Abstract
Background
The transcription factor, Sox2, is central to the behaviour of neural stem cells. It is also one of the key embryonic stem cell factors that, when overexpressed can convert somatic cells into induced pluripotent cells. Although generally studied as a transcriptional activator, recent evidence suggests that it might also repress gene expression.
Results
We show that in neural stem cells Sox2 represses as many genes as it activates. We found that Sox2 interacts directly with members of the groucho family of corepressors and that repression of several target genes required this interaction. Strikingly, where many of the genes activated by Sox2 encode transcriptional regulators, no such genes were repressed. Finally, we found that a mutant form of Sox2 that was unable to bind groucho was no longer able to inhibit differentiation of neural stem cells to the same extent as the wild type protein.
Conclusions
These data reveal a major new mechanism of action for this key transcription factor. In the context of our understanding of endogenous stem cells, this highlights the need to determine how such a central regulator can distinguish which genes to activate and which to repress.
Similar content being viewed by others
Background
Sox2 is a central player in animal development and one of only a few factors that together can initiate the formation of pluripotent cells (iPS cells) from somatic cell populations [1, 2]. This reflects its central role as a ‘node’ in the gene regulatory network that controls embryonic stem cell biology, promoting stem cell self-renewal and inhibiting differentiation. Sox2 is also one of the first genes to be active in the neural ectoderm and its expression is maintained in proliferating neural stem cells (NSCs) of the CNS throughout development and in the mature brain [3, 4]. The expression of Sox2 in these cells again seems to be associated with their self-renewal and inhibition of differentiation [5]. Sox2 is generally regarded as a transcriptional activator. However, in recent studies analysing the global response of cells to loss of Sox2 activity, the expression of many genes was seen to increase rapidly when Sox2 function was inhibited [6, 7]. ChIP-seq analysis shows that several of the genes affected in these studies are directly bound by Sox2, implying that they are direct targets [8–4A) and tested their ability to interact with Grgs (all the constructs produced Sox2 protein that still located to the nucleus, see Additional file 4: Figure S1). The subcellular translocation assay with Grg5 indicated that the C-terminal amino acids between 203 and 209 were essential for the interaction (Figure 4A). We therefore set out to generate a site-directed mutant that no longer bound Grgs, but would retain transcriptional activator activity.
Loss of Grg interaction in a 203–209 mutant
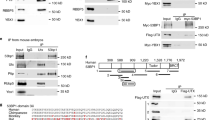
Comparison between Sox2 and the other SoxB1 members and between Sox2 orthologues from different species revealed that only four amino acids were conserved in the region from position 203–209. We therefore made a mutant, referred to as Sox2M203–209, in which these four amino acids were altered (DxxxLQY converted to VxxxAAA; Figure 4A). Although this mutant still located to the nucleus (see Additional file 4: Figure S1A ), in the subcellular translocation assay, the Sox2M203–209 mutant exhibited a dramatically reduced ability to change the subcellular localization of Grg5 (Figure 5A). Moreover, unlike WT Sox2, immune precipitation of MYC-Grg5 in COS-7 cells did not co-precipitate co-transfected Sox2M203–209 mutant (Figure 5B). In order to determine whether this mutation had disrupted a direct protein-protein interaction between the Grg proteins and Sox2 an in vitro pull down assay was used. The ability of GST-fused Grg1 or Grg5 proteins to pull down radiolabelled Sox2 or Sox2M203–209 was assessed. For both Grg proteins, the Sox2M203–209 mutant resulted in a similar 4-fold decrease in the amount of Sox2 that was co-immune precipitated (Figure 5C). It was also noted that Grg1 was able to pull down approximately 4-fold more Sox2 than did Grg5 (data not shown). These data show that residues 203–209 of the Sox2 protein are necessary for a direct interaction with both Grg1 and Grg5.
The Sox2M203–209mutant does not interact with Grgs and fails to repress a reporter construct. (A) Unlike WT Sox2, the Sox2 M203–209 mutant was unable to translocate Grg5 into the nucleus of co-transfected Cos-7 cells suggesting loss of interaction. Counting the proportion of cells exhibiting altered distribution of MYC-Grg in cells co-expressing Sox2 revealed a highly significant reduction of about 50% (p = 0.0002). (B) Immune precipitation of MYC-Grg5 co-transfected with WT Sox2 or with the Sox2 M203–209 mutant, showing that the mutant failed to be co-precipitated with Grg5. (C) In an in vitro pull down assay, immunoprecipitation of GST-fused Grg1 or Grg5 reproducibly pulled-down 4–5 fold less Sox2 M203–209 than it did WT Sox2 (D) The Sox2 M203–209 mutant retained the ability to activate luciferase expression driven by the 3xSX promoter in Cos-7 cells (the difference between this and the level induced by WT Sox2 was not statistically significant). (E) The ability of co-transfected Sox2 M203–209 mutant to induce luciferase expression driven by the REX promoter in P19 cells was very similar to that induced by WT Sox2. (F) Unlike WT Sox2, the Sox2 M203–209 mutant failed to repress luciferase expression driven from the GFAP promoter, and the addition of Grg3 or Grg5 had no effect on this. *p = <0.05; **p = <0.01; ns – not significant Scale bar approximately 20 μm.
The Sox2M203–209mutant loses repressor activity
Having established that amino acids 203–209 were required for Grg binding, we next asked whether Sox2M203–209 was defective in its ability to act as a gene repressor. Using the luciferase reporter assay described above, we found that the Sox2M203–209 mutant retained the ability to activate both the 3xSX and the REX-regulated reporter constructs (Figure 5D, E), indeed having a slightly stronger activator effect on the 3xSX promoter than WT Sox2. However, the mutant no longer significantly repressed luciferase expression of the construct driven from the GFAP promoter even in the presence of ectopic Grg3 or Grg5 (Figure 5 F). These results suggest that despite its inability to bind Grg co-repressors, Sox2M203–209 retains the transcriptional activator activity of WT Sox2, but is significantly impaired in its ability to repress transcription from the GFAP regulatory sequence.
Consistent with this, when the effect of ectopic Sox2 or the mutant Sox2 M203–209 expression on GFAP and six genes in NSCs was assessed by qPCR, a range of levels of repression was exhibited by WT Sox2. This showed repression by WT Sox2 and a reduced or lack of repression by the Sox2M203–209 mutant (Figure 6A).
A Grg binding mutant of Sox2 fails to inhibit NSC differentiation. (A) Validation of the repression of NNMT, SOX8, SERPINE1, HELLS, IGFBP2, IGFBP3 and GFAP expression using qPCR. P-values shown for 5 PCR replicates *p = <0.05; **p = <0.01. (B) Graph showing the percentage of NSCs extending MAP2 positive processes after 5 days in differentiation medium. This was inhibited by WY Sox2 but not by the Sox2 M203–209 mutant. (C) representative examples of cells after transfection; white lines indicate length of cellular extensions. (D) Model for mechanism of Sox2 action during NSC development.
Groucho-binding mutant of Sox2 fails to repress neural differentiation
Overexpression of Sox2 in NSCs has been shown to interfere with their ability to differentiate [18, 19]. We therefore compared the effect of overexpressing WT Sox2 to the Sox2M203–209 mutant. Unlike control NSCs transfected with EGFP alone, cells transfected with WT Sox2 did not extend fine, MAP2 positive processes after 5 days in differentiation medium, but instead, fewer cells extended broader, shorter processes (Figure 6B,C). However, no such inhibition of differentiation was seen in cells transfected with the Sox2M203–209 mutant.
Discussion
Sox2 is a central component of the gene regulatory network that controls a range of stem cells, most notably ESCs and NSCs. The realization that, in addition to its role as a transcriptional activator, Sox2 might also repress genes is relatively recent and little has been done to investigate this activity.
Here, we have shown that the number of genes repressed by Sox2 in NSCs is almost identical to the number activated and we have identified one mechanism (recruitment of groucho family corepressors) by which it can achieve this repression. We have consequently generated a version of Sox2 that acts as a transcriptional activator but now lacks that mechanism for repressor activity. This allows us to begin to dissect the full complexity of Sox2 activity in regulating cell behaviour.
Sox2 mechanism of repression
We chose to investigate a potential interaction with Grgs since this interaction has already been shown to mediate repression by the HMG family protein, Tcf [14, 15]. We have shown that Sox2 does indeed, directly interact with both full length and short forms of the Grgs. We used a series of deletions constructs to map the putative Grg-interacting region of Sox2 to amino acids 203–209 (YDVSALQY), which shows similarity to the Eh1 Grg interacting domain, F/YxI/VxxI/L/V [20, 21]. Targeted mutation of this region localized the interaction to the sequence, DxxxLQY, which is well conserved in the SoxB1 family.
However, alternative repressor mechanism(s) may also exist. This would provide multiple aspects to the mechanisms of action of Sox2 that could therefore be independently regulated to achieve a high level of complexity in its biological activities. It is also possible that some of the repressive effects of Sox2 are indirect via transcriptional activation of a repressor. Our results were over a short time scale so we do not feel that there was likely to be sufficient time for this to occur but it remains formally possible. This is supported by our earlier observations that the repressive effects of the very similar protein, Sox3, were mimicked by an HMG-engrailed repressor fusion protein [22].
Relative role of transcriptional repression in Sox2 functions
Since Sox2 has traditionally been regarded as a transcriptional activator, it is striking that our study revealed that the number of genes repressed by overexpression of Sox2 was a similar to the number of genes that were activated. This implies that repression plays as big a part in its biological functions as does activation. Since the numbers of genes affected by Sox2 in our study closely resembles the numbers of genes affected when Sox2 was knocked down in ES cells [6, 7] it seems probable that the effects in our study represent true targets of Sox2.
It is of note that the targets of Sox2 activation are highly enriched for regulators of Pol II transcription whereas no such genes are repressed. This implies that a large part of the activator function of Sox2 (approximately 25% of the genes affected by Sox2 overexpression) is to regulate the biological activity of NSCs indirectly through the function of downstream transcription factors, whereas its repressor function affects the cells directly though regulating effector genes. Since Sox2 is expressed in dividing progenitor cells, the enrichment for cell cycle related genes in those repressed by Sox2 looks at first to be counterintuitive. However, this observation suggests that its normal role may be in part to control the rate of mitosis in those stem cells.
Previous studies have shown that the effects of SoxB1s in inhibiting the differentiation of NSCs was mimicked by a constitutive activator form of SoxB1 protein, while an HMG-EnR construct caused cells to begin to differentiate, suggesting that the effects of the SoxB1s were entirely through its activity as a transcriptional activator [18, 19]. However, overexpression of the HMG-EnR construct did not elicit complete differentiation as shown by the absence of neurofilament or beta-tubulin expression [18]. Indeed, close inspection of the published data shows that an HMG-VP16 construct inhibits expression of early neurogenic transcription factors, but does not appear to completely inhibit expression of beta-tubulin.We therefore suggest a model in which the activator function of Sox2 promotes ‘stem cell-ness’ and so inhibits differentiation, but the repressor function of Sox2 is also required to inhibit differentiation, repressing those effector genes that would be activated soon after the cells were released from the NSC state (Figure 6C). Consistent with this model and the published data, the gene encoding neurofilament light chain was amongst those genes revealed to be repressed greater than 1.5-fold by Sox2 in our microarray analysis.
Target specificity
Our observation that some genes are activated while others are repressed in the same transfected cell population, suggests that it is the gene target sequence that determines whether Sox2 exerts its activator or repressor activity. Activation or repression is not dictated solely by the availability of cofactors for these functions in a ‘cell context’ dependent manner, but is dictated by the target gene, which presumably determines which Sox2 cofactors are available at that regulatory region to cause Sox2 to act as either an activator or repressor.
Conclusions
This study shows that transcriptional repression is a major part of the mechanism by which Sox2 acts in NSCs. In order to understand how Sox2 functions to regulate stem cell biology, we must therefore understand not only what is upstream and downstream of Sox2, but also which cell type-dependent cofactors are required for Sox2 to regulate each target and the gene sequence context that determines whether the target gene is activated or repressed.
Methods
Cell culture
The human ReNcell VM NSC line (Millipore Corp., Bedford, MA, U.S.A) was maintained as adherent cells in laminin-coated flasks (Sigma Cat. No. L-2020) with ReNcell NSC Maintenance Medium (Chemicon) supplemented with 20 ng/ml growth factors (bFGF and EGF, Invitrogen) in 50/50 mixture of Neurobasal and DMEM F12 (Gibco) medium supplemented with Penicillin/Streptomycin (Sigma), B27 and N2 (Gibco). P19CL16 mouse EC cells were maintained in Alpha Minimum Essential Medium (GIBCO) supplemented with 10% foetal bovine serum (FBS, Gibco) plus 0.5% Penicillin/Streptomycin. COS-7 cells were maintained in Dulbecco’s Modified Eagle’s medium with 10% FBS. Differentiation of NSCs was induced by removal of growth factors.
Immunofluorescent staining
Cells were cultured on poly-D-lysine coated coverslips, fixed with 4% paraformaldehyde/PBS for 10 min and permeabilised with 0.2% Triton X100/PBS for 20 min. Blocking was carried out with 10% BSA in 0.1% Triton X100/PBS for 30 min. Primary antibodies (MYC antibody (9E10), Sox2 antibody (R&D, MAB2018), MAP-2 antibody (Abcam)) and the fluorescent-conjugated secondary antibodies were incubated at room temperature for 1 h. Staining was observed after mounting in mounting medium for fluorescence with DAPI (Vector). Error bars in Figures 1C based on counting 15 cells and 3A, and 4A counting 100 cells.
Transfection
The human ReNcell VM NSC line (Millipore Corp., Bedford, MA, U.S.A) was transfected using the Mouse NSC Nucleofector® Kit (Lonza) following the manufacturer’s instructions. P19CL16 mouse EC cells were transfected using DharmaFECT transfection reagent (Dharmacon, Quiagen). COS-7 cells were transfected by square pulse electroporation; 210 volts for 50 mSec using a BTX Electro Square Porator ECM 830.
Immunoprecipitation and immunoblotting
Two days after transfection, cells were treated with the cross-linker, 1 mM dithio-bis(succinimidyl propionat) (DSP; Sigma) for 30 min. The cells were lysed, cleared by centrifugation and incubated with MYC antibody-conjugated beads (Sigma). Eluted proteins were analysed by SDS-PAGE and western blotting using anti-MYC and anti-Sox2 antibodies (See supplementary material for additional details).
In vitro pull down assays were performed using GST-fusions of Grg1 and Grg5 (cloned into the pGEX4T1 vector). These proteins were induced in BL21 E.coli cells by the addition of 0.1 mM isopropyl-D-thiogalactopyranoside. After 16 h growth at 18°C bacteria were harvested and lysed. Solubility of GST-Grg proteins was increased by an additional 1 h incubation (4°C) in lysis buffer with 1% Nonidet P-40 and 0.03% SDS. GST-fusion proteins were subsequently incubated with S35-labelled Sox2 proteins produced in vitro using a TNT T7 kit (Promega), with (Amersham Biosciences). GST-fusion protein were pulled-down using Glutathione-sepharose 4B beads (G.E Healthcare) and the presence of co-precipitated Sox proteins assessed by exposure of a PAGE gel on a phosphorimager.
Luciferase assay
The luciferase assay was carried out 24 h after transfection with the dual-luciferase-reporter assay system (Promega) following manufacturer’s instructions. Error bars represent standard deviation based on three independent transfection experiments.
Expression profiling microarray
hNSCs were transfected with EGFP with or without pcDNA-Sox2 or pcDNA- Sox2M203–209. After 14 h culture, GFP-positive cells were isolated by FACS. Total RNA was extracted using TRI reagent (Sigma) and further purified using an RNeasy kit (QIAGEN). The RNA samples were analysed using GeneChip® Human Genome U133 Arrays (Affymetrix) and data were first preprocessed using the statistical software, R with packages provide by http://www.bioconductor.org. Data was preprocessed using the RMA method [23] and filtered such that probes which gave expression outputs below control background probes (recorded in the GeneChip) were excluded. Fold differences in expression were calculated, and annotation packages were used to assign gene information to each probe set. The data was exported as a .txt file in order to be read and analysed in Excel. The Excel tool PivotTable was used to assign average expression intensity values to each gene.
Site directed mutagenesis
Point mutants were generated using the QuikChange kit (Stratagene) according to manufacturer‘s instructions.
Quantitative RT-PCR
The extraction of total RNA from transfected hNSC using TRI reagent (Sigma) was carried out according to the manufacturer’s instructions. Total RNA samples were cleaned up using the RNeasy Mini Kit (Qiagen). Purified total RNA was used for the synthesis of cDNA using SuperScript III Reverse Transcriptase (Invitrogen). The primers used in the quantitative RT-PCR are listed below. Quantitative RT-PCR was carried out by Rotor-Gene 6000 (Corbett/Qiagen) with Brilliant SYBR Green QPCR Master Mix (Agilent). The PCR programme was set at 95°C for 15 seconds, 60°C for 20 seconds and 72°C for 20 seconds. All the quantitative RT-PCR data were analysed by Rotor-Gene 6000 real-time rotary analyzer version 1.7. The relative expression level compared to cyclophilin B was calculated according to Pfaffl [24]. Error bars in Figure 6A– standard deviation based on three replicated qPCR reactions.
Details of plasmids and primers available in supplementary material.
Abbreviations
- CNS:
-
Central nervous system
- DAPI:
-
4’,6-diamidino-2-phenylindole
- EGFP:
-
Enhanced green fluorescent protein
- enR:
-
engrailed Repressor domain
- ES cell:
-
Embryonic stem cell
- FACS:
-
Fluorescent activated cell sorting
- GO:
-
Gene ontology
- HMG:
-
High mobility group
- iPS cell:
-
induced Pluripotent cell
- NSC:
-
Neural stem cell
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription PCR
- VP16:
-
Activator domain from VP16 virus
- WT:
-
Wild type.
References
Yamanaka S, Blau HM: Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010, 465: 704-712.
Hanna JH, Saha K, Jaenisch R: Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010, 143: 508-525.
Graham V, Khudyakov J, Ellis P, Pevny L: SOX2 Functions to Maintain Neural Progenitor. Identity. 2003, 39: 749-765.
Rex M, Orme A, Uwanogho D, Tointon K, Wigmore PM, Sharpe PT, Scotting PJ: Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev Dyn. 1997, 209: 323-332.
Pevny LH, Nicolis SK: Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2009, 42: 421-424.
Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov A, Ko MSH, Niwa H: Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007, 9: 625-635.
Greber B, Lehrach H, Adjaye J: Silencing of core transcription factors in human EC cells highlights the importance of autocrine FGF signaling for self-renewal. BMC Dev Biol. 2007, 7: 46.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA: Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005, 122: 947-956.
Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh Y-H, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung W-K, Clarke ND, Wei C-L, Ng HH: Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008, 133: 1106-1117.
Kim J, Chu J, Shen X, Wang J, Orkin SH: An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008, 132: 1049-1061.
Sharov AA, Masui S, Sharova LV, Piao Y, Aiba K, Matoba R, **n L, Niwa H, Ko MS: Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genomics. 2008, 9: 269.
Brantjes H, Barker N, van Es J, Clevers H: TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002, 383: 255-261.
Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z: GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009, 10: 48.
Brantjes H, Roose J, van De Wetering M, Clevers H: All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res. 2001, 29: 1410-1419.
Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A: Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998, 395: 604-608.
Shi W, Wang H, Pan G, Geng Y, Guo Y, Pei D: Regulation of the pluripotency marker Rex-1 by Nanog and Sox2. J Biol Chem. 2006, 281: 23319-23325.
Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, Wanke E, Brunelli S, Favaro R, Ottolenghi S, Nicolis SK: Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008, 135 (3): 541-557.
Bylund M, Andersson E, Novitch BG, Muhr J: Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003, 6: 1162-1168.
Graham V, Khudyakov J, Ellis P, Pevny L: SOX2 functions to maintain neural progenitor identity. Neuron. 2003, 39: 749-765.
Goldstein RE, Cook O, Dinur T, Pisante A, Karandikar UC, Bidwai A, Paroush Z: An eh1-like motif in odd-skipped mediates recruitment of Groucho and repression in vivo. Mol Cell Biol. 2005, 25: 10711-10720.
Yaklichkin S, Vekker A, Stayrook S, Lewis M, Kessler DS: Prevalence of the EH1 Groucho interaction motif in the metazoan Fox family of transcriptional regulators. BMC Genomics. 2007, 8: 201.
Shih Y, Kuo C, Hirst C, Dee C, Liu Y, Laghari Z, Scotting PJ: SoxB1 transcription factors restrict organizer gene expression by repressing multiple events downstream of Wnt signalling. Development. 2010, 137: 2671-2681.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP: Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003, 4: 249-264.
Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29 (9): e45.
Acknowledgments
We would like to thank Hans Clevers for Grg constructs and Michael Wegner for the 3xSx reporter construct. Y-RL was funded by a University of Nottingham scholarship and ZAL was funded by a Pakistan HEC scholarship (University of Sindh).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Authors declare no competing interests.
Authors’ contributions
Y-RL carried out the bulk of the biochemical and array experiments. ZAL participated in the design and interpretation of the mutants. CAN participated in the studies of NSC differentiation. JH participated in the expression array analysis and in the in vitro pull down assays. JW participated in the in vitro pull down assays. PEG participated in the studies of NSC differentiation. SW participated in the design and interpretation of the in vitro pull down assays. PJS conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Yu-Ru Liu, Zulfiqar A Laghari contributed equally to this work.
Electronic supplementary material
12868_2014_3786_MOESM1_ESM.xlsx
Additional file 1: Table S1: Affymetrix expression data from GFP and Sox2 transfected human NSCs. Data from GeneChip® Human Genome U133 Arrays. Columns G and H are anti-logged values of columns E and F allowing un-logged ratios of expression to be clear, as shown in columns I and J. (XLSX 3 MB)
12868_2014_3786_MOESM2_ESM.txt
Additional file 2: Table S2: List of GO terms of genes that increased in expression when Sox2 was over-expressed in NSCs. N is the total number of genes; B is the total number of genes associated with a specific GO term; n is the flexible cutoff, i.e. the automatically determined number of genes in the ‘target set’ (ie. affected by Sox2 overexpression) and b is the number of genes in the ‘target set’ that are associated with a specific GO term. Enrichment is defined as (b/n)/(B/N). FDR q-value - False Discovery Rate analogue of the p-value. (TXT 775 KB)
12868_2014_3786_MOESM3_ESM.txt
Additional file 3: Table S3: List of GO terms of genes that decreased in expression when Sox2 was over-expressed in NSCs. N is the total number of genes; B is the total number of genes associated with a specific GO term; n is the flexible cutoff, i.e. the automatically determined number of genes in the ‘target set’ (ie. affected by Sox2 overexpression) and b is the number of genes in the ‘target set’ that are associated with a specific GO term. Enrichment is defined as (b/n)/(B/N). FDR q-value - False Discovery Rate analogue of the p-value. (TXT 346 KB)
12868_2014_3786_MOESM4_ESM.tiff
Additional file 4: Figure S1: Nuclear localization of various Sox2 deletion mutants (B) and the Sox2 M203–209 mutant (C) as compared to WT Sox2 (A). Sox2 proteins were overexpressed in COS-7 cells and visualized after 20 h using anti-sox2 antibody (green). Nuclei were counter stained with DAPI (blue). All proteins localized exclusively to the nuclei. Scale bar approximately 10 μm. (TIFF 138 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, YR., Laghari, Z.A., Novoa, C.A. et al. Sox2 acts as a transcriptional repressor in neural stem cells. BMC Neurosci 15, 95 (2014). https://doi.org/10.1186/1471-2202-15-95
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2202-15-95