Abstract
Background
The objective was to determine the repeatability and stability of capnography interfaced with human exposure facility.
Methods
Capnographic wave signals were obtained from five healthy volunteers exposed to particle-free, filtered air during two consecutive 5 min intervals, 10 min apart, within the open and then the sealed and operational human exposure facility (HEF). Using a customized setup comprised of the Oridion Microcap® portable capnograph, DA converter and AD card, the signal was acquired and saved as an ASCII file for subsequent processing. The minute ventilation (VE), respiratory rate (RR) and expiratory tidal volume (VTE) were recorded before and after capnographic recording and then averaged. Each capnographic tracing was analyzed for acceptable waves. From each recorded interval, 8 to 19 acceptable waves were selected and measured. The following wave parameters were obtained: total length and length of phase II and III, slope of phase II and III, area under the curve and area under phase III. In addition, we recorded signal measures including the mean, standard deviation, mode, minimum, maximum – which equals end-tidal CO2 (EtCO2), zero-corrected maximum and true RMS.
Results
Statistical analysis using a paired t-test for means showed no statistically significant changes of any wave parameters and wave signal measures, corrected for RR and VTE, comparing the measures when the HEF was open vs. sealed and operational. The coefficients of variation of the zero-corrected and uncorrected EtCO2, phase II absolute difference, signal mean, standard deviation and RMS were less than 10% despite a sub-atmospheric barometric pressure, and slightly higher temperature and relative humidity within the HEF when operational.
Conclusion
We showed that a customized setup for the acquisition and processing of the capnographic wave signal, interfaced with HEF was stable and repeatable. Thus, we expect that analysis of capnographic waves in controlled human air pollution exposure studies is a feasible tool for characterization of cardio-pulmonary effects of such exposures.
Similar content being viewed by others
Background
The primary objective of current and future air pollution studies is to further understand the mechanisms for epidemiologic data on increased morbidity and mortality due to exposure to a ambient pollutants, e.g. fine particulate air pollution, i.e. particles of aerodynamic diameter < 2.5 μm (PM2.5) [1–5]. In addition, the next objective is to link the data from animal studies [6] with clinical evidence related to adverse cardio-pulmonary and vascular events; in particular, coronary and cerebro-vascular events related with such exposure [7–10]. To this end, environmental air pollution studies aim to reveal the physiologic changes and molecular mechanisms that follow inhalation of gaseous and particulate pollution and lead to a systemic inflammatory response. Moreover, they aim to provide plausible explanations of the association between air pollution exposure and morbidity/mortality from cardio-pulmonary and vascular etiology. Understanding these outcomes is of paramount importance in susceptible populations such as children, the elderly, asthmatics, patients suffering from chronic obstructive pulmonary disease (COPD), cardiovascular diseases and diabetes.
Different health outcomes and indices have been assessed during controlled human exposures to gaseous and particulate air pollution: ECG and lung function changes [11, 12], heart rate variability [13–15], blood and sputum markers of inflammation – cellular variability and mediators including interleukin 6 (IL-6), leukotriene B4 (LTB4), tumor necrosis factor alpha (TNFα,) interleukin 8 (IL-8), endothelins and fibrinogen, [13, 16].
Acute PM2.5 exposure can promote systemic inflammation by activating the capillary endothelium in the pulmonary circulation [17, 18]. Therefore, we redirected our interests in detecting acute effects of PM2.5 exposure from lung mechanics to gas exchange surface of the alveolar-capillary membrane. Our fine concentrated ambient particles (CAP) exposure facility can concentrate particles sized 0.15 – 2.5 μm in aerodynamic diameter. Due to geometry of the airways, fine particles (PM2.5) can be inhaled deep into the small conducting bronchioles and the alveoli. If adverse effects of PM2.5 are to be measured, a major site that could be examined is the alveolar-capillary membrane. Therefore, we considered capnography as the most suitable procedure that can provide real-time information about alveolar ventilation, pulmonary perfusion, respiratory pattern, and CO2 elimination/production. Clinically, capnography has been used in concert with pulse oxymetry to detect adverse respiratory events during anesthesia [19, 20]. In addition, capnography has been used in emergency rooms and during emergency transportation [21–24]. However, although widely used in clinical and pre-hospital settings, an extensive literature search confirmed that capnography has not been used as a procedure or outcome measure in settings of controlled human air pollution studies.
In our previous studies [17, 25], we could not detect any statistically significant changes in standard pulmonary function measures, such as forced expiratory volume in the 1st second (FEV1) or lung diffusing capacity for carbon monoxide (DLCO). However, we have reported a constriction of the brachial artery immediately following a 2-hour CAP + O3 exposure [18] and an acute increase in diastolic blood pressure in healthy adults during controlled exposure to CAP ± O3 [26]. We hypothesize that "real-time" capnography would be a more sensitive or even specific marker of the inflammation induced by pollutants (CAP ± O3) at the alveolar-capillary membrane, as well as pollutant-induced airway constriction, rather than more gross, effort dependent functional tests such as spirometry and lung diffusion. Since events at the alveolar-capillary membrane are an instantaneous reflection of ventilatory/cardiovascular function, any compromise of the ventilation-perfusion relationship, which may result after breathing CAP ± O3, could have some detectable consequences. Therefore, capnography as a non-invasive, instantaneous physiological measurement, which could provide insight into alveolar ventilation, physiological dead space changes, pulmonary perfusion, ventilation-perfusion mismatch, changes of cardiac output and respiratory patterns, seems to be a potentially valuable and needed procedure in air pollution research.
In this study, we tested acquisition of the capnographic wave signal for stability and repeatability through customized hardware & software components interfaced with the human exposure facility of The Gage Occupational and Environmental Health Unit, St. Michael's Hospital and University of Toronto. The HEF design and exposure characterization have been described in details elsewhere [17, 25]. See Figure 1 showing HEF with the door open.
Methods
Hardware and Software Setup
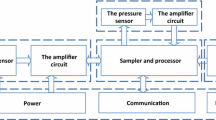
See Figure 2 for the schematic of the hardware interfaced with the HEF, which was used for this procedure. In order to record and analyze instantaneous CO2 digitized waveforms, we chose the commercially available Oridion Microcap® CO2 portable bedside capnograph (Oridion Systems Ltd, Microcap®, model #CS-04178) [27]. It uses Microstream® Technology with low-flow, sidestream aspiration vs. traditional high-flow or mainstream monitors [28, 29]. This monitor has a low sampling rate of 50 ml/min and an accuracy of EtCO2 readings of ± 2 mmHg in the range of 0–38 mmHg and ± 5% of the reading in the range of 39–99 mmHg, when steady state is reached. EtCO2 accuracy is maintained for up to 80 breaths/min. The system response time, including delay and rise time, is typically 2.45 seconds, with a maximum 2.9 seconds. In addition, proprietary Microbeam IR source generates only the specific wavelengths characteristic of the CO2 absorption spectrum. This IR source illuminates the microsample cell and reference channel, where the infrared emission exactly matches the absorption spectrum of the CO2 molecule, thus other gases in the sample such as N2O, O2 and water vapor, do not affect the CO2 reading. The capnograph has a built-in self-check procedure for verification of validity of the recorded signal. These features make it suitable for our HEF setting, in which we expose non-intubated healthy subjects and subjects with mild pulmonary or cardiovascular disease.
The capnograph we used, had a factory digital-output-only, although, as an option the manufacturer provided a Digital-to-Analog Converter (CS-07143) [30], which was used to process out 4 digital signals including ground, CO2 wave, respiratory rate and CO2 measurement validity (indicates invalidity raising output voltage to full scale). At full scale, 0.9 V equals 100 mmHg for the CO2 output and 150 breaths/min for the respiratory rate output. A voltage of 1.0 V indicates invalidity due to sampling line disconnection, auto zero, and/or malfunction in all channels. An analog output from DA converter was directed to the terminal board ADAM – 3968 SCSI 68P by Super Logics Inc. and further by an expansion cable CS-G56 as a single-ended connection to ADAC MF-5500, 12-bit, 100-kHz PCI data acquisition board [31]. The CO2 wave signal resolution obtained through entire setup (capnograph, DA converter and AD card) was 0.27874 mmHg over the full scale of 100 mmHg. The acquired signal was captured by WinView software v. 2.9 from Super Logic Inc [32], using the continuous sampling mode with 20 samples per second, board DMA and smooth scanning mode. Logged data were saved as an ASCII file on the hard drive. The computer used was a Dell Optiplex GX270 with 3.2 GHz Pentium 4 processor and 512 Mb RAM. Saved ASCII files were imported into WinDaq Waveform Browser version 2.39 by DATAQ Instruments Inc. [33], where capnographic waves were analyzed for acceptability according to a Landis score [34]. Variables of interest were measured and entered into an MS Access 2003 database.
The exhaled CO2 was sampled using two customized Microstream® EtCO2 adult NIV Lines™ [35], joined together. We did not want to place the capnograph itself within the sealed HEF, which is at sub-atmospheric pressure (11 mmHg), and slightly higher temperature and relative humidity compared to laboratory and ambient conditions, respectively. The first line (NIV-1) had the capnograph end cut off and that end was attached inside the HEF to the 20G diameter custom needle port, installed through Plexiglas window. The subject used the patient end as a nasal probe with prongs placed comfortably within the nostrils. The second line (NIV-2) had the patient end cut off and that end was attached to the other side of the needle port. The capnograph end of this line was attached to the sampling line port of the capnograph itself. The total length of sampling line, joined in this fashion, was 4.3 m.
Subjects
Two tests (EXP#1 and EXP#2) were carried out on five healthy, non-smokers (4 males and 1 female), aged 35–46. Continuous capnographic wave recordings were obtained twice on each subject, 10 min apart, while seated inside the HEF, first with the door open and then with the door sealed under operational conditions. Subjects had the sampling line nasal prongs placed inside their nostrils, with a particle delivery/CO2 removal mask over their nose and mouth. Monitoring and routine testing was carried out as in our human exposure studies during a filtered air control exposure [17, 25]. One subject had variable and completely unacceptable waveforms due to periodical sniffling during the tests, thus we analyzed data for four subjects. Immediately before and after each capnographic waveform recording, resting expired tidal volume and respiratory rate were measured using a VMM-401 flow turbine by Interface Associates [37]. It was essential to directly measure tidal volume and respiratory rate, as changes in these parameters can modify the EtCO2 [38]. The protocol was approved by the Research Ethics Board of St. Michael's Hospital, Toronto.
Data and Statistical Analysis
We measured acceptable capnographic waves for two sets of variables (wave parameters and wave statistics) and two ventilatory variables, shown in Table 1. Selected capnographic wave parameters are described in the literature with defined physiologic meaning and with the ability to characterize certain ventilatory changes [34, 39, 40]. Capnographic wave signal statistics represented standard statistical parameters, noting that RMS is derived as true RMS by means of an appropriate algorithm within WINDAQ software, and in this case represented mean expired CO2 (expressed in mmHg) over the expiratory phase of one breath. The mathematical definition of true RMS used by WINDAQ to derive this statistics is where: vn = instantaneous voltage and W = window size [41]. Measurements such as mean blood pressure and/or flow from a pulsatile pressure, airflow waveform and of course capnographic wave are non-sinusoidal waveforms that require a true RMS calculation. Breathing pattern, low respiratory rate and large tidal volume, can influence the shape of the capnographic wave, particularly the slope of phase III of the capnographic wave [42]; therefore, we recorded these parameters for the purpose of the quality control of the recorded capnographic wave parameters and statistics during the two tests. See Figure 3 showing the example of typical capnographic wave analysis, using WinDaq software.
From the list of variables in Table 1, we selected 18 variables for statistical analyses. Nine describe aspects of the entire waveform and include the variables: tot_len, tot_AUC, EtCO2, CORRMAX, MIN, MEAN, STDEV, MED and RMS. Three describe phase II, from the start of the wave upstroke to the beginning of the alveolar plateau, and include len2, slope2 and absolute difference for slope2. Four describe phase III, the start to the end of the alveolar plateau, and include len3, slope3, diff3 and AUC3. Two were obtained from the minute ventilation test, immediately before and after the capnography test, reported as an average value, and include average RR and average VTE. Means, standard deviations, coefficients of variation (CV) and the number (N) of waveforms for each subject's test (in total EXP #1 = 60 waves and EXP #2 = 54 waves) were calculated for each of the 18 variables. The prefix M1 and M2 are the means for tests #1 and #2, S1 and S2 are the standard deviations and CV1 and CV2 the coefficients of variation (100 × standard deviation/mean). Data are reported for the four subjects, each of whom had two 5-minute capnography tests (8 tests in total). Among the 8 tests, the number of waveforms ranged from 8–19, depending on the subject's respiratory rate and acceptability of waveforms. Statistical analysis was performed using the SAS System for Windows v. 8.2 (SAS Institute Inc., Cary, NC., USA). The differences between the wave parameters, ventilatory parameters and signal statistics obtained within the open (EXP#1) and sealed (EXP#2) HEF, were evaluated using a paired t-test. A p-value < 0.05 was considered to indicate a statistically significant difference.
Results
The results of the measurements for monitored variables are presented in Tables 2, 3, and 4. Summary data for EtCO2 parameters, comparing EXP#1 and EXP#2 for the four subjects are shown in Table 2. The two variables with the lowest CV were zero-corrected EtCO2 (CORRMAX), 3.89% for the first and 3.62% for the second measurement, and EtCO2 4.14% for the first and 4.48% for the second measurement, respectively. The phase II absolute difference (diff2) had a slightly higher CV, 6.48% and 6.52%. The eight other variables all had maximum CVs >10%, with the highest CV for the phase III slope (slope3), 52.57% and 57.96%. None of the mean values for these parameters measured with the HEF open and sealed showed any significant statistical difference. The p-values ranged from 0.16 for diff3 (phase III absolute difference) to 0.98 for len2 (length of the phase II).
Summary data for the ventilatory parameters, VTE and RR comparing EXP#1 and EXP#2 for the four subjects are shown in Table 3. The coefficients of variation for these two variables were above 10%, 21.87% for the CV of RR for the first measurement and 33.25% for CV of VTE for the second measurement. In addition, none of the mean values for these parameters was significantly different comparing the open versus sealed and operational HEF.
Descriptive statistics for EtCO2 signal variables measured within the open and sealed HEF (EXP#1 and EXP#2) are presented in Table 4. The coefficient of variation for RMS is smaller than the CV for MEAN, for both exposures, while the EtCO2 signal zero (MINIMUM) has the highest CV of all variables. The paired t-test for means showed no significant statistical difference between measurements within open and sealed HEF for any of these variables.
Discussion
We added customized hardware and software components to interface with the HEF in order to utilize the advantages of capnography over the standard tests of lung function. We chose to use capnography in the HEF as we expect that capnography will be a useful tool for the detection of changes in ventilation during human exposure studies. Capnography, as a non-invasive, real-time physiologic measurement of adequacy of ventilation and perfusion, has found its role in a variety of medical disciplines including anesthesiology, critical care, emergency medicine, cardiology, pediatrics, neurology and respiratory care. Current capnographic hardware and software is designed for these aforementioned environments. As such, we did not find a commercially available, ready-to-go solution for the implementation of capnography in the experimental environment of human air pollution exposure studies. Our goal was to use relatively inexpensive and commercially available hardware components to acquire EtCO2 wave signals within sealed and operational HEF, but with acceptable signal quality. Our first choice for the capnograph was the Microcap® CO2 portable bedside monitor, with nominal performance features adequate for our study's setup. However, due to the absence of an analog output, this monitor needed an additional digital-to-analog converter. We used one supplied as an option by the original manufacturer, designed to be used with this particular model of capnograph. Further processing of the four analog signals (EtCO2 wave, RR, EtCO2 wave signal validity and ground) utilized the standard solution (terminal board, expansion cable and MF 5500 AD card). Although the signal was less than 1.0 V, due to the low level of noise, we found that a single ended connection was adequate. We performed zero CO2 calibration of the capnograph with 100% O2, in order to measure the system signal noise for the overall hardware and software setup. The average noise level was 0.0052 ± 0.016 V and RMS was 0.0168 V. Signal-to-noise ratio (SNR) over the full scale of 100 mmHg was 34.59 dB. We found the WinView data acquisition software easy to install and setup using the MF 5500 AD board. The EtCO2 wave signal was saved as an ASCII file and imported into WinDaq Waveform Browser. This software was quite adequate for EtCO2 wave signal analysis.
An additional concern, related to the EtCO2 sampling line required a customized solution. We used two original sampling lines to make one custom line, which attaches on both sides of the custom-made needle port screwed into the plexiglas HEF window. Accordingly, we did not observe additional delay added to frequency response of the monitor. We considered the possibility that sampling through a longer line than originally designed and recommended by the manufacturer could also affect the performance of the capnography monitor. However, we did not observe that sampling in this way affected the reading of EtCO2 and RR on the capnograph itself. Mason et al. [36] showed that an experimental sampling line as long as 9.0 m was equally reliable as the standard 3.0 m long one, which agrees with our observation.
Comparisons of the differences between capnographic measurements obtained under two different conditions, relevant to our design of human exposure studies (open and sealed HEF), did not yield any statistical significance. Yet, some differences existed in absolute values, but allocation of these differences among observed measures was to be expected. In the group of EtCO2 wave parameters, the variable with the lowest CV was the zero-corrected EtCO2, 3.89% and 3.62%, followed closely by EtCO2, 4.14% and 4.48%. Verschuren et al. [43] considered CV of the EtCO2 less than 5% as an indicator of steady-state status for their patients The EtCO2 is the main output value of the capnograph and correction of this value with the minimum-recorded value, for a particular wave, increased the accuracy of true EtCO2 reading. Among seven out of eight tests, we found that the range of recorded zero values for EtCO2 was between 0 and 0.55664 mmHg (1.42% of maximum-recorded EtCO2 of 39.167 mmHg). Surprisingly, we found that one subject's exposure (EXP#2 for that subject, with 15 acceptable analyzed waves) had recorded a zero range between 1.4274 to 2.8547 mmHg (7.29% of the maximum-recorded EtCO2 of 39.167 mmHg). Reviewing the lab log for this test, we found that 5 min after the enclosure was sealed, the recorded CO2 within the HEF was approximately 0.38%. This could have happened, because the particle delivery/CO2 removal mask either was not in place or correctly positioned, or in the case of the CO2 removal pump system malfunction. Thus, the maximum recoded zero for EtCO2 was approximately the background concentration of CO2 in the sealed HEF (749 mmHg × 0.0038 = 2.846 mmHg). Later, we were able to simulate this pattern of zero shift with the particle delivery/CO2 removal mask incorrectly positioned and this explained the unexpected CV of the MINIMUM variable for the EtCO2 signal. See Figure 4 for the EtCO2 tracing of that original test in trend format.
CV for the slopes of phase II and III, for both measurements, showed that these two variables are also the ones with the largest physiologic variability even in the steady state. Thus, they have the potential to be considered as variables capable of detecting a deviation from the normal pattern as well. Changes in RR, VTE, and ventilation maldistribution expressed as ventilation/perfusion (V/Q) mismatch, can result in changes of phase II and III of the EtCO2 wave [42]. For both, EXP#1 and EXP#2, RR and VTE were virtually identical, allowing us to have slopes of phase II and III clearly comparable between the two measurements. The CV for RR and VTE (range 21.87 to 33.25) were somewhat higher than reported by Verschuren at al. [43] for their patients. We believe that this might be possibly due to the psychological effect of the limited space in the HEF, which might affect the variability of the breathing pattern.
In the group of EtCO2 signal variables, we found that the CV for RMS was smaller than for the MEAN, because RMS has been calculated as the true RMS and better represents the "average" value of expired CO2 than the MEAN value obtained by the standard equation. The true RMS, in this case, better approximates mixed-expired CO2 (PECO2) per one breath or over the time that the EtCO2 signal was "averaged". The PECO2 and arterial partial pressure for CO2 (PaCO2) are used to calculate dead space to tidal volume ratio (VD/VT).
Conclusion
We have described a customized, inexpensive and feasible hardware and software implementation of standard capnography, as an adjunct to our human exposure facility, which is used in air pollution studies. Our hardware and software setup for acquisition and analysis of EtCO2 wave signal was demonstrated as reliable and stable. We would have preferred that the selected capnograph monitor have an analog output, so we could avoid digital to analog signal conversion. The instrument was meant to be used in standard pre-hospital or hospital environments, therefore the manufacturer had not considered that option. However, extending the use of capnography into research labs like ours, where we carry out air pollution human exposure studies, has been long overlooked. Using capnography in the way we described, we may be able to detect and better characterize the effect of exposure to air pollution on cardiopulmonary system, particularly on lung ventilation and perfusion. We plan to use this technique in our on-going and subsequent controlled human exposure studies as a physiologic outcome measure.
References
Cohen AJ, Ross Anderson H, Ostro B, Pandey KD, Krzyzanowski M, Kunzli N, Gutschmidt K, Pope A, Romieu I, Samet JM, Smith K: The global burden of disease due to outdoor air pollution. J Toxicol Environ Health A 2005, 68: 1301–1307. 10.1080/15287390590936166
Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM: Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A 2005, 68: 1071–1092. 10.1080/15287390590935932
Peters A, Goldstein IF, Beyer U, Franke K, Heinrich J, Dockery DW, Spengler JD, Wichmann HE: Acute health effects of exposure to high levels of air pollution in eastern Europe. Am J Epidemiol 1996, 144: 570–581.
Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ: Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109: 71–77. 10.1161/01.CIR.0000108927.80044.7F
Burnett RT, Brook J, Dann T, Delocla C, Philips O, Cakmak S, Vincent R, Goldberg MS, Krewski D: Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol 2000,12(Suppl 4):15–39. 10.1080/089583700750019495
Vincent R, Kumarathasan P, Goegan P, Bjarnason SG, Guenette J, Berube D, Adamson IY, Desjardins S, Burnett RT, Miller FJ, Battistini B: Inhalation toxicology of urban ambient particulate matter: acute cardiovascular effects in rats. Res Rep Health Eff Inst 2001, 104: 5–54. discussion 55–62
Chang CC, Tsai SS, Ho SC, Yang CY: Air pollution and hospital admissions for cardiovascular disease in Taipei, Taiwan. Environ Res 2005, 98: 114–119. 10.1016/j.envres.2004.07.005
Zanobetti A, Schwartz J: The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect 2005, 113: 978–982.
Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani DC: Effects of air pollutants on acute stroke mortality. Environ Health Perspect 2002, 110: 187–191.
Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW: Air pollution and incidence of cardiac arrhythmia. Epidemiology 2000, 11: 11–17. 10.1097/00001648-200001000-00005
Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, Verrier RL, Nearing BD, Canner MJ, Suh H, Stone PH: Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect 2005, 113: 883–887.
Schulz H, Harder V, Ibald-Mulli A, Khandoga A, Koenig W, Krombach F, Radykewicz R, Stampfl A, Thorand B, Peters A: Cardiovascular effects of fine and ultrafine particles. J Aerosol Med 2005, 18: 1–22. 10.1089/jam.2005.18.1
Pope CA 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ: Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 2004, 112: 339–345.
Pope CA 3rd, Eatough DJ, Gold DR, Pang Y, Nielsen KR, Nath P, Verrier RL, Kanner RE: Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect 2001, 109: 711–716.
Pope CA 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW: Heart rate variability associated with particulate air pollution. Am Heart J 1999, 138: 890–899. 10.1016/S0002-8703(99)70014-1
Pope CA 3rd: Particulate air pollution, C-reactive protein, and cardiac risk. Eur Heart J 2001, 22: 1149–1150. 10.1053/euhj.2001.2593
Urch B, Brook JR, Wasserstein D, Brook RD, Rajagopalan S, Corey P, Silverman F: Relative contributions of PM2.5 chemical constituents to acute arterial vasoconstriction in humans. Inhal Toxicol 2004, 16: 345–352. 10.1080/08958370490439489
Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F: Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 2002, 105: 1534–1536. 10.1161/01.CIR.0000013838.94747.64
Guo X, Luo A, Ren H, Ye T, Smalhout B: Simultaneously monitoring end tidal CO2 and other parameters versus anesthesia management. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1998, 20: 76–80.
Li CH, Zhao W, Zhang JH, Jia NG: Detection of esophageal intubation-assessment of several methods in clinical anesthesia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2003, 25: 197–200.
Castioni C: Capnography in EMS. Emergency medical services 2003, 32: 80–2. 84, 86–8
Krauss B: Capnography in EMS. A powerful way to objectively monitor ventilatory status. JEMS 2003, 28: 28–30. 32–8, 41
Thaxton JN, Kyle J, Thomas J, Fouty T, Tyler W: Initiation of capnography in a rural EMS system. Prehosp Emerg Care 1999, 3: 23–26.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay M: Pulmonary Dead-Space Fraction as a Risk Factor for Death in the Acute Respiratory Distress Syndrome. N Engl J Med 2002, 346: 1281–1286. 10.1056/NEJMoa012835
Petrovic S, Urch B, Brook J, Datema J, Purdham J, Liu L, Lukic Z, Zimmerman B, Tofler G, Downer E, Corey P, Tarlo S, Broder I, Dales R, Silverman F: Cardio-respiratory effects of concentrated ambient PM 2.5 : A pilot study using controlled human exposures. Inhalation Toxicology 2000,12(S1):173–88.
Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD: Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 2005, 113: 1052–1055.
Oridion Systems Ltd [http://www.oridion.com/global/english/products/portable_bedside_monitors/microcap/features_benefits/]
Colman Y, Krauss B: Microstream capnography technology: a new approach to an old problem. J Clin Monit Comput 1999, 15: 403–409. 10.1023/A:1009981115299
Hagerty JJ, Kleinman ME, Zurakowski D, Lyons AC, Krauss B: Accuracy of a new low-flow sidestream capnography technology in newborns: a pilot study. Journal of perinatology; official journal of the National Perinatal Association 2002, 22: 219–225.
Oridion Systems Ltd [http://www.oridion.com/download/d_a_converter.pdf]
IOtech Inc [http://www.iotech.com/catalog/daq/adac.html]
Super Logic Inc [http://www.superlogics.com/low-cost-data-acquisition-software/pcmcia-usb-pci/143.htm]
DATAQ Instruments Inc [http://www.dataq.com/products/software/playback.htm]
Landis B, Romano PM: A scoring system for capnogram biofeedback: preliminary findings. Appl Psychophysiol Biofeedback 1998, 23: 75–91. 10.1023/A:1022195721961
Oridion Systems Inc [http://www.oridion.com/global/english/products/filterline_circuits_adapters/nivline/product_specifications/]
Mason KP, Burrows PE, Dorsey MM, Zurakowski D, Krauss B: Accuracy of capnography with a 30 foot nasal cannula for monitoring respiratory rate and end-tidal CO 2 in children. Journal of clinical monitoring and computing 2000, 16: 259–262. 10.1023/A:1011436329848
Interfaceusa Associates [http://www.interfaceusa.com/media/media85/pdf/VMM-401.pdf]
Gisolf J, Wilders R, Immink RV, van Lieshout JJ, Karemaker JM: Tidal volume, cardiac output and functional residual capacity determine end-tidal CO 2 transient during standing up in humans. J Physiol 2004, 554: 579–590. 10.1113/jphysiol.2003.056895
Kalenda Z: Mastering infra-red capnography. Zeist, The Netherlands: Kerckebosch BV; 1989.
You B, Peslin R, Duvivier C, Vu VD, Grilliat JP: Expiratory capnography in asthma: Evaluation of various shape indices. Eur Respir J 1994, 7: 318–323. 10.1183/09031936.94.07020318
DATAQ Instruments Inc [http://www.dataq.com/applicat/articles/freq_rms.htm]
Stromberg NO, Gustafsson PM: Ventilation inhomogeneity assessed by nitrogen washout and ventilation-perfusion mismatch by capnography in stable and induced airway obstruction. Pediatr Pulmonol 2000, 29: 94–102. 10.1002/(SICI)1099-0496(200002)29:2<94::AID-PPUL3>3.0.CO;2-B
Verschuren F, Liistro G, Coffeng R, Thys F, Roeseler J, Zech F, Reynaert Marc: Volumetric Capnography as a Screening Test for Pulmonary Embolism in the Emergency Department. Chest 2004, 125: 841–850. 10.1378/chest.125.3.841
Acknowledgements
The authors would like to thank Mr. Vladimir Lukic of Wortahn Studios, Toronto for the illustration depicting the design of the HEF with added capnography components. This project was supported by Health Canada contract # 4500075293 and The Ontario Thoracic Society Block Term Grant in 2004. MEF is supported by the St. Michael's Hospital Research Institute and the Nelson Arthur Hyland Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
KZL initiated and designed the study, designed capnographic hardware & software setup, performed data analysis and interpretation, and drafted the manuscript. BU contributed to overall aspects of the study and specifically in statistical analysis, results interpretation and manuscript drafting. MF contributed in technical design modifications and conducted all experimental procedures and measurements. MEF contributed to study design and implementation with valuable clinical input and comments on physiologic aspects of capnography, while FS, the principal investigator, contributed valuable discussion and suggestions throughout this project. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lukic, K.Z., Urch, B., Fila, M. et al. A novel application of capnography during controlled human exposure to air pollution. BioMed Eng OnLine 5, 54 (2006). https://doi.org/10.1186/1475-925X-5-54
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-925X-5-54