Abstract
We proposed a facile strategy to synthesize porous zinc-doped copper sulfide (Zn–CuS) nanoflowers using a cationic surfactant (CTAB) by hydrothermal route. The end product is used as electrode material in supercapacitor in KOH as electrolyte. Initially it is found that inorganic dopant (Zn2+) plays a vital role in controlling or modifying the morphology of CuS which has resulted in the newer materials exhibiting multi-functional, high-performance stabilizer character further than what traditional single nanomaterials possess. Morphological analysis illustrates flower-like structures that the size of Zn–CuS nanostructure is about 14–20 nm. When utilized as an electroactive material for electrochemical capacitors, the Zn–CuS electrode delivers a specific capacitance (Cs) 826.31 Fg−1 at 5mAcm−2, which is higher than the CuS electrode 328.26 Fg−1 at 5mAcm−2. The outstanding electrochemical behavior is essentially by reason of the porous nature of synthesized nanostructures, which gives quick diffusion pathway to electronic and ionic transport on electrode and electrolyte interfaces. This exploration work affirms that the Zn–CuS electrode can serve as more attractive material for supercapacitive applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the expanding utilization of electronic devices and vehicles, explore research have been made to investigate superior storage frameworks. Among these charge storage systems, SCs have been shown to be most favorable power sources attributable to their outstandingly high energy density, small charge and discharge times, and ultra-long cycle life [1,2,3,4,5]. Be that as it may, the energy density of commercially used SCs is about 5 wh kg−1, which is lower than other energy storage devices like Li-ion batteries [6]. The charge in SCs is stored by the electro active materials. So it is attractive and vital to design superior electrode materials so as to advance the energy density of SCs.
Carbon-based materials, transition metal oxides/hydroxide, and conducting polymers are among the most seriously investigated SC’s electode materials. Carbon materials show low capacitance because of their surface predominant EDLCs mechanism [7,8,9]. Transition metal oxides show higher capacitance inferable from their redox-response improved energy storage system. Be that as it may, these materials have poor electrochemical strength, a reality that has, to a great extent constrained their far reaching applications in SCs. Therefore, it is vital to grow new electrode materials with attractive supercpacitor properties, such as high electrical conductivity, porous structure, substantial capacitors, and great electrochemical stability.
Recently sulfide materials have been examined as novel SC’s electrode materials with enhanced electrochemical performance. The metal sulfide nanostructures such as ZnS, CuS, and CoS were utilized as electrode materials for energy storage applications [10,11,12]. The substitution of two or more valance states of metal constituents present in sulfides and the sulfur have high theoretical capacity than that of oxides and it can provide better capacitance behavior.
The covellite copper sulfide is exhaustively studied material attributable to great potential applications in several fields. Recently unary transition metal sulfides viz., copper cobalt sulfides [13] and iron copper sulfides [14] have been examined as new electrode materials for supercapacitor. Huang et al. synthesized CuS hexagonal nanosheets by solvothermal route and based on these CuS electrode showed a Cs of 833 Fg−1 under a high current density of 1 Ag−1 [15]. Peng et al. synthesized flower-like CuS for SCs with high Cs of 597 Fg−1 at a current density of Ag−1 [16]. Zhu et al. reported CuS nanoneedles on carbon nanotubes by a multiple step and template method, and supercapacitors via this composite demonstrated a capacitance of 114 Fg−1 at a scan rate of 2 mv/s in the three-electrode tests [17].
It was reported that the binary sulfides such as Zn–CuS have an electronic conductivity of about 2 orders higher than that of the oxide partners and a lot higher conductivity than that of the binary sulfides [18,19,20]. Also, the electrochemical commitments from both Zn and Cu ions in the binary sulfides are relied upon to give more extravagant redox responses than that of the single ternary sulfides. Pu et al. built up Zn–CuS hallow nanostructure that was sulfurized from a Zn–Cu precursor by a two-step sacrificial template method. This Zn–CuS hallow structure manifested a high Cs of 437 Fg−1, when tried as a SC’s material at a current density of 1 Ag−1 [21].
In this work, a novel mesoporous flower-like Zn–CuS and CuS nanostructure was synthesized via hydrothermal treatment using organic cationic surfactant (CTAB) as structure promoting agent. The first important point is no researcher uses flower-like mesoporous nanostructure as the electrode material to attain high Cs compared to spherical CuS nanostructure. The formation mechanism of CuS and Zn–CuS was elaborately discussed. The electrochemical behavior of the CuS and Zn–CuS was investigated as electrode material for supercapacitors in 2 M KOH electrolyte solution. As estimated, the conventional composites bear the cost of generous upgrade and prompting to enough redox reactions. Such special highlights enable the fusion electrode to display considerably enhanced energy storage ability when compared CuS and earlier reports. The results obtained from this nanocomposite demonstrated promising execution of hybrid structure of mesoporous binary metal sulfide for future energy storage applications.
The synthesis of Zn–CuS has the following aims:
-
1.
The high electrical conductivity of Zn–CuS will assist to overcome the limited electrical conductivity.
-
2.
The Zn–CuS will relax ion transfer for the reason that the short ion diffusion paths and give reversible, quick faradic reactions.
-
3.
The Zn–CuS will improve the utilization rates of electrode materials due to the porous nature.
The outcomes in this investigation demonstrate that the fabricated Zn–CuS show high specific capacitance, and long cycle life, and are potential electrode materials for SCs.
2 Experimental sections
2.1 Synthesis of CuS and Zn–CuS nanostructures
In a typical procedure, 1 mM of Cu (NO3)0.3H2O, 2 mM of Tu,Sc(NH2)2 and 0.1 mM of CTAB were dissolved in 40 ml of ethylene glycol and magnetic stirring for 1 h. The solution into a Teflon-lined autoclave is maintained at 140 °C for 9 h. The prepared black powder was washed many times and dried at 70 °C for 6 h. The three different concentrations of Zn–CuS were synthesized under same conditions with the addition of Zn (NO3)0.3H2O.
2.2 Materials characterization
The diffraction patterns were recorded on X’Pert-PRO using Cu Kα radiation. X-ray diffractometer was at a scan rate of 1° min−1 over the range 10°–70° (2θ) at room temperature. The optical properties were determined by UV–Vis spectroscopy (Shimadzu UV1700). The change in morphology on increasing the concentration of Zn-ion-doped CuS was inspected through a (JEOL-JSM – 5610 LV with INCA EDS) SEM and the elemental analysis was studied using EDX, and the morphology was confirmed with (SUPRA 55) field emission scanning electron microscope (FESEM). The TEM morphologies were analyzed on a TEM CM-200. Chemical compositions were investigated by XPS (Kratos Analytical). The BET parameters of the Zn–CuS surface area were measured in Micrometrics ASAP-2010 system using the liquid nitrogen adsorption–desorption method.
2.3 Electrochemical measurements
In our previous paper, the electrode preparation and electrochemical characterization techniques of prepared samples were discussed [22].
3 Results and discussion
3.1 XRD
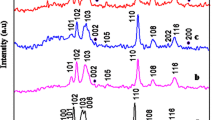
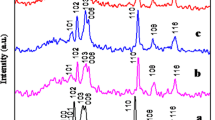
The crystal phase, purity and crystallographic structures of all synthesized samples were tested by X-ray diffraction (XRD) measurements. Figure 1a depicts the XRD spectra of synthesized CuS nanostructure and Fig. 1b–d shows the synthesized Zn–CuS nanostructures. The well-crystalline diffraction patterns with 2θ values at 26.53º, 27.41º, 29.24º, 31.75º, 32.85º, 47.98º, 52.54º, and 59.05º are oriented along the (100), (101), (102), (103), (006), (110), (108), and (116) planes of covellite CuS and Zn–CuS nanostructures, with lattice constants of a = 3.792 Å and c = 16.344 Å (JCPDS No.06–0464). All diffraction planes in good crystalline quality are in good agreement with the previous report [23]. The diffraction peak of the (006) crystal plane with (0.05 mM) Zn-doped CuS is considered. This shoulder peak will be suppressed compared to pure CuS as shown in Fig. 1b. When the Zn concentration increases (0.15 mM and 0.30 mM), the (006) crystal plane also increases. Some extra peaks appear in the (0.30 mM) Zn-doped CuS. Notably, a slight left shift of the peak intensity of (0.05 mM) Zn–CuS contributes to the Zn2+ incorporated into CuS and the substitutional cationic sites are occupied and the peak intensity is not shifted for Zn (0.15 and 0.30 mM)-doped CuS nanostructures. These results may suggest that the Zn (0.15 and 0.30) do** concentration is appreciably incorporated into CuS lattice structure. This observation may suggest that the lower concentration of Zn2+ (0.05 mM) is easier to replace Cu2+. Results show that (0.05 mM) Zn-doped CuS has optimum concentration as compared to other Zn (0.15 mM and 0.30 mM)-doped CuS. Absence of impurity peaks is monitored, signifying the high purity of the samples by this simple method.
The average grain sizes were found to be 14, 7, 12, and 17 nm for CuS, CuS/Zn (0.05), CuS/Zn (0.15), and CuS/Zn (0.30), respectively, and were quantified using Scherrer Eq. (1)
The lattice parameters of CuS and Zn–CuS crystals are estimated using Eq. (2):
For the hexagonal system, volume of the unit cell was quantified by Eq. (3) [24].
Table 1 summarizes the lattice parameters, grain size and volume of the unit cell. When dopant (Zn2+) concentration increases, lattice parameters and the unit cell volume increase, which reveal the well incorporation of Zn2+ in lattice sites.
The substitution of the Cu2+ ions (rCu2+ = 0.74 Å) [25] with similar radius Zn2+ ions (rZn2+ = 0.74 Å) [26] outcome in a minute diminish in lattice constant of CuS. But, the lattice parameters of Zn–CuS (d103 = 3.791, d110 = 16.352) are similar to that of CuS (d103 = 3.789, d110 = 16.345). It might be caused by same radii of Cu (rCu2+ = 0.74 Å) and Zn (rZn2+ = 0.74 Å). The lattice constants of the Zn–CuS are more extensively increased than the CuS nanostructures. It provides a strong confirmation for the merging of Zn2+ ion into the CuS lattice.
3.2 XPS
For chemical states and elemental composition of the obtained samples, X-ray photoelectron spectroscopy (XPS) measurements were conducted. Figure 2 shows the survey spectrum and the peaks recognized Zn, Cu, S, O, and C elements. The peaks for Cu, Zn, and S elements are identified in the EDS spectra (Fig. 3c, f, i, l), which confirms that the sample is generally composed Cu, Zn, and S elements. Elemental composition of the Zn–CuS is quantified by XPS, and shown in Fig. 2a. The full spectrum reveals that the survival of peaks is attributed to S, Zn, Cu, O and additionally C elements. From this survey, no other element is found which implies the high purity.
Figure 2b shows that core-level spectrum of Cu 2p is split into two main peaks Cu 2p3/2 (931.71) and Cu 2p1/2 (951.79) eV. The intensity peaks of Cu 2p3/2 and Cu 2p1/2 are separated by an energy difference of 20 eV which is fundamentally the same binding energy [27]. Figure 2c reveals the core-level spectrum of Zn 2p lines. The main peak Zn 2p3/2 appears at 1022.35 eV and another peak Zn 2p1/2 is located at 1045.23 eV [28]. In Fig. 2d, the core-level spectrum of S 2p indicates the assignment of the binding energy at 161.9 and 162.9 eV can be allocated to 2p3/2 and 2p1/2, respectively, which is typical the co-ordination of S ions with metal ions. Obviously, the XPS data demonstrate that Zn–CuS material has a composition containing S2+, Zn2+, and Cu2+, which is consistent with the previous reference for CuS [29]. The atomic ratio of Zn, S, and Cu elements as, 1:2:1, equivalent to the chemical formula of Zn S Cu. The weight percentage and atomic percentage of Zn(0.05 mM)–CuS nanocomposite are tabulated in Table 2.
3.3 Surface morphological analysis
3.3.1 SEM/EDX
The representative SEM images reveal the formation of CuS and Zn–CuS structures to a size of 1 and 0.5 µm as shown in Fig. 3a–k. The SEM micrographs reveal the uniformly dispersed with cluster structure of CuS microstructures are shown in Fig. 3 (a, b). The SEM micrographs of Zn (0.05 mM)-doped CuS are shown in Fig. 3 (d, e). The flower like structure comprises irregular nanoflakes are well defined flower like Zn doped CuS which are obtained and the regular petal growth and each microflower is arranged identical interlaced nanoflakes compared with other Zn (0.15 and 0.30)-doped CuS. The cationic surfactant is supplied as a “soft” template material to synthesize the flower-like nanostructures. The creation of transitional pores was observed on the surface, which is the specific feature for obtaining high Cs. Figure 3g, h, j, and k) depicts the remaining two samples Zn (0.15 and 0.30)–CuS micrographs which reveal irregular nanoflakes agglomerated and sheet-like accumulations are arranged and distributed zigzag patterns of the microstructures in a coherent form.
The elemental compositions were analyzed with EDX. The EDX spectra of CuS confirmed the presence of Cu and S peaks alone (Fig. 3c). The elemental compositions of Zn–CuS are shown in Fig. 3f, i and l which indicates subsistence of Cu, Zn, and S element. Absence of other elements in all the spectrums implies high purity of the samples.
3.3.2 FESEM
Figure 4a, b shows the FESEM images of Zn (0.05 mM)-doped CuS nanostructures are flower-like CuS nanostructure that is about 200 nm.
The FESEM image shows compactly combined flower urchin-like Zn (0.05 mM)–CuS architectures in the existence of CTAB. At high magnification, the image (Fig. 4b) presents each hierarchical CuS nanoflowers built up by many interleaving and slight bending nanoflakes with the thickness of about 14–20 nm, which entangle together to behave a network in the bud-like flower. The surface of the Zn–CuS appears much rough, which is essentially credited to the quick ion interactions accompanied with the Kirkendall effects amid sulfidation [30]. The rough surface has been additionally affirmed by the TEM image. The architecture might present generous upgrade in electron transport and providing active sites for redox reduction.
3.3.3 TEM/SAED
The TEM images of Zn (0.05 mM)–CuS nanostructures are flower-like morphologies in the range of 100–500 nm are shown in Fig. 4c–e. TEM images show that each nanoflower surface and outer nanostructure consists of the aggregations of darker secondary branches (nanoflakes). Moreover, these nanoflakes are interconnected to form a flower-like porous architecture. A clear dissimilarity among the pale center and dark edges can be observed, which confirms CuS nanoflowers have a hollow interior. There are many nanopores on the shell of the hierarchical nanostructures.
The SAED pattern (Fig. 4f) reveals well-recognized diffraction rings of Zn (0.30)–CuS representing highly structured subunits and polycrystalline nature. The SAED patterns display a set of diffraction rings which can be indexed as (101), (102), (103), (110), (108), and (116) corresponding to the Zn–CuS phase. Actually Zn (0.05 mM)–CuS signifies its crystal structure.
3.3.4 Growth mechanism
To know the growth mechanism, the reaction conditions are monitored. The SEM images demonstrate that the shape and size of the material change gradually with Zn2+ concentration. Based on the results obtained above, the growth mechanism of the flower-like Zn-doped CuS architectures are presented in Scheme 1.
The CTAB served as template for the formation of microstructures and thiourea serves as a sulfur source, which combines with Cu2+ and ethylene glycol. Initially Cu2+ is coordinated with Zn2+, leading to the formation of metallic complexes. Hydrolysis takes place and leads to the formation of Zn and CuS nuclei when heating the solution and it contains a large number of dangling bonds and favors the introducing of Zn2+ to the CuS crystalline and leads to its anisotropic growth. As the reaction continues, the nanoflakes self-assembled to the hierarchical flower-like mesoporous microstructures are formed.
3.4 Optical properties
The semiconducting materials are highly dependent on the nature and it is well known that structure sensitive and the quantity of limitations are present in a crystal. Figure 5a–d depicts the UV–Vis spectra of samples CuS and Zn–CuS in the range of 200–800 nm.
The UV–Vis spectra depend on the nature of dopant concentration as well as copper and sulfur composition of CuS nanostructures. All the absorption curves appeared at 270 nm for CuS and 272, 271, 270 nm for Zn–CuS nanostructures [31] are compared with bulk CuS [32]. Especially Zn (0.05 mM)–CuS a broad absorption at 500 nm is due to the covellite phase [33] which reveals that there are distinct red shifts compared with other Zn–CuS [34, 35]. This clearly shows that the absorption edges are red shifted. The maximum red shift of the Zn–CuS nanostructures may be associated with the construction of CuS nanoflakes and it signifies decrease in the crystallite size and change in shape (nanoflowers) obtained. The ionic radius of Zn2+ is similar to Cu2+, which indicates Zn2+ ion doped in CuS lattice. The unusual phenomena in the UV–Vis spectra may have many applications in the optical field.
Figure 6 shows the bandgap plots of (αhѵ)2 vs. hѵ as per Tauc’s equation for all the samples and bandgaps are 2.65, 3.60, 2.82, and 3.50 eV for CuS and Zn–CuS nanostructures, respectively. The bandgap energy values of synthesized samples are estimated [36] by the following Eq. (4):
The highest absorption curve is detected at Zn (0.05 mM)–CuS (Fig. 5b) which has excellent optical property, as a result of good agreement with Zn (0.05 mM)–CuS, which is supported by XRD pattern (Fig. 1b).
3.5 BET measurements
The porous structure and specific surface area of the as-synthesized CuS and Zn (0.05 mM)–CuS nanoflowers are investigated using N2 adsorption–desorption isotherm and the consequent results are shown in Fig. 7a, b. The hysteresis circles at a general weight of 0.3–1.1 shows that the isotherms can be assigned sort IV, and the ordinary isotherm bend watched Zn–CuS exhibited the mesoporous qualities of the anode [37]. The normal pore diameter of Zn–CuS nanofower is – 3–71 nm and for (0.1 mM) CTAB–CuS is – 3–76 nm. The surface area of the Zn–CuS is 18.23 m2/g and for (0.1 mM) CTAB–CuS is 8.32m2/g.
However, at high relative pressure from 0.95 to 1.0, the uptake is attributed to the pore spaces between the nanoflowers. The porous structure and high surface area provide low-resistance pathways and more electroactive sites are beneficial for the Zn–CuS, which enhances charge transport and power capability. Therefore, the Zn–CuS sample utilized as electrode materials can expect better electrochemical properties for supercapacitors.
3.6 Electrochemical studies
3.6.1 Effect of scan rate
For electrochemical measurements, the as-prepared CuS and Zn–CuS nanostructures were fabricated as incorporated electrodes and explored in the three-electrode test in a voltage window between 0.0 to 0.5 V (vs. SCE) with 2 M KOH as aqueous electrolyte solution. However, Fig. 8a shows the CV curve of the CuS electrode [22] which exhibits roughly rectangular shape with weak redox curves with broad faradaic curve in the range of 0.12 to 0.41 V [38]. We assumed that the intimate bonding of CuS electrode reduced the contact resistance among them; thus, Faraday processes of the electrochemically active species become easy [39]. Figure 8b shows the redox peaks present in Zn–CuS electrode at all sweep rates which may supply fast transport and facile accessibility to active sites for electrolyte species.
With an increasing sweep rate, a pair of redox curves was observed and better response relationship of the specific current of redox peak and a typical pseudocapacitance become visible in all the CV curves caused by Faradaic reactions inside the electrode materials [40]. It is notable that the Zn–CuS displays more positive cathodic peak potential than the CuS electrode which suggests their excellent intrinsic electrochemical ability and conductivity of the Zn–CuS electrode. At high scan rate, the CV curve has a larger area compared to the lower scan rate and resulted the capacitance decrease during the redox process [41]. Contrasted to CuS, the curve of Zn–CuS CV shows more clear redox curves and larger region, which implies better reversibility and higher capacitance in light of the fact that the electroconductibility of Zn–CuS is superior to that of CuS.
Notably the CV curves show non-rectangular shape, and Zn–CuS electrode exhibits a pseudocapacitance behavior due to Faradaic reactions which are liable for charge storage. At high scan rate, broader redox curves are obtained in Zn–CuS CV than the low scan rate. When compared with the CTAB–CuS electrode, the CTAB–Zn-doped CuS electrode exhibits a greater area of CV curves, indicating the CTAB/Zn–CuS electrodes acquire higher Cs. The smallest variations in redox curve positions indicate low polarization effect which may feature the difference in electrode polarization behaviors. This behavior is closely related to the chemical composition and physical morphology of the electrode material. This excellent Cs may be ascribed to the mesoporous structure of Zn–CuS. Porous structure can be considered to get better consumption and the Cs of electroactive materials [42].
3.6.2 Chronopotentiometry test
The chronopotentiometric measurements were done to quantify the Cs of CuS and Zn–CuS materials. Figure 8c, d illustrates GCD curves of two electrodes at a current density of 5 mA cm−1 signifying the Faradaic reaction. Obviously, the discharge curve can be separated into two districts, a rapid potential fall caused by the internal resistance of electrode materials and a time-consuming potential decay owing to the Faradic redox reaction. The Cs of both CuS and Zn–CuS electrodes is determined from the discharge curves according to the following Eq. (5): [43, 44]
The CTAB/CuS modified GCE delivers 328.26 Fg−1 at 5 mA cm−2 and the CTAB/Zn–CuS modified GCE delivers 826.31 Fg−1 at 5 mA cm−2. Cs of the CTAB/Zn–CuS is higher than that of CuS electrode material. The highest Cs enhanced owing to two reasons: (1) Improved electrical conductivity provides fast charge transfer in Zn–CuS and (2) Porous structure of Zn–CuS nanostructures can considerably extend the consumption and the Cs of electroactive materials [45]. Comparison of specific capacitance for synthesized CuS and Zn–CuS electrode materials with other reported electrode materials are tabulated in Table3.
3.6.3 EIS
Figure 9 displays the EIS results of the CuS and Zn–CuS electrodes in the fitting circuit (see inset the equivalent circuit of the supercapacitor in Fig. 9), Rs is solution resistance and Rct is charge transfer resistance, Cdl is the double layer capacitance and Zw is the Warburg impedance.
Nyquist plot of CuS and Zn–CuS electrodes is shown in Fig. 9a, b. In samples (a and b), at high-frequency region a semicircle is obtained and goes to (Rct) of the electrode. This Rct is mostly relay to the surface region of the Zn–CuS, because of the Faradic process of CuS and Zn–CuS. Both electrodes exhibit a linear portion in the lower frequency region and semicircle in the high-frequency region corresponding to Warburg impedance (Zw) [46]. Both the materials have ideal capacitor behavior. Also the electrochemical impedance spectra analyzed by nyquist plot confirmed by capacitance behavior. The current study diameter of the semicircle for CTAB/CuS increases as compared with Zn–CuS. From this EIS analysis, Zn–CuS electrode exposes suitable electrode for SCs.
4 Highlights
-
Development of new useful Zn–CuS nanocomposites electrode materials for supercapacitor will be the outcome of this paper.
-
Porous nature of Zn–CuS nanocomposites will play major role can provide fast ion/ electron transfer leads to improved reaction kinetics and high performance compared to other hybrid composites. The mesopores in these materials can supply numerous catalytically active sites, facilitating the oxygen diffusion and electrolyte throughout the electrode.
-
Major drawback of supercapacitor is low energy density compared to batteries. To overcome this draw back by changing suitable electrode material, this may enhance the energy density and stability of the supercapacitors in future.
-
Therefore, it is extremely important to develop alternative metal sulfide electrode material with a combination of low cost, larger capacitance and excellent electrochemical stability.
-
Combining all the above results, it has been concluded that the Zn–CuS nanocomposites used in the present work proves to be a low cost and environment friendly in nature for its application as an electrode materials in supercapacitors.
5 Conclusion
In summary, a novel hierarchical Zn–CuS mesoporous nanoflowers are synthesized by simple one-pot mild hydrothermal approach. The crystal phase, purity and grain size are estimated by XRD. The SEM, FESEM and TEM images of Zn–CuS show that flower-like nanostructures are built up by many interleaving slightly bending nanoflakes. Profiting from the progressive and mesoporous structures have demonstrated considerable upgrade in active sites for redox responses. The growth mechanism is discussed elaborately. The pore diameter of Zn–CuS nanoflower is – 3–71 nm. The BET surface area of the Zn–CuS sample is 18.23 m2/g. Moreover, the Zn–CuS samples exhibit superior specific capacitance, and the Zn2+ ion concentration on CuS is optimized. The electrochemical behavior of the mesoporous nanoflowers can be ascribed to the effect of these factors viz., high surface area, the presence of mesopores and the existence of nanoflakes in the flower. The existence of CTAB behaves as shape promoting and is also favorable for mesoporous structures. The Zn–CuS sample exhibits high specific capacitance 826.31 Fg−1 at 5 mA cm−2. The consequences of supercapacitors with the Zn–CuS electrode propose that such effortless strategy holds a promising electrode for superior supercapacitors.
References
Hsu YK, Chen YC, Lin YG (2014) Synthesis of copper sulfide nanowire arrays for high-performance supercapacitors. Electrochim Acta 139:401–407
Heydari H, Moosavifard SE, Elyasi S, Shahraki M (2017) Nanoporous CuS nano-hollow spheres as advanced material for high-performance supercapacitors. Appl Surf Sci 394:425–430
Peng H, Ma G, Mu J, Sun K, Lei Z (2014) Controllable synthesis of CuS with hierarchical structures via a surfactant-free method for high-performance supercapacitors. Mater Lett 122:25–28
He D, Wang G, Liu G, Bai J, Suo H, Zhao C (2017) Facile route to achieve mesoporous Cu(OH)2 nanorods on copper foam for high-performance supercapacitor electrode. J Alloy Compd 699:706–712
Krishnamoorthy K, Veerasubramani GK, Pazhamalai P, Kim SJ (2016) Designing two dimensional nanoarchitectured MoS2 sheets grown on Mo foil as a binder free electrode for supercapacitors. Electrochim Acta 190:305–312
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845
Peng S, Li L, Tan H, Cai R, Shi W, Li C, Mhaisalkar SG, Srinivasan M, Ramakrishna S, Yan Q (2014) Hollow spheres: MS2 (M = Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv Funct Mater 24:2155
**a X, Zhu C, Luo J, Zeng Z, Guan C, Ng CF, Zhang H, Fan HJ (2014) Synthesis of free-standing metal sulfide nanoarrays via anion exchange reaction and their electrochemical energy storage application. Small 10:766
**ao J, Wan L, Yang S, **ao F, Wang S (2014) Design hierarchical electrodes with highly conductive nico2s4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett 14:831
Jayalakshmi M, Rao MM (2006) Synthesis of zinc sulphide nanoparticles by thiourea hydrolysis and their characterization for electrochemical capacitor applications. J Power Sources 157:624–629
Tsamouras D, Kobotiatis L, Dalas E, Sakkopoulos S (1999) An impedance study of the metal sulfide CuxZn(1– x )S electrolyte interface. J Electroanal Chem 469:43–47
Wang B, Park J, Su D, Wang C, Ahn H, Wang G (2012) Solvothermal synthesis of CoS2–graphene nanocomposite material for high-performance supercapacitors. J Mater Chem 22:15750–15756
Moosavifard SE, Fani S, Rahmanian M (2016) Hierarchical CuCo2S4 hollow nanoneedle arrays as novel binder- free electrodes for high-performance asymmetric supercapacitors. Chem Commun 52:4517–4520
Pei LZ, Wang JF, Tao XX, Wang SB, Dong YP, Fan CG, Zhang Q-F (2011) Synthesis of CuS and Cu1.1Fe1.1S2 crystals and their electrochemical properties. Mater Charact 62:354–359
Huang K-J, Zhang J-Z, Fan Y (2015) One-step solvothermal synthesis of different morphologies CuS nanosheets compared as supercapacitor electrode materials. J Alloys Compd 625:158–163
Peng H, Ma G, **g**g Mu, Sun K, Lei Z (2014) Controllable synthesis of CuS with hierarchical structures via a surfactant-free method for high-performance supercapacitors. Mater Lett 122:25–28
Zhu T, **a B, Zhou L, Lou XWD (2012) Arrays of ultrafine CuS nanoneedles supported on a CNT backbone for application in supercapacitors. J Mater Chem 22:7851
Wang X, Liu WS, Lu X, Lee PS (2012) Dodecyl sulfate induced fast faradic process in nickel cobalt oxide/reduced graphite oxide composite material and its application for asymmetric supercapacitor device. J Mater Chem 22:23114
Ding R, Qia L, Jia M, Wang H (2013) Facile and large-scale chemical synthesis of highly porous secondary submicron/micron-sized NiCo2O4materials for high-performance aqueous hybrid AC-NiCo2O4 electrochemical capacitors. Electrochim Acta 107:494
Tang C, Tang Z, Gong H (2012) J Electrochem Soc 159:A651
Pu J, Cui FL, Chu SB, Wang TT, Sheng EH, Wang ZH (2014) ACS Sustainable Chem Eng 2:809–815
Surekha P, Geetha D, Ramesh PS (2017) One-pot synthesis of CTAB stabilized mesoporous cobalt doped CuS nano flower with enhanced pseudocapacitive behavior. J Mater Sci: Mater Electron 28:15387–15397
Du H, Jiao L, Cao K, Wang Y, Yuan H (2013) Polyol-mediated synthesis of mesoporous α-Ni (OH)2 with enhanced supercapacitance. Appl Mater Interfaces 5:6643
Gandhi V, Ganesan R, Syedahamed AHH, Thaiyan M (2014) Effect of cobalt do** on structural, optical, and magnetic properties of ZnO nanoparticles synthesized by coprecipitation method. J Phys Chem C 118:9715–9725
Shannan RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751
Wang P, Gao Y, Li P, Zhang X, Niu H, Zheng Z (2016) Do** Zn2+ in CuS nanoflowers into chemically homogeneous Zn0.49Cu0.50S1.01 superlattice crystal structure as high-efficiency n-type photoelectric semiconductors. Appl. Mater. Interfaces. 8:15820–15827
Feng X, Li Y, Liu H, Li Y, Cui S, Wang N, Jiang L, Liu X, Yuan M (2007) Controlled growth and field emission properties of CuS nanowalls. Nanotechnology 18:145706
**ao Y, Lei Y, Zheng B, Gu L, Wang Y, **ao D (2015) Rapid microwave-assisted fabrication of 3D cauliflower-like NiCo2S4architectures for asymmetric supercapacitors. RSC Adv 5:21604
Tanveer M, Cao C, Ali Z, Aslam I, Idrees F, Khan WS, But FK, Tahira M, Mahmood N (2014) Template free synthesis of CuS nanosheet-based hierarchical microspheres: an efficient natural light driven photocatalyst. Cryst Eng Comm 16:5290
Yang J et al (2014) Electrochemical performances investigation of NiS/rGO composite as electrode material for supercapacitors. Nano Energy 5:74–81
Chen C, Li Q, Wang Y, Li Y, Zhong X (2011) Room temperature synthesis of flower-like CuS nanostructures under assistance of ionic liquid. Front Optoelectron China 4:150–155
Xu H, Wang W, Zhu W, Zhou L (2006) Synthesis of octahedral CuS nanocages via a solid–liquid reaction. Nanotechnology 17:3649–3654
Silvester EJ, Grieser F, Sexton BA, Healy TW (1991) Spectroscopic studies on copper sulfide sols. Longmuir 7:2917
Gao J, Li Q, Zhao H, Li L, Liu C, Gong Q, Qi L (2008) One-pot synthesis of uniform Cu2O and CuS hollow spheres and their optical limiting properties. Chem Mater 20:6263–6269
Haram SK, Mahadeshwar AR, Dixit SG (1996) Synthesis and characterization of copper sulfide nanoparticles in triton-X 100 water-in-oil microemulsions. J Phys Chem 100:5868–5873
Pankove JI (1971) Optical processes in semiconductors. Prentice- Hall, Englewood Cliffs, NJ
Song K, Song WL, Fan LZ (2015) Scalable fabrication of exceptional 3D carbon networks for Supercapacitors. J Mater Chem A 3:16104–16111
Silambarasan M, Ramesh PS, Geetha D, Venkatachalam V (2016) A report on 1D MgCo2O4 with enhanced structural, morphological and electrochemical properties. J Mater Sci: Mater Electron 28:6880–6888
Fang Y, Luo B, Jia Y, Li X, Wang B, Song Q, Kang F, Zhi L (2012) Renewing functionalized graphene as electrodes for high-performance supercapacitors. Adv Mater 24:6348–6355
Yang J, Guo W, Li D, Qin Q, Zhang J, Wei C, Fan H, Wu L, Zheng W (2014) Hierarchical porous NiCo2S4 hexagonal plates: formation via chemical conversion and application in high performance supercapacitors. Electrochimi Acta 144:16–21
Silambarasan M, Ramesh PS, Geetha D (2016) Facile one step synthesis structural, optical and electrochemical properties of NiCo2O4 nanostructure. J Mater Sci: Mater Electron 28:323–336
Surekha P, Geetha D, Ramesh PS (2017) Preparation and characterization of porous hollow sphere of Ni doped CuS nanostructures for electrochemical supercapacitor electrode material. Springer Proc Phys 189:277–288
Behzadi PG, Fekri AL, Mirzaee M (2018) Flexible graphene supercapacitor based on the PVA electrolyte and aTiO3/PEDOT:PSS composite separator. J Mater Sci: Mater Electron 29:17432–17437
Mirzaee M, Pour GB (2018) Design and fabrication of Ultracapacitor based on paper substrate and BaTiO3/PEDOT: PSS separator film. Recent Patent Nanotechnol 12(3):192–199
Silambarasan M, Padmanathan N, Ramesh PS, Geetha D (2016) Spinel CuCo2O4 nanoparticles: facile one-step synthesis, optical, and electrochemical properties. Mater Res Express 3(9):1–11
Silambarasan M, Ramesh PS, Geetha D (2017) Co-precipitation synthesis and enhanced electrochemical pseudocapacitive properties of spinel nickel cobaltite nanostructures modified glassy carbon electrodes. Springer Proc Phys 189:219–231
Fekri AL, Ghoranneviss M, Behzadi PG (2018) Graphite nanoparticles paper supercapacitor based on gel electrolyte. Mater Renew Sustain Energy 7:29
Nazarpour FH, Behzadi PG, Nasiri SM, Esmaili P (2019) PVA-based supercapacitors. Int J Ion Sci Technol Ion Motion 25(7):2951–2963
Fekri AL, Ghoranneviss M, Pour BG (2018) Highperformance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 4:e00862
Acknowledgement
This work was financially supported by University grants commission (UGC), India, (F.No- 43–533/2014 (SR) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Podili, S., Geetha, D. & Ramesh, P.S. Tuning the dopant (Zn2+) composition for uniform mesoporous Zn–CuS nanoflower via hydrothermal approach as a novel electrode material for high-rate supercapacitor. SN Appl. Sci. 2, 1023 (2020). https://doi.org/10.1007/s42452-020-2668-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2668-5