Abstract
Develo** and applying a novel plant growth–promoting agent (PGPA; a micronutrient-amino acid chelated compound developed from autolysis yeast cells) in alleviating salt stress toxicity can be the best alternative option environmentally and economically. High-performance liquid chromatography (HPLC) showed that the assembled PGPA is rich in nucleobases than yeast extract (> 56-fold). This study, as a first investigation, was aimed to evaluate PGPA’s potential role in reducing oxidative injury induced by salt stress. Barley (Hordeum vulgare L. cv. Giza 123) plants were grown under non-saline or saline conditions (6.0 and 12.0 dS m−1) with and without PGPA foliar application. The PGPA application mitigated salt-induced oxidative stress by enhancing the activity of superoxide dismutase, catalase, guaiacol peroxidase, ascorbate peroxidase, monodehydroascorbate reductase, dehydroascorbate reductase, glutathione reductase, glutathione peroxidase, and glutathione S-transferase, as well as the content of ascorbate, glutathione, proline, and glycinebetaine. Moreover, PGPA protected salt-stressed plants from the deleterious effects of methylglyoxal by up-regulating the glyoxalase enzymes activity. The PGPA alleviated membrane damage by decreasing reactive oxygen species (ROS) accumulation, lipid peroxidation, protein oxidation, electrolyte leakage, and NADP+ content. The protection of photosynthesis by PGPA was closely associated with the improved chlorophyll fluorescence parameters, leaf water content, membrane stability index, and NADPH content. The PGPA-treated plants also exhibited higher stomatal conductivity together with improved transpiration and photosynthetic rates under saline conditions. Overall, PGPA regulated the antioxidant machinery, glyoxalase system, and photosynthetic capacity, implying that it plays a critical role in salt stress mitigation. Therefore, it could be a useful agent to alleviate the harmful effects of salinity stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Salt stress is a major yield-limiting factor, as it covers 33% of the irrigated agricultural lands worldwide (Kromdijk and Long 2016). It is a complicated abiotic stress that causes osmotic stress, specific ionic impacts, and nutritional imbalances. Salinity decreases soil osmotic potentials and hinders water uptake by roots, resulting in a physiological drought in the plant (Talaat 2015; Rady et al. 2019). Photosynthesis, the most critical physico-chemical mechanism in higher plants, is extremely vulnerable to salt stress (Talaat 2019a). Salinity-induced suppression of photosynthetic electron transport causes overproduction of harmful reactive oxygen species (ROS). Excessive accumulation of ROS accelerates chlorophyll breakdown and inhibits photochemical efficiency of photosystem II (Talaat 2021). Furthermore, ROS, as a strong oxidant, can react with lipids, proteins, and nucleic acids, causing lipid peroxidation, protein denaturation, and DNA mutation, respectively (Kohli et al. 2019). Plants possess integrated machinery for ROS detoxification, which can be categorized into antioxidant enzymes (superoxide dismutase (SOD), guaiacol peroxidase (GPOX), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione S-transferase (GST), monodehydroascorbate reductase (MDHAR), dehydroascorbate (DHAR)) and non-enzymatic antioxidants (α-tocopherol, carotenoids, ascorbic acid (AsA), reduced glutathione (GSH), flavonoids, total phenols) (Fan et al. 2016; Todorova et al. 2016). Another adverse effect of salt stress on plants is the accumulation of methylglyoxal (MG) which is very reactive cytotoxic compound (Nisarga et al. 2017; Talaat and Todorova 2022). To eliminate excessive MG, a GSH-dependent glyoxalase system, including glyoxalase I (Gly I) and glyoxalase II (Gly II) has evolved in plant cells (Kamran et al. 2018; Zaheer et al. 2020; Ahmad et al. 2020). Furthermore, under stressful conditions, foliar spray of aminochelate was superior to its soil application (Souri and Aslani 2018; Souri and Hatamian 2019).
PGPA is also enriched with nucleotides. In fact, nucleotides are one of the most important class of metabolites because their complete oxidation releases carbon and nitrogen, which can be recycled into central metabolism (Casartelli et al. 2018). Moreover, nucleotides are building blocks for nucleic acid synthesis, an energy source, as well as precursors of alkaloids, sucrose, polysaccharides, phospholipids, coenzymes, phytohormones, and secondary products (Stasolla et al. 2003). Evidence indicate that adenosine can manufacture large amounts of ATP to aid the removal and/or transport of salts into vacuoles and to synthesis the compatible solutes that modify the plant osmotic potential (Stasolla et al. 2003). A previous study by Casartelli et al. (2018) identified purines as a drought responsive metabolite that accumulates in drought-tolerant rice varieties. However, there is no enough information currently available about nucleobases ability to be applied as a stress-ameliorative agent in plants.
Barley (Hordeum vulgare L.) is one of the world’s most extensively cultivated crops. Soil salinization is becoming an important factor affecting agricultural sustainability, thereby overcoming its injuries impact on crop production is a major challenge worldwide (Talaat 2019b). The use of natural and safety biostimulants to induce adaptability is the most innovative strategy that protects plants from saline conditions. Therefore, the aim of this investigation was to develop and disclose the influence of formulated PGPA on barley plants grown in salty soils. Specifically, our novel purpose was to evaluate the potential role played by PGPA on reducing oxidative injury induced by salt stress. To address this aim, we investigated the presence of important chemical compounds using HPLC and gas chromatography-mass spectrometry (GC–MS) in the developed PGPA. We also investigated the influence of PGPA on the antioxidant machinery, including activity of antioxidant enzymes (SOD, CAT, GPOX, APX, DHAR, MDHAR, GR, GPX, GST), content of antioxidant molecules (AsA, GSH, proline, glycinebetaine), ROS accumulation, lipid peroxidation, protein oxidation, electrolyte leakage, membrane stability index, relative water content, as well as nicotinamide adenine dinucleotide phosphate oxidized (NADP+) and nicotinamide adenine dinucleotide phosphate reduced (NADPH) content of barley plants grown under both non-saline and saline conditions. Furthermore, the PGPA effect on the glyoxalase defense system, including activity of (Gly I and II) and content of MG, was also detected in stressed and non-stressed plants. The response of photosynthetic activity including the gas exchange parameters and chlorophyll fluorescence system was also evaluated. Indeed, no study regarding mitigation of salinity stress by the use of PGPA has been reported. Accordingly, as a first investigation, it has been hypothesized that the application of this developed PGPA can help plants to overcome the deleterious effects of salt stress on plant growth and production by altering ROS level, antioxidant machinery, methylglyoxal detoxification system, and photosynthetic capacity. Correlation between PGPA application as well as antioxidant, glyoxalase defense, and photosynthetic machineries could provide a novel insight that can help to promote plant production under stressful conditions.
2 Materials and Methods
2.1 Preparation of the PGPA Agent
For PGPA preparation, 200 g l−1 bakery yeast (Saccharomyces cerevisiae) was dissolved in deionized water and left to autolysis for 12 h at room temperature. To inhibit enzymes from further digestion and acquire a large amount of useful compounds and nucleobases, the suspension was heated at 100 °C for 10 min before being quickly cooled on ice. Unbroken cells, partially disrupted cells, and cell debris were removed by centrifugation at 4 °C. Ascorbic acid (250 ppm l−1), citric acid (900 ppm l−1), and salicylic acid (100 ppm l−1) were all added to the suspension to make the PGPA. One millimolar of phosphoric acid and zinc chloride was added to this mixture after 12 h of room temperature incubation. The Egyptian patent office (no. 1232/2020) received the detailed method for the preparing of the PGPA. Yeast extract was prepared as a control as described by Zarei et al. (2016).
2.2 Chemical Analysis of the PGPA Agent
The chemical composition of the PGPA was determined by GC–MS using Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS-fused silica capillary column with electron ionization mass detector (Aktar et al. 2009). High-performance liquid chromatography using Agilent HPLC 1100 system equipped with an auto sampler, diode array detector (DAD), and ChemStation software was used to separate and quantify the nucleobases in the PGPA suspension (Huang et al. 2012).
2.3 Plant Material and Experimental Design
A pot experiment was conducted at the greenhouse of Plant Physiology Department, Faculty of Agriculture, Cairo University, Egypt, under natural light and temperature conditions with an average day/night temperature of 25/15 ± 2 °C and average humidity of 65%. The experiment was repeated twice, in November 30 of 2019 and 2020. Barley (Hordeum vulgare L. cv. Giza 123) seeds were kindly supplied by the Agriculture Research Center, Egyptian Ministry of Agriculture. This cultivar was selected based on its high yield productivity. Its salt tolerance was tried to increase by using PGPA foliar application. The pots were 30 cm in diameter and 35 cm in height and contained 15 kg of clay loamy soil (sand 37%, silt 28%, clay 35%). Table S1 shows the soil chemical analysis, which performed according to Cottenie et al. (1982).
Before sowing, pots were divided into three groups. The first one assigned as control (non-saline; 0.1 dS m−1) and the other two groups were assigned as two levels of salinity treatment (6.0 and 12.0 dS m−1 salinity level; which obtained by adding to the soil a mixture of NaCl, CaCl2, and MgSO4 at the molar ratio of 2:2:1, respectively).
At each salinity treatments, barley plants at 45 (stem elongation stage), 60 (booting stage), and 90 (heading stage) days from sowing were subjected to two PGPA’ treatments: treated with PGPA and untreated plants. Untreated plants were sprayed with distilled water. Tween-20 (0.05%) was added as surfactant at the time of treatment. All the plants were watered regularly, and different intercultural operations were conducted when needed. Recommended fertilization and management practices were followed.
The experimental layout was completely randomized design with two factors: three levels of salinity and two spraying treatments. The plants were sampled after 75 days of sowing to assess the shoot height, leaves number, total leaf area (using a portable leaf area meter (LI-COR 3000, Lambda Instruments Corporation, Lincoln, Nebraska, USA), and shoot dry weight. After maturation, number of grains and grain yield were estimated. Data were collected from four replicates, and each replicate includes six plants gathered from the same pot.
The following physiological and biochemical traits were determined in 75-day-old (after 15 days of PGPA second application) barley leaves. Data were collected from four replicates, each of which contained six plants gathered from the same pot.
2.4 Gas Exchange Measurement
The gas exchange of attached leaves was measured at 8:30–11:30 am using an infrared gas analyzer, Li-Cor-6400 (Li-Cor Inc., Lincoln, NE, USA). The photosynthetic photon flux density (PPFD) was set at 1000 µmol m−2 s−1. Air temperature, air relative humidity, and CO2 concentration were set at ambient conditions in the greenhouse. Net photosynthetic rate (Pn, μmol CO2 m−2 s−1), stomatal conductance (Gs, mol H2O m−2 s−1), and transpiration rate (Tr, mmol H2O m−2 s−1) were recorded simultaneously.
2.5 Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence parameters were measured in leaves with a Portable Chlorophyll Fluorometer (PAM2500; Heinz Walz, Effeltrich, Germany) after a 30-min dark adaptation. Chlorophyll fluorescence in dark- and light-adapted leaves was excited and measured. The maximum quantum efficiency of PSII photochemistry (Fv/Fm), electron transport rate (ETR), actual photochemical efficiency of PSII (ΦPSII), photochemical quenching coefficient (qP), effective quantum yield of PSII photochemistry (Fv′/Fm'), and non-photochemical quenching coefficients (qN) were calculated according to Pfündel et al. (2008).
2.6 Estimation of Stress Markers
Fresh barley leaves of 0.1 g were ground in a mortar with 900 µL buffer to estimate hydrogen peroxide (H2O2) and malondialdehyde (MDA), following the instructions in H2O2 and MDA kits, according to the procedure described by Nawaz et al. (2018). The contents of H2O2 and MDA were measured at wavelengths of 405 and 532 nm, respectively. For measuring superoxide radical (O2• −), fresh barley leaves of 0.1 g were homogenized in 900 µL buffer and centrifuged at 4000 × g for 10 min. The O2•− content was measured according to the instructions in O2•− kits, as described by Gao et al. (2019). At a wavelength of 550 nm, the O2•− content was measured. Oxidative damage to proteins was estimated as the content of carbonyl groups (Reznick and Packer 1994). At 370 nm, the carbonyl content was measured. The protein content was evaluated according to the method of Bradford (1976). The NADP+ and NADPH contents in barley leaves were measured according to the instructions of the kit using a spectrophotometric assay (Liu et al. 2019).
2.7 Extraction and Assay of Antioxidant Enzymes
Barley fresh leaves (0.5 g) were homogenized in 5 mL of ice-cold 100 mM phosphate buffer (pH 7.4) containing 1% polyvinyl pyrrolidine and 1 mM EDTA and then centrifuged at 15,000 × g for 10 min at 25 °C. The supernatant was collected and used in the enzymatic analyses. According to the method of Rao and Sresty (2000), the SOD activity was determined by monitoring its inhibition of the photochemical reduction of nitroblue tetrazolium. One unit of enzyme activity was defined as the amount of enzyme bringing about a 50% inhibition in the reduction rate of nitroblue tetrazolium detected at 560 nm. The CAT activity was determined by monitoring the decrease in absorbance at 240 nm due to decomposition of H2O2 (Cakmak and Marschner 1992). According to the method of Flocco and Giulietti (2007), the GPOX activity was measured as oxidation of guaiacol in the presence of hydrogen peroxide. The kinetic evolution of absorbance at 470 nm was measured during 1 min. One unit of peroxidase was defined as the amount of enzyme that caused the formation of 1 mM of tetraguaiacol per min. The APX activity was measured by monitoring the decrease in absorbance at 290 nm due to ascorbate oxidation according to Ramel et al. (2009). The MDHAR activity was measured by examining NADH oxidation at 340 nm (Hossain et al. 1984). The DHAR activity was measured by examining ascorbate formation at 265 nm (Doulis et al. 1997). The GR activity was assayed by detecting the oxidation of NADPH at 340 nm (Zhu et al. 2007). The activities of GPX and GST were evaluated according to (Nagalakshmi and Prasad 2001). 1-Chloro-2,4-dinitrobenzene was used to start the reaction, and the absorbance change at 340 nm was measured to calculate GPX and GST activity.
2.8 Estimation of the Contents of Reduced (AsA) and Oxidized (DHA) Ascorbate as well as Reduced (GSH) and Oxidized (GSSG) Glutathione
Samples (0.5 g) were homogenized with 5 ml 5% (w/v) sulfosalicylic acid at 4 °C. The homogenate was centrifuged at 4 °C for 20 min at 20,000 × g, and the supernatant was stored. The 5,5-dithiobis-(2-nitrobenzoic acid)-GR recycling procedure was used to determine GSH and GSSG. The increase in absorbance of the reaction mixtures was measured at 412 nm Fe3+ can be reduced to Fe2+ by anticyclic acid. Fe2+ and dipyridine form a colored complex, which has a maximum absorption value at 525 nm. The DHA was measured according to this method after being reduced to reduced ascorbic acid by adding dithiothreitol (Hernandez et al. 2010).
2.9 Determination of MG Content and Its Relevant Enzymes
The content of MG was measured at 288 nm by observing the utilization of N-α-acetyl-S-(1-hydroxy-2-oxo-prop-1-yl) cysteine following the method of Yadav et al. (2005). The activities of Gly I and Gly II were estimated as described previously by Li et al. (2018). The absorbance of the assay mixture was measured at 240 nm and 412 nm for Gly I and Gly II, respectively.
2.10 Determination of Proline and GB
Proline was determined in fresh leaf samples according to Bates et al. (1973), and the absorbance value was done at 520 nm. Glycinebetaine (GB) was determined in dried ground leaves by the method of Grieve and Grattan (1983), and the absorption value was determined at 365 nm.
2.11 Estimation of EL, MSI, and RWC
The electrolyte leakage (EL) was measured by an electrical conductivity meter as described by Li et al. (2015). The membrane stability index (MSI) was estimated following the method of Sairam et al. (2002). The procedure described by Hayat et al. (2007) was followed to appraise the relative water content (RWC).
2.12 Statistical Analysis
Four replicates per treatment were employed in a completely randomized design. Because the findings of the two growing seasons followed a similar pattern, a combined analysis was performed. The two-way ANOVA test was used to statistically assess all measured parameters, where the first factor was the salt treatments, and the second was the foliar application treatments. The least significant difference (LSD) test at a level of significance p < 0.05 was used to determine whether there were any differences between the treatments. The data are presented as means ± standard error (SE). The SAS software (SAS Inc., Cary, NC) was used for the statistical analysis.
3 Results
3.1 PGPA Chemical Composition
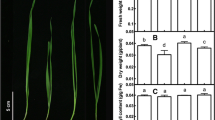
The presence of bioactive ingredients that have essential roles in enhancing tolerance against stressful conditions can be seen in the GC–MS results (Table 1). Moreover, the five nucleobases (adenine, thymine, uracil, guanine, and cytosine) in nucleic acids identified using HPLC–DAD demonstrated that formed PGPA is rich in nucleobases (> 56 fold) than yeast extract (Fig. 1).
3.2 PGPA Treatment Alleviates the Growth and Productivity Reduction Induced by Salt Stress
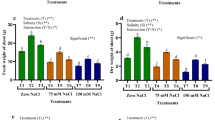
Saline conditions (6.0 and 12.0 dS m−1) drastically reduced the shoot height, leaves number, leaf area, shoot dry weight, grains per plant, and grain yield. On the contrary, PGPA treatment significantly (p < 0.05) alleviated the salt toxicity and attenuated the inhibitory impact of salt on these parameters (Table 2). It significantly improved the shoot height (57.4%), leaves number (62.2%), leaf area (84.4%), shoot dry weight (92.9%), grains per plant (69.1%), and grain yield (88.6%) in barley plants subjected to 12.0 dS m−1 salinity level, compared with untreated plants. Moreover, PGPA treatment not only ameliorated the deleterious effects of saline conditions on plant growth and productivity but also considerably improved the growth and yield related traits under non-saline condition, when compared with untreated plants.
3.3 PGPA Treatment Improves Gas Exchange Traits in Salt-Stressed Plants
Gas exchange parameters were considerably affected under salt stress (Table 3). On the contrary, PGPA application significantly increased Pn, Gs, and Tr by 65.7%, 83.3%, and 54.8%, respectively, in barley plants subjected to 12.0 dS m−1 salinity level, compared with untreated plants.
3.4 PGPA Treatment Meliorates Chlorophyll Fluorescence Attributes Under Salt Stress
Plants grown under saline conditions exhibited reduced Fv/Fm, ΦPSII, Fv′/Fm', ETR, and qP compared to values of control plants. However, PGPA application to salt-stressed plants proved effective in improving these attributes (Table 3). PGPA treatment to barley plants subjected to 12.0 dS m−1 salinity level improved Fv/Fm, ΦPSII, Fv′/Fm', ETR, and qP by 84.6%, 136.0%, 87.2%, 90.2%, and 114.3%, compared to values of control plants. In contrast, qN increased under salt stress and decreased by PGPA. It was significantly (p < 0.05) decreased in treated plants by 30.4%, at 12.0 dS m−1 salinity levels, respectively, when compared with untreated plants.
3.5 PGPA Treatment Relives Salt-Induced Stress by Controlling the Over-accumulation of ROS
To investigate if PGPA alleviates salt stress–induced oxidative stress, the generation of H2O2 and O2•− in barley leaves was detected. Salt stress caused a considerable increase in their concentrations. Conversely, PGPA treatment significantly (p < 0.05) mitigated this adverse effect and restore their production to a similar level as the unstressed plants (Fig. 2a and b). It neutralized salt-generated toxic effects by reducing H2O2 and O2•− content by 29.4% and 26.7%, respectively, under 12.0 dS m−1 salinity level compared with control treatment.
Influence of novel plant growth–promoting agent (PGPA) on the content of (a) hydrogen peroxide (H2O2) and (b) superoxide (O2• −) in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.6 PGPA Treatment Improves the Antioxidant Enzyme Activity of Barley Under Saline Conditions
To elucidate the mechanism underlying how PGPA alleviates salt stress–induced oxidative damage, the activity of different antioxidant enzymes was assayed. The results showed that while salt treatments increased the activity of SOD, CAT, GPOX, APX, GR, GPX, and GST (Fig. 3a-i ), it decreased the activity of MDHAR and DHAR (Fig. 3e and f). However, PGPA application significantly (p < 0.05) enhanced the activity of SOD, CAT, GPOX, APX, GR, MDHAR, DHAR, GPX, and GST under saline conditions (Fig. 3a-i). PGPA treatment under 6.0 and 12.0 dS m−1 salinity levels led to an increase in the activity of SOD (36.3% and 69.0%), CAT (29.2% and 60.0%), GPOX (56.8% and 86.4%), APX (29.9% and 57.4%), GR (44.3% and 85.7%), MDHAR (53.1% and 135.7%), DHAR (60.9% and 120.0%), GPX (40.9% and 79.6%), and GST (52.8% and 88.1%), respectively, relative to control plants.
Influence of novel plant growth–promoting agent (PGPA) on the activity of (a) superoxide dismutase (SOD), (b) catalase (CAT), (c) guaiacol peroxidase (GPOX), (d) ascorbate peroxidase (APX), (e) monodehydroascorbate reductase (MDHAR), (f) dehydroascorbate reductase (DHAR), (g) glutathione reductase (GR), (h) glutathione peroxidase (GPX), and (i) glutathione S-transferase (GST) in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.7 PGPA Treatment Meliorates AsA–GSH Cycle Under Salt Stress
To explicate how PGPA eliminates the adverse effects of salt stress, the content of non-enzymatic antioxidants was quantified. The GSH and AsA contents showed increases by salinity and/or exogenous application (Fig. 4a and b). Salt stress increased the content of GSSG and DHA (Fig. 4c and d) but decreased the ratio of GSH/GSSG and AsA/DHA (Fig. 4e and f) in barley leaves. PGPA significantly increased the content of GSH and AsA as well as the ratio of GSH/GSSG and AsA/DHA but decreased GSSG and DHA content under saline conditions (Fig. 4a-f). It alleviated the adverse effects of salinity and significantly (p < 0.05) enhanced the ratio of GSH/GSSG by 172.8% and 367.5% and that of AsA/DHA by 214.1% and 532.5% at 6.0 and 12.0 dS m−1 salinity levels, respectively, compared with that in untreated plants.
Influence of novel plant growth–promoting agent (PGPA) on the content of (a) reduced glutathione (GSH), (b) ascorbate (AsA), (c) oxidized glutathione (GSSG), and (d) dehydroascorbate (DHA), as well as the ratios of (e) GSH/GSSG and (f) AsA/DHA in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.8 PGPA Treatment Promotes Proline and GB Production as well as Leaf RWC of Salt-Stressed Plants
Compared to non-saline condition, an increase was detected in the concentration of proline and GB under salt stress conditions. Additionally, PGPA treatment promoted their accumulation in salt-stressed plants (Fig. 5a and b). Foliar application of PGPA under 6.0 and 12.0 dS m−1 salinity levels led to an increase in proline concentration by 65.7% and 96.0% and GB concentration by 72.3% and 108.2%, respectively, relative to control plants.
Influence of novel plant growth–promoting agent (PGPA) on the concentrations of (a) proline (mg g−1 FW) and (b) glycinebetaine (GB; µmol g−1 DW) as well as in (c) the relative water content (%) in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
Furthermore, salinity stress sharply decreased the leaf RWC compared with the control; however, the PGPA treatment alleviated the salt induce stress (Fig. 5c). It significantly (p < 0.05) improved in treated plants by 23.7% and 48.9%, at 6.0 and 12.0 dS m−1 salinity levels, respectively, when compared with untreated plants.
3.9 PGPA Treatment Modulates Methylglyoxal Detoxification System in Salt-Stressed Plants
In view of the effect of salt treatments on glyoxalase machinery, it was postulated that salt stress induced high MG accumulation in barley leaves (Fig. 6a), whereas it considerably reduced the activity of Gly I and Gly II (Fig. 6b and c). Conversely, the application of PGPA significantly restored MG content (Fig. 6a). Moreover, PGPA significantly enhanced Gly I and Gly II activities in leaves of stressed plants compared to that in untreated ones (Fig. 6b and c). It significantly (p < 0.05) reduced the MG content by 43.9% and 56.3%, whereas it was significantly increased the Gly I activity by 43.9% and 69.7%, and Gly II activity by 69.4% and 171.1% compared to values of control plants at 6.0 and 12.0 dS m−1 salinity levels, respectively.
Influence of novel plant growth–promoting agent (PGPA) on the content of (a) methylglyoxal (MG) as well as the activity of (b) glyoxalase I (Gly I) and (c) glyoxalase II (Gly II) in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
3.10 PGPA Treatment Alleviates the Membrane Damage of Plants Under Stressful Conditions
Alteration of cellular membrane was assessed by determining the content of MDA, carbonyl, NADP+, and NADPH as well as the EL and MSI. Salt stress led to membrane lipid peroxidation and protein oxidation (Fig. 7a and b) as well as increased the EL (Fig. 7c). In contrast, PGPA treatment significantly reduced the EL (Fig. 7c) as well as the MDA and carbonyl accumulation (Fig. 7a and b) induced by salt stress. It significantly (p < 0.05) reduced the MDA content by 18.8% and 29.0%, carbonyl content by 12.5% and 26.0%, and EL by 10.7% and 20.0% compared to values of control plants at 6.0 and 12.0 dS m−1 salinity levels, respectively.
Influence of novel plant growth–promoting agent (PGPA) on the (a) lipid peroxidation, (b) protein oxidation, (c) electrolyte leakage (%), (d) membrane stability index (%), (e) nicotinamide adenine dinucleotide phosphate oxidized (NADP+), and (f) nicotinamide adenine dinucleotide phosphate reduced (NADPH) in leaves of barley plants grown under salt stress (6 and 12 dS m−1). Data are mean ± standard error (n = 4). Asterisks represent significant differences between treatments at p < 0.05 level according to LSD test
Salt treatments sharply reduced the value of MSI; however, PGPA foliar treatment was associated with a significant increase in its level (Fig. 7d). MSI was enhanced in treated plants by 24.4% and 45.5%, at 6.0 and 12.0 dS m−1 salinity levels, respectively, when compared with untreated plants.
Salt stress considerably increased NADP+ content, while it decreased NADPH content in barley leaves (Fig. 7e and f). On the contrary, PGPA application reduced the NADP+ content by 16.1% and 30.0% and elevated the NADPH content by 43.8% and 82.9% compared to values of control plants at 6.0 and 12.0 dS m−1 salinity levels, respectively.
4 Discussion
The current study’s novel achievement is the development of an environmentally friendly approach that counteracts the negative effects of salinity on plant development. Salt stress disrupts metabolic balance of cells, inducing overproduction of ROS and MG, and causing oxidative stress that severely affects many plant metabolic processes (Kamran et al. 2018). Stearic acid, oleic acid, and dodecanoic acid are key components in cell membranes, energy sources, precursors of lipid mediators, and signal transducers, thus promoting adaptability against oxidative stress, which together with squalene can regulate biophysical properties and dynamic membrane organization (He and Ding 2020). Another compound dihydroxanthin is a carotenoid and has antioxidant properties (Khan et al. 2019). Citric acid and butanedioic acid confer plant abiotic stress tolerance by improving photosynthetic rates, reducing ROS burst, inducing antioxidant defense systems, and involving in the biosynthesis of phytohormones, amino acids, signaling molecules, and other secondary metabolites (Tahjib-Ul-Arif et al. 2021). In addition, cis-vaccenic acid, cis-9-hexadecenoic acid, n-hexadecanoic acid, and 4-(dimethylamino) benzaldehyde have antioxidant and anti-inflammatory properties (Guerrero et al. 2017; Latif et al. 2019). 2-Myristnoyl pantetheine involved in the synthesis of coenzyme A, which is required for many biochemical processes (Czumaj et al. 2020).
Growth and yield reduction can be used as signs to assess the level of salt-induced injuries in plants (Talaat and Shawky 2012; Acosta-Motos et al. 2017). In the current study, salt stress considerably reduced the plant growth and productivity that may be resulted from inducing oxidative damage in barley plants, as indicated by the overproduction and accumulation of H2O2, O2•−, MDA, carbonyl, and MG. Nevertheless, salt-stressed PGPA-treated plants showed better growth performance and lower cellular injury than those stressed untreated ones. The reason for this is thought to be owing to upregulating the antioxidant defense system, including enzymatic and non-enzymatic activities that scavenged the ROS and kept a proper balance within the cells as well as improving the MG detoxification system to detoxify MG through the activity of Gly I and Gly II. Taken together, abovementioned results mainly suggest that the treatment of plants with PGPA helped to induce reprogramming of some important stress-related pathways, such as antioxidant and glyoxalase defense machineries, thereby facilitating their interconnectivity for a more efficient cellular ROS detoxification through the activation of oxidative metabolism. Furthermore, it is known that aminochelates represent an excellent source of nitrogen and micronutrients for plant, which may impact on plant growth by regulating cell division and expansion, nutritional status, and metabolism (Souri and Aslani 2018; Souri and Hatamian 2019). Other mechanism explains how PGPA facilitates plant growth is through its role on ATP production. Indeed, PGPA may provide the stressed plants with sufficient amount of ATP to counteract the deleterious effects of salt stress, as it contains nucleotides, which play an important role in ATP synthesis (Werner and Witte 2011). Probably for all these reasons, PGPA as a biological technique would represent an efficient adaptation to salt stress.
In the present study, it was found that saline conditions proved toxic effect on photosynthetic activity of barley plants, apparently due to high oxidative stress as shown by higher ROS, MDA, carbonyl, and MG contents. On the contrary, application of PGPA increased photosynthetic efficiency of salt-stressed plants by increasing both the gas exchange traits (Pn, Gs, Tr) and the chlorophyll fluorescence attributes (Fv/Fm, ΦPSII, Fv′/Fm', ETR, qP). However, qN increased under salt stress and decreased by PGPA treatment. Salt stress-induced photo-inhibition and reduced photosynthetic efficiency may be reversed by the PGPA treatment. The present work demonstrated that PGPA treatment caused up-regulation of antioxidant defense and methylglyoxal detoxification systems, which consequently mitigate oxidative damage caused by salinity. This alleviation of oxidative stress helped in protecting photosynthetic activity. These results provide evidence that PGPA can involve in maintaining photosynthetic efficiency of salt-stressed plants by blocking ROS and MG burst.
Additionally, in the chloroplast thylakoid membrane, the function of photosynthetic electron transport is to produce NADPH from NADP+ (Yamori and Shikanai 2016). In the present study, salt stress may disrupt the process of NADPH production since NADP+ content was higher while NADPH content was lower in salt-stressed plants compared to values of non-stressed ones. On the contrary, application of PGPA resulted in an increase in NADPH content while a decrease in NADP+ content, suggesting that this treatment may be able to mitigate the deleterious effects of salt stress on the NADPH generation. It is interesting to note that PGPA treatment may be able to restore the damaged cell membrane, lessen oxidative damage brought by salt, and protect the photosynthetic machinery from salt damage.
Under salt stress conditions, the excessive reduction of electron transfer in chloroplasts and the overall decline of photosynthetic electron transfer lead to the overformation of ROS (Fan et al. 2016; Talaat 2019b). In this study, we found that barley leaves accumulated more ROS (H2O2 and O2•−) by increasing salinity level, whereas PGPA treatment significantly reduced their contents, proving that PGPA can play an important role in regulating ROS accumulation, thus conferring the salt tolerance in treated plant. This lower O2•− accumulation could contribute to the higher SOD activity in leaves of treated plants under saline conditions. A previous study by Gill and Tuteja (2010) reported that SOD is the major O2•− scavenger. Moreover, it is notable that the lower H2O2 level was coordinated with the higher APX, MDHAR, DHAR, and GR activity in these plants. This finding corroborates with that of You and Chan (2015) who postulated that the participation of APX in H2O2 removal requires the AsA–GSH cycle, which includes APX, MDHAR, DHAR, and GR as well as AsA and GSH as enzymatic and non-enzymatic components. The present study suggested that PGPA treatment might exert its roles in enhancing barley salt tolerance probably due to its effect on blocking ROS burst.
Various antioxidative strategies have been developed in plants to cope with the oxidative stress induced by salinity. Previous studies suggested that plant salt tolerance was normally associated with plant antioxidative capacity, namely ROS-scavenging or-detoxification ability (Talaat 2019b; Talaat and Todorova 2022). Our results revealed that when barley plants facing the oxidative injury caused by salt stress, change in the antioxidant enzymes activity was detected. Moreover, this antioxidant strategy was altered by PGPA treatment in salt-stressed plants. In the current study, the activity of SOD, CAT, GPOX, APX, MDHAR, DHAR, GR, GPX, and GST was significantly enhanced in salt-stressed treated plants compared with the control, that contributed partly to reduce the ROS accumulation and manage the oxidative damage. This higher level of antioxidant enzymes activity in salt-stressed treated plants than those in untreated ones could decrease cellular and photo oxidative damage, which is an important reason for the enhanced plant growth.
In addition to enhancing the activities of antioxidant enzymes, PGPA also contributes to AsA–GSH cycle modulation. Both AsA and GSH play a key role in ROS detoxification as well as in osmoregulation, water use efficiency, and photosynthetic activity (Fan et al. 2016). In the present study, when barley plants encounter salt-stressed conditions, AsA–GSH cycle showed variable response. GSSG and DHA contents were increased, whereas the ratios of GSH/GSSG and AsA/DHA were decreased, which could be caused by the overproduction of MG which may induced GSH oxidation and GSSG formation, as was also reported by (Kalapos et al. 1992). In contrast, PGPA treatment significantly meliorated the AsA–GSH cycle under salt stress, suggesting that treated plants could rely on antioxidants to maintain cellular redox homeostasis under saline condition. Our results reveal that PGPA application to stressed plants maintained AsA regeneration that could be not only as a result of increased APX, MDHAR, DHAR, and GR activities, but also due to enhanced proline and GB accumulation. Previous studies have postulated that both proline and GB can involve in AsA regeneration in the presence of MDHAR and DHAR (Fan et al. 2016). Taken together, these results suggest that PGPA treatment resulted in unique antioxidant profile. Proline, GB, AsA, GSH, and antioxidant enzymes are interconnected with a tight coordination to maintain an optimal cellular redox balance to trigger ROS production.
One of the mechanisms whereby PGPA improve plant growth is through an interconnection between leaf water status and osmotic regulators accumulation. In this study, we found that proline and GB concentrations were significantly elevated in stressed treated plants, suggesting a possible role in mitigation the water loss in barley leaves. Thus, PGPA may has the potential to help leaves to maintain a higher water level and lower cellular osmotic potential by the organic solute accumulation. Furthermore, it is postulated that proline and GB not only contribute to the osmotic adjustment under salt stress, but also have the function of quenching ROS (Ashraf and Foolad 2007; Talaat 2021), and therefore, they can contribute in the metabolism of ROS and stabilization of membrane structure. Our results revealed a positive correlation between the production of these organic solutes and the elimination of ROS, that resulted into the reduction of stress-mediated growth inhibition in barley plants.
Salt stress can also induce oxidative stress by MG overproduction (Kamran et al. 2020; Talaat and Todorova 2022). Consistent with this report, our study indicated a significant increase in barley MG content under 6.0 and 12.0 dS m−1 salinity levels. Salinity might reduce the activity of enzymes responsible for eliminating MG (Gly I and Gly II) which ultimately causes by the decrease in GSH content. In contrast, we found that the PGPA spraying significantly reduce the content of oxidative stress marker MG in salt-stressed plants through upregulating the activity of MG detoxification system (Gly I and Gly II) and this may be another mechanism involved in increasing barley salt tolerance. This protective effect of PGPA on stress-induced MG accumulation could be related to its positive influence on GR activity and GSH regeneration. This is in line with Kamran et al. (2020), who postulated that GSH plays a pivotal role in the regulation of glyoxalase enzyme system, which in turn repressed the MG content in plants. Similarly, Mostofa et al. (2015) reported that there was a positive correlation between the enhanced activity of Gly II and GSH level as well as GSH/GSSG ratio.
MDA, carbonyl, and EL are the key indicators of oxidative damage that negatively affect the membrane’s integrity (Talaat 2021; Talaat and Shawky 2022). In the present study, we found that salt stress increased lipid peroxidation, protein oxidation, and EL in barley leaves, indicating aggravated oxidative injury under saline conditions. However, their levels in leaves of stressed treated plants were lower than those in leaves of stressed untreated ones, suggesting a greater ability of treated plants to counteract oxidative stress. Our results clearly reveal that PGPA facilitated ROS scavenging by increasing antioxidant enzyme activity and antioxidant molecules content in barley leaves. These responses, in turn, resulted in the maintenance of membrane integrity and reduced cell membrane lipid peroxidation and protein oxidation under salt stress, as evidenced by an increased MSI in barley leaves. Indeed, PGPA-mediated improvement of antioxidant properties are also in line with the findings of Hammad and Ali (2014) and Abdel Latef et al. (2019), who reported that ROS-caused membrane damage was alleviated by yeast extract. Overall, our results clearly indicate that ROS-caused membrane damage was alleviated by PGPA.
According to the results of this study, we can conclude that PGPA alleviates the salt-induced barley yield inhibition by activating a battery of enzymatic and non-enzymatic detoxification systems, strengthening methylglyoxal detoxification machinery, maintaining a higher water level, enhancing photosynthetic efficiency, and maintaining cellular membrane structure (Fig. 8).
A model showing salt stress induced oxidative stress in barley plant by increasing reactive oxygen species (ROS; hydrogen peroxide (H2O2) and superoxide radical (O2• −)) generation and methylglyoxal (MG) formation. Meanwhile, the novel plant growth–promoting agent (PGPA) reduced salt stress damage to the plant by activating antioxidant defense machinery and strengthening methylglyoxal detoxification system via affecting the activity of superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glyoxalase I (Gly I), and glyoxalase II (Gly II), as well as the content of ascorbate (AsA), dehydroascorbate (DHA), reduced glutathione (GSH), oxidized glutathione (GSSG), and methylglyoxal (MG). The protection effect of PGPA was also closely associated with the improved photosynthetic efficiency, plant water content, and cellular membrane stability
5 Conclusions
Our study suggests that application of plant growth–promoting agent (a micronutrient-amino acid chelated compound developed from autolysis yeast cells) significantly increased the barley salt tolerance by alleviating salt stress–induced oxidative stress through reprogramming antioxidant defense machinery and strengthening methylglyoxal detoxification system, which facilitates the scavenging of excess reactive oxygen species and thus increased stability of the cellular membrane. The plant growth–promoting agent is also involved in enhancing photosynthetic activity and mitigating the water loss in barley leaves that promotes the plant growth and yield under saline conditions. According to the results, it is concluded that plant growth–promoting agent as innovative product can act as an effective, low-cost, and environmentally friendly agent to mitigate salt stress in crops.
References
Abdel Latef AAH, Mostofa MG, Rahman MM, Abdel-Farid IB, Tran LP (2019) Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physio-biochemical characteristics of stressed plants. J Plant Growth Regul 38:966–979. https://doi.org/10.1007/s00344-018-9906-8
Acosta-Motos JR, Ortuno MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. https://doi.org/10.3390/agronomy7010018
Ahmad R, Ishaque W, Khan M, Ashraf U, Riaz MA, Ghulam S, Ahmad A, Rizwan M, Ali S, Alkahtani S, Abdel-Daim MM (2020) Relief role of lysine chelated zinc (Zn) on 6-week-old maize plants under tannery wastewater irrigation stress. Int J Environ Res Public Health 17:5161. https://doi.org/10.3390/ijerph17145161
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid - a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2(1):1–12. https://doi.org/10.2478/v10102-009-0001-7
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bates LS, Waldren RP, Teare LD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 7:248–254. https://doi.org/10.1006/abio.1976.9999
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Casartelli A, Riewe D, Hubberten HM, Altmann T, Hoefgen R, Heuer S (2018) Exploring traditional aus-type rice for metabolites conferring drought tolerance. Rice 11:9. https://doi.org/10.1186/s12284-017-0189-7
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of plants and soils. In: Laboratory of analytical and agrochemistry. State University, Ghent, pp 14–24. https://lib.ugent.be/catalog/rug01:000239299
Czumaj A, Szrok-Jurga S, Hebanowska A, Turyn J, Swierczynski J, Sledzinski T, Stelmanska E (2020) The pathophysiological role of CoA. Int J Mol Sci 21:9057. https://doi.org/10.3390/ijms21239057
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize. Plant Physiol 114:1031–1037. https://doi.org/10.1104/pp.114.3.1031
Fan Y, Bose J, Zhou M, Shabala S (2016) ROS production, scavenging, and signaling under salinity stress. In: Shabir, H.W., Mohammad, A.H. (Eds.), Managing salt tolerance in plants: molecular and genomic perspectives. CRC Press, Boca Raton, Florida, USA, pp. 187–205. https://doi.org/10.1201/b19246.
Fernandez-San Millan A, Farran I, Larraya L, Ancin M, Arregui LM, Veramendi J (2020) Plant growth-promoting traits of yeasts isolated from Spanish vineyards: benefits for seedling development. Microbiol Res 237:126480. https://doi.org/10.1016/j.micres.2020.126480
Flocco CG, Giulietti AM (2007) In vitro hairy root cultures as a tool for phytoremediation research. In: Willey, N. (eds) Phytoremediation. Methods in Biotechnology, vol 23. Humana Press. https://doi.org/10.1007/978-1-59745-098-0_14
Gao W, Feng Z, Bai Q, He J, Wang Y (2019) Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int J Mol Sci 20:1176. https://doi.org/10.3390/ijms20051176
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Guerrero RV, Abarca-Vargas R, Petricevich VL (2017) Chemical compounds and biological activity of an extract from Bougainvillea x buttiana (var rose) Holttum and Standl. Int J Pharm Pharm Sci 9(3):4246. https://doi.org/10.22159/ijpps.2017v9i3.16190
Hammad SAR, Ali OAM (2014) Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extract. Ann Agric Sci 59:133–145. https://doi.org/10.1016/j.aoas.2014.06.018
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41. https://doi.org/10.1016/j.envexpbot.2006.06.002
He M, Ding N-Z (2020) Plant unsaturated fatty acids: multiple roles in stress response. Front Plant Sci 11:562785. https://doi.org/10.3389/fpls.2020.562785
Hernandez M, Fernandez-Garcia N, Diaz-Vivancos P, Olmos E (2010) A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J Exp Bot 61:521–535. https://doi.org/10.1093/jxb/erp321
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395. https://doi.org/10.1093/oxfordjournals.pcp.a076726
Huang Q, Kaiser K, Benner RA (2012) A simple high performance liquid chromatography method for the measurement of nucleobases and the RNA and DNA content of cellular material Limnol Oceanogr. Methods 10:608–616. https://doi.org/10.4319/lom.2012.10.608
Kalapos MP, Garzo T, Antoni F, Mandl J (1992) Accumulation of S-D-lactoylglutathione and transient decrease of glutathione level caused by methylglyoxal load in isolated hepatocytes. Biochim Biophys Acta 1135(2):159–164. https://doi.org/10.1016/0167-4889(92)90132
Kamran M, **e K, Sun J, Wang D, Shi C, Lu Y, Gu W, Xu P (2020) Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L). Ecotoxicol Environ Saf 188:09877. https://doi.org/10.1016/j.ecoenv.2019.109877
Khan S, Richa Kaur H, Jhamta R (2019) Evaluation of antioxidant potential and phytochemical characterization using GC-MS analysis of bioactive compounds of Achillea filipendulina (L) leaves. J Pharmacogn Phytochem 8(3):258–265
Kohli SK, Khanna K, Bhardwaj R, Abd-Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8:641. https://doi.org/10.3390/antiox8120641
Kromdijk J, Long SP (2016) One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proc Biol Sci 283:20152578. https://doi.org/10.1098/rspb.2015.2578
Latif MA, Tofaz T, Chaki BM, Tariqul Islam HM, Saddam Hossain Md, Kudrat-E-Zahan Md (2019) Synthesis, characterization, and biological activity of the Schiff base and its Ni(II), Cu(II), and Zn(II) complexes derived from 4-(dimethylamino) benzaldehyde and S-benzyldithiocarbazate. Russ J Gen Chem 89:1197–1201. https://doi.org/10.1134/S107036321906015X
Li X, Ahammed GJ, Zhang YQ, Zhang GQ, Sun ZH, Zhou J, Zhou YH, **a XJ, Yu JQ, Shi K (2015) Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biol 17:81–89. https://doi.org/10.1111/plb.12211
Li Z-G, Nie Q, Yang C-L, Wang Y, Zhou Z-H (2018) Signaling molecule methylglyoxal ameliorates cadmium injury in wheat (Triticum aestivum L) by a coordinated induction of glutathione pool and glyoxalase system. Ecotoxicol Environ Saf 149:101–107. https://doi.org/10.1016/j.ecoenv.2017.11.021
Liu CL, Dong HG, Zhan J, Liu X, Yang Y (2019) Multi-modular engineering for renewable production of isoprene via mevalonate pathway in Escherichia coli. J Appl Microbiol 126:1128–1139. https://doi.org/10.1111/jam.14204
Lozano-Grande MA, Gorinstein S, Espitia-Rangel E, Dávila-Ortiz G, Martínez-Ayala AL (2018) Plant sources, extraction methods, and uses of squalene. Int J Agron 2018:1829160. https://doi.org/10.1155/2018/1829160
Mostofa MG, Fujita M, Tran LSP (2015) Nitric oxide mediates hydrogen peroxide-and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul 77:265–277. https://doi.org/10.1007/s10725-015-0061-y
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299. https://doi.org/10.1016/s0168-9452(00)00392-7
Nawaz MA, Jiao Y, Chen C, Shireen F, Zheng Z, Imtia M, Bie Z, Huang Y (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon on seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220:115–127. https://doi.org/10.1016/j.jplph.2017.11.003
Nisarga KN, Vemanna RS, Kodekallu Chandrashekar B, Rao H, Vennapusa AR, Narasimaha A, Makarla U, Basavaiah MR (2017) Aldo-ketoreductase 1 (AKR1) improves seed longevity in tobacco and rice by detoxifying reactive cytotoxic compounds generated during ageing. Rice (NY) 10:11. https://doi.org/10.1186/s12284-017-0148-3
Pfündel E, Klughammer C, Schreiber U (2008) Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl Notes 1:21–24
Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky EM (2019) Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J Hortic Sci Biotech 94:777–789. https://doi.org/10.1080/14620316.2019.1626773
Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G (2009) Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol 9:28. https://doi.org/10.1186/1471-2229-9-28
Rao KVM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363. https://doi.org/10.1016/S0076-6879(94)33041-7
Saleem MH, Ali S, Rehman M, Rizwan M, Kamran M, Mohamed IA, Bamagoos AA, Alharby HF, Hakeem KR, Liu L (2020) Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus Capsularis L) seedlings. Environ Technol Innov 19:100895. https://doi.org/10.1016/j.eti.2020.100895
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163(5):1037–1046. https://doi.org/10.1016/S0168-9452(02)00278-9
Souri MK, Hatamian M (2019) Aminochelates in plant nutrition: a review. J Plant Nutr 42:67–78. https://doi.org/10.1080/01904167.2018.1549671
Souri MK, Aslani M (2018) Beneficial effects of foliar application of organic chelate fertilizers on French bean production under field conditions in a calcareous soil. Adv Hort Sci 32(2):265–272. https://doi.org/10.13128/ahs-21988
Stasolla C, Katahira R, Thorpe TA, Ashihara H (2003) Purine and pyrimidine nucleotide metabolism in higher plants. J Plant Physiol 160:1271–1295. https://doi.org/10.1078/0176-1617-01169
Taha RS, Seleiman MF, Alhammad BA, Alkahtani J, Alwahibi MS, Mahdi AHA (2021) Activated yeast extract enhances growth anatomical structure and productivity of Lupinus termis L plants under actual salinity conditions. Agronomy 11:74. https://doi.org/10.3390/agronomy11010074
Talaat NB (2015) Effective microorganisms modify protein and polyamine pools in common bean (Phaseolus vulgaris L.) plants grown under saline conditions. Sci Hortic 190:1–10. https://doi.org/10.1016/j.scienta.2015.04.005
Talaat NB (2019a) Effective Microorganisms: an innovative tool for inducing common bean (Phaseolus vulgaris L.) salt-tolerance by regulating photosynthetic rate and endogenous phytohormones production. Sci Hortic 250:254–265. https://doi.org/10.1016/j.scienta.2019.02.052
Talaat NB (2019b) Role of reactive oxygen species signaling in plant growth and development. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. John Wiley & Sons, Ltd, UK. pp 225–266. https://doi.org/10.1002/9781119468677.ch10
Talaat NB (2021) Co-application of melatonin and salicylic acid counteracts salt stress-induced damage in wheat (Triticum aestivum L.) photosynthetic machinery. J Soil Sci Plant Nutr 21:2893–2906. https://doi.org/10.1007/s42729-021-00576-z
Talaat NB, Shawky BT (2012) Influence of arbuscular mycorrhizae on root colonization, growth and productivity of two wheat cultivars under salt stress. Arch Agron Soil Sci 58:85–100. https://doi.org/10.1080/03650340.2010.506481
Talaat NB, Shawky BT (2022) Synergistic effects of salicylic acid and melatonin on modulating ion homeostasis in salt-stressed wheat (Triticum aestivum L) plants by enhancing root H+-pump activity. Plants 11:416. https://doi.org/10.3390/plants11030416
Talaat NB, Todorova D (2022) Antioxidant machinery and glyoxalase system regulation confers salt stress tolerance to wheat (Triticum aestivum L) plants treated with melatonin and salicylic acid. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00907-8
Tahjib-Ul-Arif M, Zahan MI, Karim MM, Imran S, Hunter CT, Islam MS, Mia MA, Hannan MA, Rhaman MS, Hossain MA, Brestic M, Skalicky M, Murata Y (2021) Citric acid-mediated abiotic stress tolerance in plants. Int J Mol Sci 22:7235. https://doi.org/10.3390/ijms22137235
Todorova D, Talaat, NB, Katerova Z, Alexieva V, Shawky BT (2016) Polyamines and brassinosteroids in drought stress responses and tolerance in plants. In: Water stress and crop plants: a sustainable approach, Volume 2. (ed. P. Ahmad)., John Wiley & Sons, Ltd, UK. pp. 608–627. https://doi.org/10.1002/9781119054450.ch35
Werner AK, Witte CP (2011) The biochemistry of nitrogen mobilization: purine ring catabolism. Trends Plant Sci 16:381–387
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337:61–67. https://doi.org/10.1016/j.bbrc.2005.08.263
Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67:81–106. https://doi.org/10.1146/annurev-arplant-043015-112002
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
Zaheer IE, Ali S, Saleem MH, Ali M, Riaz M, Javed S, Sehar A, Abbas Z, Rizwan M, El-Sheikh MA, Alyemeni MN (2020) Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol Mol Biol Plants 26:2435–2452. https://doi.org/10.1007/s12298-020-00912-0
Zarei O, Dastmalchi S, Hamzeh-Mivehroud MA (2016) A simple and rapid protocol for producing yeast extract from Saccharomyces cerevisiae suitable for preparing bacterial culture media. Iran J Pharm Res 15:907–913
Zhu H, Cao Z, Zhang L, Trush MA, Li Y (2007) Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells: chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury. Mol Cell Biochem 301:47. https://doi.org/10.1007/s11010-006-9396-z
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
NBT conceptualized and coordinated the research, conceived the idea, designed and carried out the experiments, generated and analyzed the data, and wrote the manuscript. AAM and SNAE performed, studied, and designed PGPA agent. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Talaat, N.B., Mostafa, A.A. & El-Rahman, S.N.A. A Novel Plant Growth–Promoting Agent Mitigates Salt Toxicity in Barley (Hordeum vulgare L.) by Activating Photosynthetic, Antioxidant Defense, and Methylglyoxal Detoxification Machineries. J Soil Sci Plant Nutr 23, 308–324 (2023). https://doi.org/10.1007/s42729-022-00993-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00993-8