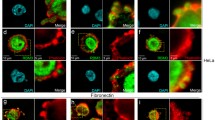
BMP2-induced PIP3 acts as a cellular compass at the leading edge and recruits LL5β
Numerous cellular activities have been reported to depend on BMP2-induced PI3K signalling [9–11, 51–56]. Most previous studies focused on the role of PI3K-induced Akt activity with Akt being the major PI3K effector. In the present study, we investigated the role and function of PIP3 beyond Akt activation and focused on PIP3 localisation and recruitment of cytoskeletal regulators. We visualised BMP2-dependent PIP3 production in a spatiotemporal manner to gain further insight into its function. We found PIP3 became quickly enriched in BMP2-induced lamellipodia at the cytocortex, especially in cells that displayed strong PCP, suggesting that PIP3 acts as a cellular compass at the leading edge of migrating cells. PIP3 recruits PH-domain-containing proteins that facilitate rearrangements of the actin cytoskeleton [57]. With this knowledge, we aimed to identify PH-domain proteins that link BMP2-induced PIP3 to actin regulators. The BMP2-induced lamellipodia are tightly cross-linked F-actin networks located at the cytocortex of the leading edge. During maturation and protrusion, these actin-rich lamellipodia form broad lamella that allow for the formation of new adhesion sites [58]. In agreement with our observations, we identified a specific interaction of PH-domain protein LL5β with PIP3. LL5β acts as a highly sensitive PIP3 effector during epidermal growth factor-induced chemotaxis and lamellipodia formation [17]. It regulates the actin cytoskeleton through interaction with and co-recruitment of filamin C [19] and filamin A [17]. Filamins orchestrate cortical actin into three-dimensional structures by cross-linking of F-actin filaments [59]. Interestingly, besides tethering filamins, LL5β also tethers Cytoplasmic linker associated proteins (CLASPs) to the leading edge [17, 18]. CLASPs attach microtubule tips to the cell cortex, which is important for microtubule stabilisation and thus PCP. Therefore, our findings provide evidence that LL5β acts as a BMP2-dependent multifunctional PIP3-sensing scaffold that eventually also orchestrates microtubule stabilisation at the cytocortex and thus links BMP2-dependent actin rearrangements to microtubule stabilisation.
p55γ and LL5β are required for BMP2-induced migration and chemotaxis
The potency of BMP2 in stimulating migration of cells with mesenchymal origin is well known. Here, we raised the question of whether our findings contribute in particular to BMP2-induced cortical actin rearrangements, PCP and chemotaxis. We demonstrated that loss of p55γ prevents cells from efficient PCP establishment during wound healing and that knock-down of p55γ or LL5β resulted in impaired BMP2-induced chemotaxis. We therefore conclude that the pro-migratory effects of BMP2 are driven by PI3K signalling leading to PIP3-dependent cytoskeletal actin rearrangements, and result mainly in directional migration explained by the ‘compass’ function of PIP3.
Conclusions
Our molecular findings provide a basis for explaining the important mechanism of BMP2-induced cortical actin rearrangements and chemotaxis, which we have graphically summarised (Figure 8). The novel in vitro data presented here close gaps in our current understanding of how BMP2 gradients influence the cellular cytoskeleton and hence mesenchymal progenitor cell chemotaxis. Interestingly, PIP3 production increases the efficacy of cells in detecting and processing shallow chemokine gradients [60]. This suggests that the molecular mechanism identified here is important for mesenchymal progenitor cells that respond to BMP2 gradients in vivo where they might originate from distant locations. To visualise this in vivo in the context of our novel molecular findings will be the future goal and a translation of this knowledge towards the fields of developmental biology and regenerative medicine is expected.
Methods
Chemicals, recombinant growth factors and inhibitors
All chemicals were purchased from Sigma Aldrich unless stated otherwise. Recombinant human BMP2 was kindly provided by Walter Sebald (University of Würzburg, Würzburg, Germany). The inhibitor LDN-193189 was a kind gift from Paul Yu (Harvard Medical School, Boston, MA, USA) and described elsewhere [61]. LY294002 was purchased from Cell Signaling Technology (Cell Signaling Technology Inc., Danvers, MA, USA) and PI103 was purchased from Echelon Bioscience (Echelon Bioscience Inc., Salt Lake City, USA).
Antibodies
Phospho-specific antibodies, as well as protein- and tag-specific antibodies, were used and applied as recommended by the manufacturer. A detailed list of all antibodies used in this study is provided in Additional file 7.
Cell culture
C2C12 cells and HEK293T cells (both from American Type Culture Collection) were cultivated in Dulbecco’s modified Eagle’s Medium (DMEM) (Biochrom GmbH, Berlin, Germany) supplemented with 10% (v/v) foetal calf serum and 100 U/ml penicillin/streptomycin. To maintain highest plasticity, C2C12 cells were kept undifferentiated and competent for BMP-induced signalling by subculture conditions that maintained a low density corresponding to approximately 150,000 cells per 182 cm2. Cells were split every other day when reaching 30% to 40% confluency and not used at passages higher than 20. Seeding in higher densities such as required for scratch wound healing was performed 12 hours prior to the experiment. C2C12 cells were transfected 48 hours prior to seeding in six-well plates with 0.5 to 3 μg plasmid DNA or 50nM siRNA (Dharmacon, GE Healthcare, Lafayette, CO, USA) (see Additional file 8: Table T1) using Lipofectamine2000 and Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. HEK293T cells were transfected using polyethyleneimine and expanded in high glucose (4,500 mg/l glucose) DMEM, 48 hours prior to experiment. All experiments requiring BMP2 stimulation were conducted after 6 hours starvation in DMEM without serum. Cells were grown on uncoated cell culture plastic unless stated otherwise.
Expression plasmids
The plasmids encoding human BMPRII-LF-HA or mouse BMPRIb-HA were described previously [20, 62, 63]. Single point mutations used to generate kinase dead receptors were generated by cyclic mutagenesis PCR as described in [64]. The construct encoding N-terminal flag-tagged p55γ was generated by cloning the full-length open reading frame of mouse p55γ into the TOPO-TA vector (Invitrogen, Carlsbad, CA, USA) before ligation via EcoRI/NotI into pcDNA3.1 basic. Cloning primers used in this paper are available upon request. The construct encoding HA-tagged p85α was a kind gift from Bart Vanhaesebroeck (QMUL, London, UK). The construct encoding GFP-tagged PH-domain of Akt was a kind gift from Kerstin Danker (Charité Berlin, Germany). All constructs were verified by DNA sequencing.
Immunoprecipitation assays
Immunoprecipitation of expressed proteins from HEK293T cells was performed using a modified radio-immunoprecipitation assay buffer containing 0.5% (w/v) sodium dodecyl sulphate and 0.1% Nonidet P-40. Immunoprecipitation from C2C12 cell extracts was performed using a modified radio-immunoprecipitation assay with 0.1% sodium dodecyl sulphate and 0.5% Nonidet P-40. A detailed description of the immunoprecipitation and immunoblotting procedures can be found in Additional file 7. PIP bead assay was purchased from Echelon Bioscience and precipitation was performed according to manufacturer’s instructions.
Mass spectrometry
Identification of p55γ binding to GST-BMPRII was performed as described in [20]. PIP bead-binding proteins were identified by matrix-assisted laser desorption ionisation-time of flight mass spectrometry-based peptide mass fingerprinting as described previously [65].
Scratch wound healing
The scratch wound healing assay was performed using cell culture inserts (ibidi GmbH) according to the manufacturer’s instructions on uncoated tissue culture plastic. A detailed description of the procedure can be found in Additional file 7. The rate of cell migration was measured by quantifying the intensity translocation values for three independent biological replicates per condition using a selective mask filter (Slidebook).
Boyden chamber assay
The assay was performed in a similar manner to [10] with a detailed description of the procedure in Additional file 7.
Chemotaxis assays
Two-dimensional chemotaxis was assayed using the μ-slide chemotaxis chamber system (ibidi GmbH, Martinsried, Germany) according to accompanying instructions with the following modifications: 1 day prior to seeding, chambers were coated with 0.5% gelatin solution in humidified atmosphere washed for 1 hour and dried at 37°C. Pictures were taken using a 4× objective in bright field modus. Measurements were performed using an automated sample table mounted on an Axiovert 200 M (Carl Zeiss, Jena, Germany) in combination with Axiovision Mark&Find tool. Manual cell tracking was performed using the open source ImageJ plugin Manual tracking v2.0.
Immunofluorescence and live cell imaging
For detection of fluorescent signals, we used the Alexa-conjugated secondary antibody system (Invitrogen, Carlsbad, CA, USA) and an inverted fluorescence Axiovert 200 microscope (Carl Zeiss, Jena, Germany) equipped with a live cell imaging heating and CO2 chamber mounted to a CoolSnapHQ CCD camera (Roper Scientific, Martinsried, Germany). Confocal images were taken using a Zeiss LSM519 laser scanning confocal using 63× magnification Plan Apochromat objective. A detailed description is provided in Additional file 7.
Statistics and bioinformatics
Detailed information and description of statistical analysis on co-localisation studies, intensity translocation values, western blot quantification, used databases and artwork programmes is provided in Additional file 7.
We provide an inventory of supplemental information, supplemental experimental procedures, supplemental information and supplemental references (Additional file 7).
Abbreviations
- BISC:
-
BMP-induced signalling complex
- BMP2:
-
Bone morphogenetic protein 2
- BMPRI/II:
-
Bone morphogenetic protein receptor type I/II
- BMPRII-LF:
-
BMP receptor type II-long form
- BMPRII-SF:
-
BMP receptor type II-short form
- CLASPs:
-
Cytoplasmic linker associated proteins
- DiI:
-
Fluorescent lipophilic cationic indocarbocyanine dye I
- DiO:
-
Fluorescent lipophilic cationic indocarbocyanine dye O
- GST:
-
Glutathione S-transferase
- HA-tag:
-
Human influenza hemagglutinin-tag
- iSH2:
-
Inter-Src homology 2 domain
- p110α:
-
p110 catalytic subunit p110 alpha
- F-actin:
-
Filamentous actin
- p55γ:
-
PI3K regulatory subunit p55 gamma
- PCP:
-
Planar cell polarity
- PDK1:
-
3-phosphoinositide-dependent kinase-1
- PH:
-
Pleckstrin homology domain
- PHLDB2 (also known as LL5β):
-
Pleckstrin homology-like domain family B member 2
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PIP2:
-
Phosphatidylinositol 4,5-bisphosphate
- PIP3:
-
Phosphatidylinositol (3,4,5)-trisphosphate
- pSmad1:
-
Phospho-Smad1
- pTyr:
-
Phospho-tyrosine
- SH2:
-
Src homology 2 domain
- TC:
-
Truncation
- TGF-β:
-
Transforming growth factor beta
- wt:
-
Wild type.
References
Ruschke K, Hiepen C, Becker J, Knaus P: BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res. 2012, 347: 521-544.
Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M: Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007, 236: 2493-2501.
Fiedler J, Roderer G, Gunther KP, Brenner RE: BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002, 87: 305-312.
Finkenzeller G, Hager S, Stark GB: Effects of bone morphogenetic protein 2 on human umbilical vein endothelial cells. Microvasc Res. 2012, 84: 81-85.
Mishima Y, Lotz M: Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res. 2008, 26: 1407-1412.
Horbelt D, Denkis A, Knaus P: A portrait of transforming growth factor beta superfamily signalling: background matters. Int J Biochem Cell Biol. 2012, 44: 469-474.
Sieber C, Kopf J, Hiepen C, Knaus P: Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009, 20: 343-355.
Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R: Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002, 7: 949-960.
Fong YC, Li TM, Wu CM, Hsu SF, Kao ST, Chen RJ, Lin CC, Liu SC, Wu CL, Tang CH: BMP-2 increases migration of human chondrosarcoma cells via PI3K/Akt pathway. J Cell Physiol. 2008, 217: 846-855.
Gamell C, Osses N, Bartrons R, Ruckle T, Camps M, Rosa JL, Ventura F: BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J Cell Sci. 2008, 121: 3960-3970.
Perron JC, Dodd J: ActRIIA and BMPRII Type II BMP receptor subunits selectively required for Smad4-independent BMP7-evoked chemotaxis. PLoS One. 2009, 4: e8198-
Dey BR, Furlanetto RW, Nissley SP: Cloning of human p55 gamma, a regulatory subunit of phosphatidylinositol 3-kinase, by a yeast two-hybrid library screen with the insulin-like growth factor-I receptor. Gene. 1998, 209: 175-183.
Pons S, Asano T, Glasheen E, Miralpeix M, Zhang Y, Fisher TL, Myers MG, Sun XJ, White MF: The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol Cell Biol. 1995, 15: 4453-4465.
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B: The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010, 11: 329-341.
Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, Neel BG, Birge RB, Fajardo JE, Chou MM, Hanafusa H, Schaffhausen B, Cantley LC: SH2 domains recognize specific phosphopeptide sequences. Cell. 1993, 72: 767-778.
Vanhaesebroeck B, Alessi DR: The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000, 346: 561-576.
Takabayashi T, **e MJ, Takeuchi S, Kawasaki M, Yagi H, Okamoto M, Tariqur RM, Malik F, Kuroda K, Kubota C, Fujieda S, Nagano T, Sato M: LL5beta directs the translocation of filamin A and SHIP2 to sites of phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) accumulation, and PtdIns(3,4,5)P3 localization is mutually modified by co-recruited SHIP2. J Biol Chem. 2010, 285: 16155-16165.
Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, Akhmanova A: CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006, 11: 21-32.
Paranavitane V, Coadwell WJ, Eguinoa A, Hawkins PT, Stephens L: LL5beta is a phosphatidylinositol (3,4,5)-trisphosphate sensor that can bind the cytoskeletal adaptor, gamma-filamin. J Biol Chem. 2003, 278: 1328-1335.
Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S: Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004, 4: 1346-1358.
Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K: Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995, 92: 7632-7636.
Panayotou G, Bax B, Gout I, Federwisch M, Wroblowski B, Dhand R, Fry MJ, Blundell TL, Wollmer A, Waterfield MD: Interaction of the p85 subunit of PI 3-kinase and its N-terminal SH2 domain with a PDGF receptor phosphorylation site: structural features and analysis of conformational changes. Embo J. 1992, 11: 4261-4272.
Obenauer JC, Cantley LC, Yaffe MB: Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003, 31: 3635-3641.
Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P: The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002, 277: 5330-5338.
Guzman A, Zelman-Femiak M, Boergermann JH, Paschkowsky S, Kreuzaler PA, Fratzl P, Harms GS, Knaus P: SMAD versus non-SMAD signaling is determined by lateral mobility of bone morphogenetic protein (BMP) receptors. J Biol Chem. 2012, 287: 39492-39504.
Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, Ehrlich M, Henis YI, Sebald W, Knaus P: Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am. 2003, 85-A: 44-51.
Liu F, Ventura F, Doody J, Massague J: Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995, 15: 3479-3486.
Kavanaugh WM, Turck CW, Klippel A, Williams LT: Tyrosine 508 of the 85-kilodalton subunit of phosphatidylinositol 3-kinase is phosphorylated by the platelet-derived growth factor receptor. Biochemistry. 1994, 33: 11046-11050.
Sun M, Hillmann P, Hofmann BT, Hart JR, Vogt PK: Cancer-derived mutations in the regulatory subunit p85alpha of phosphoinositide 3-kinase function through the catalytic subunit p110alpha. Proc Natl Acad Sci U S A. 2010, 107: 15547-15552.
Jung J, Kim HY, Kim M, Sohn K, Kim M, Lee K: Translationally controlled tumor protein induces human breast epithelial cell transformation through the activation of Src. Oncogene. 2011, 30: 2264-2274.
Ueki K, Yballe CM, Brachmann SM, Vicent D, Watt JM, Kahn CR, Cantley LC: Increased insulin sensitivity in mice lacking p85beta subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci U S A. 2002, 99: 419-424.
Cunningham NS, Paralkar V, Reddi AH: Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992, 89: 11740-11744.
Myers DC, Sepich DS, Solnica-Krezel L: Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002, 243: 81-98.
Lind M, Eriksen EF, Bunger C: Bone morphogenetic protein-2 but not bone morphogenetic protein-4 and −6 stimulates chemotactic migration of human osteoblasts, human marrow osteoblasts, and U2-OS cells. Bone. 1996, 18: 53-57.
Boergermann JH, Kopf J, Yu PB, Knaus P: Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010, 42: 1802-1807.
Yi JY, Shin I, Arteaga CL: Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005, 280: 10870-10876.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S: The protein kinase complement of the human genome. Science. 2002, 298: 1912-1934.
Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C: Activin signaling pathways in ovine pituitary and LbetaT2 gonadotrope cells. Biol Reprod. 2003, 68: 1877-1887.
** W, Yun C, Kim HS, Kim SJ: TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res. 2007, 67: 9869-9877.
Wong WK, Knowles JA, Morse JH: Bone morphogenetic protein receptor type II C-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2005, 33: 438-446.
Jimenez C, Portela RA, Mellado M, Rodriguez-Frade JM, Collard J, Serrano A, Martinez AC, Avila J, Carrera AC: Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J Cell Biol. 2000, 151: 249-262.
Inukai K, Funaki M, Nawano M, Katagiri H, Ogihara T, Anai M, Onishi Y, Sakoda H, Ono H, Fukushima Y, Kikuchi M, Oka Y, Asano T: The N-terminal 34 residues of the 55 kDa regulatory subunits of phosphoinositide 3-kinase interact with tubulin. Biochem J. 2000, 346: 483-489.
**a X, Cheng A, Akinmade D, Hamburger AW: The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol Cell Biol. 2003, 23: 1717-1725.
Li R, Gundersen GG: Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008, 9: 860-873.
Wang G, Chen C, Yang R, Cao X, Lai S, Luo X, Feng Y, **a X, Gong J, Hu J: p55PIK-PI3K stimulates angiogenesis in colorectal cancer cell by activating NF-kappaB pathway. Angiogenesis. 2013, 16: 561-573.
Langenfeld EM, Langenfeld J: Bone morphogenetic protein-2 stimulates angiogenesis in develo** tumors. Mol Cancer Res. 2004, 2: 141-149.
Raida M, Clement JH, Leek RD, Ameri K, Bicknell R, Niederwieser D, Harris AL: Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res Clin Oncol. 2005, 131: 741-750.
Rothhammer T, Bataille F, Spruss T, Eissner G, Bosserhoff AK: Functional implication of BMP4 expression on angiogenesis in malignant melanoma. Oncogene. 2007, 26: 4158-4170.
Lai TH, Fong YC, Fu WM, Yang RS, Tang CH: Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate. 2008, 68: 1341-1353.
Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK: Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005, 65: 448-456.
Ghosh-Choudhury N, Mandal CC, Choudhury GG: Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007, 282: 4983-4993.
Kang MH, Kim JS, Seo JE, Oh SC, Yoo YA: BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Exp Cell Res. 2010, 316: 24-37.
Langenfeld EM, Kong Y, Langenfeld J: Bone morphogenetic protein-2-induced transformation involves the activation of mammalian target of rapamycin. Mol Cancer Res. 2005, 3: 679-684.
Perron JC, Dodd J: Structural distinctions in BMPs underlie divergent signaling in spinal neurons. Neural Dev. 2012, 7: 16-
Sugimori K, Matsui K, Motomura H, Tokoro T, Wang J, Higa S, Kimura T, Kitajima I: BMP-2 prevents apoptosis of the N1511 chondrocytic cell line through PI3K/Akt-mediated NF-kappaB activation. J Bone Miner Metab. 2005, 23: 411-419.
Vinals F, Lopez-Rovira T, Rosa JL, Ventura F: Inhibition of PI3K/p70 S6K and p38 MAPK cascades increases osteoblastic differentiation induced by BMP-2. FEBS Lett. 2002, 510: 99-104.
Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR: P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002, 108: 809-821.
Ridley AJ: Life at the leading edge. Cell. 2011, 145: 1012-1022.
van der Flier A, Sonnenberg A: Structural and functional aspects of filamins. Biochim Biophys Acta. 2001, 1538: 99-117.
Postma M, Bosgraaf L, Loovers HM, van Haastert PJ: Chemotaxis: signalling modules join hands at front and tail. EMBO Rep. 2004, 5: 35-40.
Cuny GD, Yu PB, Laha JK, **ng X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT: Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008, 18: 4388-4392.
Gilboa L, Nohe A, Geissendorfer T, Sebald W, Henis YI, Knaus P: Bone morphogenetic protein receptor complexes on the surface of live cells: a new oligomerization mode for serine/threonine kinase receptors. Mol Biol Cell. 2000, 11: 1023-1035.
Nohe A, Keating E, Knaus P, Petersen NO: Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004, 16: 291-299.
Hassel S, Yakymovych M, Hellman U, Ronnstrand L, Knaus P, Souchelnytskyi S: Interaction and functional cooperation between the serine/threonine kinase bone morphogenetic protein type II receptor with the tyrosine kinase stem cell factor receptor. J Cell Physiol. 2006, 206: 457-467.
Klose P, Weise C, Bondzio A, Multhaup G, Einspanier R, Gruber AD, Klopfleisch R: Is there a malignant progression associated with a linear change in protein expression levels from normal canine mammary gland to metastatic mammary tumors?. J Proteome Res. 2011, 10: 4405-4415.
Acknowledgements
This work was supported by the Berlin Brandenburg School for Regenerative Therapies (DFG graduate school 203, fellowship to CH and AD) and by SFB958 (to PK) as well as Sonnenfeld-Stiftung (to CH) and funding from the Berlin School of Integrative Oncology (to AB). We thank Prof. Dr Sebald (Würzburg, Germany) for recombinant BMP2 and Prof. Dr Vanhaesebroeck (UCL, London, UK) for DNA constructs. We thank Gisela Wendel and Johanna Scholz for excellent technical support. We are grateful to Dr Mariona Graupera, Prof. Dr Anne Ridley and Dr David Yadin for valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CH and PK designed the experiments. CH, AB and AD performed experiments. AB and IL provided computational analysis, CW performed mass spectrometry and JHB provided valuable discussion. CH and PK wrote the manuscript. All authors read and approved the final manuscript.
Andreas Benn, Agnieszka Denkis contributed equally to this work.
Electronic supplementary material
12915_2014_777_MOESM1_ESM.pdf
Additional file 1: Figure S1: Antibody validation, quantification of co-localisation and test for BMP2 dependent tyrosine phosphorylation of endogenous BMPRII. (PDF 230 KB)
12915_2014_777_MOESM5_ESM.pdf
Additional file 5: Figure S5: Effect of small molecule inhibitors on signalling, PH-Akt-GFP translocation, phospho-Akt/phospho-PDK1 and BMPRII localisation. (PDF 614 KB)
12915_2014_777_MOESM7_ESM.pdf
Additional file 7: Inventory of supplemental information, supplemental experimental procedures, supplemental references.(PDF 112 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hiepen, C., Benn, A., Denkis, A. et al. BMP2-induced chemotaxis requires PI3K p55γ/p110α-dependent phosphatidylinositol (3,4,5)-triphosphate production and LL5β recruitment at the cytocortex. BMC Biol 12, 43 (2014). https://doi.org/10.1186/1741-7007-12-43
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1741-7007-12-43