Abstract
Background
Methotrexate (MTX) commonly used in rheumatoid arthritis (RA) has severe adverse effects. Ortho-vanillin, an inhibitor of Toll-like receptors (TLR), can prevent inflammation. Glucan is a cereal fiber recognized by dectin-1 or β-glucan receptors of phagocytic macrophages. The purpose of the current project was to study the effect of co-administration of MTX and vanillin by targeted delivery to macrophages using β-glucan microspheres to reduce inflammation of RA.
Methods
MTX and vanillin nanoparticles in bovine serum albumin (BSA) or gelatin were doped in glucan particles (GPs) and characterized for their physical properties. Twenty-four hours after induction of RA in paw of rats, they received normal saline (1 mg/kg, ip), MTX (2 mg/kg/week, ip), β-glucan (1 mg/kg/week, ip), GPs-MTX (2 mg/kg/week, ip), GPs-vanillin (200 mg/kg/day, po), and GPs-MTX (2 mg/kg/week, ip) plus GPs-vanillin (200 mg/kg/day, po). The last group received free MTX ip and vanillin po for 14 days. Then, joint diameters, TNF-α and IL-6, were evaluated in rats.
Results
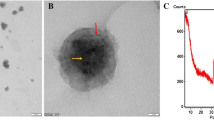
The particle size of the GPs was 5.3 µm. MTX loading efficiency in glucan microspheres was 64.5% and vanillin 44.2%. The microspheres released 88.7% of MTX and 95.1% of vanillin over 24 h. The results of in vivo studies showed a significant reduction in paw volume, TNF-α and IL-6 (p < 0.05) in animals treated with combination of MTX and vanillin-doped glucan microspheres compared to the mixture of the two drugs in free form or each drug alone.
Conclusions
Co-administration of MTX and vanillin-doped GPs may be more effective than MTX alone in RA.
Graphic abstract







Similar content being viewed by others
References
Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–99.
Rajagopalan PR, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci. 2002;99(21):13481–6.
Wessels J, Huizinga T, Guchelaar H-J. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology. 2008;47(3):249–55.
Brody M, Böhm I, Bauer R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1β to the interleukin 1 receptor on target cells. Clin Chem Lab Med. 1993;31(10):667–74.
Weisman MH, Furst DE, Park GS, Kremer JM, Smith KM, Wallace DJ, et al. Risk genotypes in folate-dependent enzymes and their association with methotrexate-related side effects in rheumatoid arthritis. Arthritis Rheum. 2006;54(2):607–12.
To H, Yoshimatsu H, Tomonari M, Ida H, Tsurumoto T, Tsuji Y, et al. Methotrexate chronotherapy is effective against rheumatoid arthritis. Chronobiol Int. 2011;28(3):267–74.
Cronstein BN, Naime D, Ostad E. The anti-inflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92(6):2675.
Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21(9):895–7.
Barich LL, Schwarz J, Barich D. Oral methotrexate in mice: a co-carcinogenic as well as an anti-tumor agent to methylcholanthrene-induced cutaneous tumors. J Invest Dermatol. 1962;39(6):615–20.
Yang L, Zhang J, Wang G. The effect of sodium hyaluronate treating knee osteoarthritis on synovial fluid interleukin-1β and clinical treatment mechanism. Pak J Pharm Sci. 2015;28(1):407–10.
Baici A, Lang A, Hörler D, Kissling R, Merlin C. Cathepsin B in osteoarthritis: cytochemical and histochemical analysis of human femoral head cartilage. Ann Rheum Dis. 1995;54(4):289–97.
Feng Z, Lian K. Identification of genes and pathways associated with osteoarthritis by bioinformatics analyses. Eur Rev Med Pharmacol Sci. 2015;19(5):736–44.
Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–12.
Andreakos E, Sacre S, Foxwell BM, Feldmann M. The toll-like receptor-nuclear factor _B pathway in rheumatoid arthritis. Front Biosci. 2005;10:2478–88.
Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233:90–6.
Seibl R, Kyburz D, Lauener RP, Gay S. Pattern recognition receptors and their involvement in the pathogenesis of arthritis. Curr Opin Rheumatol. 2004;16:411–8.
Feldmann M, Maini RN. Anti-TNF therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96.
Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14.
Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–7.
De Rycke L, Vandooren B, Kruithof E, De Keyser F, Veys EM, Baeten D. Tumor necrosis factor blockade treatment downmodulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005;52:2146–58.
Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor 2 on CD16_ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–67.
Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of Toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon. Arthritis Rheum. 2004;50:3856–65.
Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, Snyder GA, et al. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci USA. 2015;112(17):5455–60.
Soto ER, Ostroff GR. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug Chem. 2008;19(4):840–8.
Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173(2):797–806.
Huang H, Ostroff GR, Lee CK, Wang JP, Specht CA, Levitz SM. Distinct patterns of dendritic cell cytokine release stimulated by fungal beta-glucans and Toll-like receptor agonists. Infect Immun. 2009;77(5):1774–811.
Herre J, Gordon S, Brown GD. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40(12):869–76.
Figueiredo S, Moreira J, Geraldes CG, Rizzitelli S, Aimeb S, Terreno E. Yeast cell wall particles: a promising class of nature-inspired microcarriers for multimodal imaging. Chem Commun. 2011;47(38):10635–7.
Soto ER, Kim YS, Lee J, Kornfeld H, Ostroff GR. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers. 2010;2(4):681–9.
Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, et al. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol. 2012;189(1):312–7.
Smet RD, Demoor T, Verschuere S, Dullaers M, Ostroff GR, Leclercq G, et al. β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J Control Release. 2013;172(3):671–8.
Yu M, Chen Z, Guo W, Wang J, Feng Y, Kong X, et al. Specifically targeted delivery of protein to phagocytic macrophages. Int J Nanomedicine. 2015;10:1743–57.
Leo E, Vandelli MA, Cameroni R, Forni F. Doxorubicin-loaded gelatin nanoparticles stabilized by glutaraldehyde: involvement of the drug in the cross-linking process. Int J Pharm. 1997;155(1):75–82.
Willmott N, Cummings J, Florence AT. In vitro release of adriamycin from drug-loaded albumin and haemoglobin microspheres. J Microencapsul. 1985;2:293–304.
Yu Z, Yu M, Zhang Z, Hong G, **ong Q. Bovine serum albumin nanoparticles as controlled release carrier for local drug delivery to the inner ear. Nanoscale Res Lett. 2014;9(1):343.
Zhao D, Zhao X, Zu Y, Li J, Zhang Y, Jiang R, et al. Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int J Nanomedicine. 2010;5:669–77.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasr, S., Varshosaz, J. & Hajhashemi, V. Ortho-vanillin nanoparticle-doped glucan microspheres exacerbate the anti-arthritic effects of methotrexate in adjuvant-induced arthritis in rats. Pharmacol. Rep 72, 680–691 (2020). https://doi.org/10.1007/s43440-020-00099-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00099-x