Abstract
Well-defined 1D molybdenum oxide nanostructures were synthesized using the hydrothermal method with sodium dodecyl sulfate as a cap** agent with water as a solvent for the supercapacitor application at very low electrolyte concentration. The structural, morphological and optical properties of the as-prepared nanoparticles were characterized using X-ray diffraction, Field Emission Scanning Electron Microscope, and UV–visible spectroscopy. X-ray diffraction and FESEM studies revealed the formation of ‘1D Molybdenum Oxide nanorods with an average crystallite size of 31 nm. UV–visible spectroscopic analysis showed that the optical bandgap of molybdenum oxide nanorods to be 3.01 eV. The electrochemical performance of as synthesized nanorods was performed by using cyclic voltammetry, galavanostatic charge–discharge, and electrochemical impedance spectroscopy. The maximum specific capacitance obtained was 411 F g−1 in 0.1M NaOH electrolyte solution with excellent rate capability even at higher scan rates. Cyclic retention of 82.4% was observed even after 1000 cycles making it suitable electrode material for high-performance supercapacitor applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rapid urbanization and excessive reliance on fossil fuels resulted in the depletion of fossil fuels and environmental pollution, which sparked the growth of reusable and renewable energy systems. The increasing energy demand could be fulfilled by transforming natural energy from sustainable and renewable resources such as wind, tidal, and sun [1]. However, due to the geological and climatic factors, man has limited control over these natural resources. Hence energy storage is considered a promising option in harvesting the renewably generated electrical energy for future use. Also, the progress in the development of flexible, portable electronics and energy storage devices is attracting current research [2]. Two types of energy storage devices were proposed mainly Fuel Cells and Supercapacitors [3].
Supercapacitors, also known as electrochemical capacitors, are a peculiar type of energy storage device acting as a bridge between conventional capacitors and batteries [4]. In general, batteries possess superior specific energy with an inferior specific power, whereas traditional capacitors possess relatively high specific power but low specific energy. Hence supercapacitors exhibit higher power densities, short charging time, and longer discharging duration compared to traditional batteries and also excellent specific capacitance and higher energy densities than regular capacitors [5].
Based on their charge storage mechanism, supercapacitors are classified into two types, Electrochemical Double-layer Capacitors (EDLC’s) and Pseudocapacitors. Pseudocapacitors utilize fast and reversible redox reactions near the surface and on the surface of the electrodes to store the charge whereas electrochemical double-layer capacitors utilize electrostatic adsorption at the electrode and electrolyte interface to store energy [6]. Nanoporous carbonaceous materials are investigated as the unique electrode materials for EDLC’s owing to their high specific surface area, higher conductivity, and high mechanical stability [7]. Transition metal oxides and conducting polymers are promising electrodes for Pseudocapacitors. Metal oxides provide low resistance and high capacitance, making it easier to synthesize, whereas conducting polymers has more conductivity and equivalent series resistance than carbon materials [8, 9].
Pseudocapacitors exhibit higher capacitances and energy densities compared to the electrochemical double-layer capacitors [10]. Pseudocapacitors are cost-effective than electrochemical double-layer capacitors as the synthesis of carbon materials is very costly. Ruthenium oxide, manganese dioxide, and cobalt oxide are some of the transition metal oxides used as electrodes in pseudocapacitors whereas polyaniline, polypyrrole and polythiophene are the conducting polymers used as pseudocapacitor electrodes [11,12,13].
In recent years, much emphasis has been focussed on the molybdenum oxide (MoO3) and molybdenum sulfide (MoS2) related materials [14]. Molybdenum Oxide (MoO3) is a prominent transition metal oxide with rich polymorphism and structural litheness [15]. It consists of several valence states and a unique layered structure with an outstanding theoretical specific capacitance [16]. It possesses superior electrochromic and catalytic properties [17] that can potentially be applied in storage media [18], gas sensors [19] and humidity sensors [20], organic solar cells [21]. The oxidation states of molybdenum oxide range from + 2 to + 6 and predominantly exist in two primary forms, namely molybdenum (IV) oxide and molybdenum (VI) oxide [22].
In the synthesis of molybdenum oxide nanoparticles, several techniques were employed like solution combustion [23, 24], sol–gel [25], microwave [26], and green synthesis methods [27]. The hydrothermal technique [28] for the preparation of molybdenum oxide nanoparticles was commonly used among them. Different morphologies of molybdenum oxide nanoparticles such as nanobelts [29], nanoflowers [30], nanowires [31], and nanocubes [32] can be obtained. These morphologies play a substantial role in determining the specific capacitance of the material. The hydrothermal method allows studying the influence of parameters such as temperature, pressure, and reaction time on the physiochemical performance of the material [33].
Miao et al. reported the preparation of Molybdenum Trioxide nanostructures through a cost-effective metal-assisted chemical wet etching method which showed a specific capacitance of 30.85 F g−1 in 0.5M Na2SO4 electrolyte solution [34]. Wang et al. synthesized α-MoO3 nanorods through the hydrothermal method and observed that annealed α-MoO3 nanorods demonstrated excellent specific capacitance than compared to hydrothermally obtain ones [35]. Shakir et al. reported the preparation of orthorhombic molybdenum trioxide nanowires using a hydrothermal method which yielded a specific capacitance of 168 F g−1 at 0.5 Ag−1 current density and 97% cyclic retention in 1M H2SO4 electrolyte solution [36].
In the above-reported works, very high concentrations of electrolyte were used for determining the electrochemical performance of the synthesized molybdenum oxide nanomaterials. In this work, a facile hydrothermal method was employed to synthesize molybdenum oxide nanorods, and electrochemical studies were carried out using a very low concentration of electrolyte, delivering excellent rate capability and high specific capacitance.
2 Materials and methods
2.1 Chemicals and reagents
All the chemical reagents as commercially available Ammonium Heptamolybdate Tetrahydrate ((NH4)6Mo7O24·4H2O), sodium dodecyl sulfate (NaC12H25SO4) in this study are of analytical grade and are used as without any further purification. Distilled water was obtained from the distilled water purification system.
2.2 Synthesis and electrode preparation
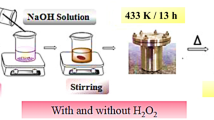
Molybdenum oxide nanostructures were synthesized employing the hydrothermal method. Typically, 0.02M Ammonium Heptamolybdate Tetrahydrate was dispersed in 20 ml distilled water and subjected to continuous stirring for 30 min. Now, 0.01M sodium dodecyl sulfate dispersed in 20 ml distilled water is added drop by drop to the above solution and stirred continuously for 20 min. The pH of the above mixture is adjusted to 3 with the dropwise addition of dilute HCl under vigorous stirring. The transparent mixture was transferred into a 120 ml Teflon lined stainless steel autoclave which is subjected to the hydrothermal treatment of 180 °C for 24 h. After 24 h, the autoclave was naturally cooled down to room temperature. A blue color precipitate was formed during this process which was washed with distilled water and ethanol several times. Finally, the precipitate was centrifuged and dried at 60 °C overnight to get dry powder. The obtained nanoparticles were collected and stored for further characterization studies.
2.3 Characterization and measurements
The morphology of the as-synthesized molybdenum oxide nanorods was determined by Field Emission Scanning Electron Microscopy (JEOL JSM-7600F). Bruker D8 Advanced X-Ray Diffractometer employing CuKα radiation is used to determine the XRD patterns. The maximum absorbance of Molybdenum Oxide nanorods was determined using UV–visible spectroscopy employing LAMBDA 1050 UV/Vis spectrophotometer. FTIR spectra of the samples were obtained at ambient temperature using the KBr disk method employing Bruker FTIR spectrophotometer. Cyclic-voltammetry, Galvanostatic charge–discharge, and Electrochemical-impedance-spectroscopy were performed using AUTO LAB PG stat workstation.
2.4 Electrode preparation
Electrochemical measurements were carried out employing three-electrode configurations where Pt wire serves as a counter electrode, and Ag/AgCl serves as a reference electrode in 0.1M NaOH aqueous electrolyte within a potential window of − 0.2 V to 1 V. The working electrode was prepared by taking off 85:10:5 composition of active material, PVDF (Polyvinylidene fluoride) acting as binder and carbon black along with few drops of NMP (N-Methyl-2-Pyrrolidone) to make the slurry. The slurry was coated on the working electrode and dried at 60 °C for 4 h.
3 Results and discussions
3.1 Structural and morphological studies
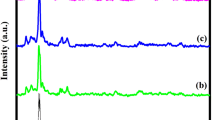
Figure 1 represents the XRD patterns of MoO3 nanorods. XRD measurements are used to estimate the crystallographic structure of the synthesized molybdenum oxide nanorods. The inset show diffraction peaks at 12.67°, 23.32°, 25.67°, 27.23°, 33.73°, 38.66°, 46.20°, 49.06° and 55.03° corresponding to the lattice planes of (020), (110), (040), (021), (111), (060), (041), (200) and (002) which is indexed to the orthorhombic structure of molybdenum oxide with lattice parameters a = 3.96 Å, b = 13.78 Å and c = 3.74 Å which is in well agreement with JCPDS card no.: 05-0508. The orientation of (020), (040) and (060) planes reveal the highly anisotropic growth of Molybdenum Oxide nanoparticles possessing a 1D structure [37]. The sharp, well-defined peaks indicate the high degree of crystallinity of the obtained nanorods. The average crystallite size of the obtained nanoparticles calculated using Debye Sherrer equation which is given by
where β is Full-Width Half Maximum (FWHM), k is the shape factor, λ is the wavelength of Cu Kα radiation, and θ is the diffraction angle. The average crystallite size 31 nm was obtained using the above Eq. (1).
Figure 2 displays the high magnification FESEM images of molybdenum oxide nanorods. The hydrothermal technique has a unique edge over other chemical routes in a way capability in controlling the morphology ranging from 0D to 3D structures by carefully selecting the reaction time and temperature. The morphological phenomena of the surface were investigated using Field emission scanning electron microscopy. From the figure, it can be observed that the synthesized molybdenum oxide nanorods consist of a 1D rod-like morphology. FESEM analysis further confirmed the growth of molybdenum oxide crystallites to be of 100–180 nm diameters approximately and length of ~ 2 µm.
3.2 UV–visible spectroscopy
Figure 3a shows the UV–visible spectrum along with the Tauc plot. The optical absorbance and energy bandgap of the synthesized molybdenum oxide nanorods were investigated employing UV–visible spectroscopy. The maximum absorbance was observed at 227 nm which confirms the formation of molybdenum oxide nanorods due to d–d transition in molybdenum oxide. The energy bandgap of the material obtained using the Tauc plot was Eg = 3.01 eV. The obtained bandgap value indicates that the synthesized molybdenum oxide nanorods were a perfect semiconductor. The calculated energy bandgap is considerably higher than the bulk material, which can be attributed to reduced particle size [38] due to quantum confinement effect. The bandgap of the obtained Molybdenum oxide nanorods is higher than MoO3 nanobelts and is lower than the MoO3 prism-like structures [39] and nanoribbons [40].
3.3 Fourier transform infra-red and Raman spectroscopy
Figure 3b shows that FTIR spectroscopy is used to analyze the functional groups present in the MoO3 nanostructures captured in the wavenumber region between 4000 and 400 cm−1. The peak at 534 cm−1 is attributed to the vibrations of the Mo–O–Mo bond of oxygen atoms of molybdenum oxide. The peaks at 870 cm−1 and 988 cm−1 are associated with Mo–O–Mo bond symmetric vibrations and characteristic stretching vibration of Mo=O bond, which is the essential characteristic of the layered orthorhombic MoO3 phase [36]. Peaks at 1630 cm−1 and 3448 cm−1 attributes to stretching and bending vibrations of OH groups in water molecules. The distant peaks at 2926 cm−1 and 1396 cm−1 correspond to the stretching and bending vibrations of N–H groups in NH4+.
Figure 4 shows the Raman spectra of molybdenum oxide nanostructures recorded at an excitation wavelength of 632.8 nm. Raman spectroscopy is a useful tool in examining the quantum confinement effect in nanostructured metal oxides. From the figure, it can be said that the high-intensity peak at 816 cm−1 (Ag, B1g) is attributed to the doubly coordinated oxygen. Mo2=O stretching modes of corner shared oxygen atoms that are shared by two MoO6 octahedrons. The peak at 991 cm−1 (Ag, B1g) is due to the asymmetric stretching of terminal oxygen atoms. The peaks at 246 cm−1 and 283 cm−1(B2g, B3g) are attributed to the twisting mode and wagging mode of O=Mo=O bonds. The peak at 474 cm−1 corresponds to the asymmetric stretching/bending mode of the O–Mo–O bond. The Raman peak at 335 cm−1 is due to O–Mo–O bending mode and peak at 377 cm−1 gives O–Mo–O scissoring mode. The peak at 660 cm−1(B2g, B3g) is attributed to the Mo3–O stretching vibrations of triply coordinated oxygen caused by the edge shared oxygen atoms of O–Mo–O stretch [41].
3.4 Growth mechanism of MoO3 nanorods
Figure 5 explains the mechanism involved in the formation of molybdenum oxide nanorods. Nucleation begins with the reaction between Ammonium Heptamolybdate Tetrahydrate with dilute HCl. The increase in reaction temperature under hydrothermal conditions generates sufficient energy for the oxidation to form MoOx moieties with an anisotropic growth of nanoparticles in a preferred direction. Self-assembled 1D growth of MoO3 nanorods is aided by expanding the duration of reaction time and temperature during hydrothermal treatment. The detailed morphological changes that occur in the formation of molybdenum oxide nanorods are explained as follows:
-
At the initial stage of hydrothermal treatment, MoOx entities are formed in the solution.
-
Over the course of time, flakes or sheet-like structures are formed due to condensation and nucleation.
-
With further increase in the time, the layered structure of Mo8O23 turns to twirl due to the increase in the hydrostatic pressure inside the autoclave, which is the crucial step in the formation of 1D morphology.
-
Further increase in the reaction time results in the destruction of tubular microrods by the dissolution under hydrothermal conditions allowing the formation of cluster/chunk of nanorods.
Hence, reaction time is vital in deciding the length, diameter of nanorods.
3.5 Electrochemical studies
The electrochemical measurement of as synthesized MoO3 nanorods were analyzed by cyclic voltammetry, galvanometric charge–discharge, and electrochemical impedance spectroscopy in Fig. 6. Cyclic voltammetry was performed at different scan rates within a potential window of − 0.2 V to 1 V at room temperature. Figure 6a shows the cyclic voltammograms representative of the as-synthesized molybdenum oxide nanorods. It is observed that MoO3 has undergone redox reaction and the redox peaks appear at 0.45 V and 0.32 V (vs. Ag/AgCl) in the forward and reverse scans respectively, specifying different valence states of molybdenum ions and the oxidation peak appears in between 0.45 V is attributed to Mo (vi) oxidation state. These peaks mark the phase transition during Na+ ions intercalation and deintercalation into the host MoO3 in the electrolyte. However, there is only one oxidation peak, probably due to the overlap**. Using Cyclic Voltammetry, the specific capacitance of the material has been calculated using the following equation:
where CS is the specific capacitance, I is the current density used for charge–discharge, Vs is the scan rate, m is the mass of the active material, and \(\Delta Vm\) is the potential window.
The specific capacitance of the molybdenum oxide nanorods synthesized by the hydrothermal method was calculated as 411 F g−1 at a scan rate of 5 mVps. The specific capacitance of the as-synthesized molybdenum oxide nanorods is significantly higher than the values previously reported [18, 34, 36, 42]. This significant amount of electrochemical performance can be attributed to the nanorods structure, which plays an essential role in the accessibility of electrolyte ions (Na+) to the MoO3 active material. Also, the area of the CV loop increases when the scan rate is increased from 5 to 100 mV/s in a 0.1M NaOH electrolyte solution because with increasing scan rate, the current increases with the voltage. This increase in specific current results in the decrease in specific capacitance of the material since at lower scan rate i.e., at 5 mV/s all the active sites(both on the surface and inside the surface) in MoO3 nanorods are accessible for the electrolyte ions during charge transfer process resulting in high specific capacitance and also prominent redox peaks. However, as the scan rate increases, only few number of active sites is available for the charge transfer process leading to a decrease in capacitance value. Also, the interaction between electrode and electrolyte is time-limited; hence, the utilization of the surface area of the material is also limited or reduced with increasing scan rate resulting in the decrease in specific capacitance. Nevertheless, the redox and oxidation peaks are still evident at high scan rates of 100 mV/s, which is the evidence of excellent rate capability. The inverse relationship between scan rate and specific capacitance is shown in Fig. 6b.
Figure 6c shows the Nyquist plot of the MoO3 electrode measured in 0.1M NaOH solution in three-electrode configuration within the frequency spectrum of − 0.2 Hz to 100 kHz. Electrochemical Impedance spectroscopy is the key parameter to interpret the interfacial performance of the electrode and electrochemical kinetics of the entire system. The charge transfer resistance associated with a semicircle in a higher frequency region with a resistance of 1.64 Ω signifies better conductivity of the electrode material. It can be noted that MoO3 has very low internal resistance and good ion response in high-frequency response. This low charge transfer resistance emphasizes that molybdenum oxide nanorods are better for electrochemical applications. The linear response in the low-frequency region corresponds to electrolyte diffusive resistance inside the electrode, which can be attributed to the ideal capacitive behavior of the MoO3 electrode.
Figure 7a displays the charge–discharge curve of molybdenum oxide nanorods in 0.1M NaOH aqueous electrolyte within a potential window of − 0.2 V to 1 V at different densities which are used to assess the properties of MoO3 nanorods for their use in supercapacitors. The specific capacitance (Cg) of the electrode material using three-electrode configurations for a discharging curve is calculated using the following equation:
where I is the current density used for charge–discharge, \(\Delta {\text{t}}\) is the discharging time of the material, m is the mass of the active material, and \(\Delta V\) is the potential window.
According to the equation, the specific capacitance of MoO3 nanorods measured at a current density of 1 m Ag−1 is 389 F/g, which is approximately equal to the specific capacitance calculated from cyclic voltammetry measurements. At lower current densities, all the active sites that are present both on the surface and inside the active material are fully accessed and diffused by the Na+ ions in the electrolyte which significantly reduces the ionic mobility pathway deriving high specific capacitance. The nonlinear discharge or slight plateau in the charge–discharge curve can be attributed to the faradic redox kinetics. As the current density increased, the charge–discharge time decreased since, at higher current density, the up-rise of the output voltage happened much faster, which resulted in the faster charging-discharging times.
In this electrochemical analysis, the interaction between MoO3 and aqueous electrolyte (in this case, NaOH) can be described as given below:
The charge storage in the material happened either by the surface limited non-faradic reaction (i.e., adsorption) or by the intercalation of ions of the electrolyte into the interlayer spacing of the material. The reslts of present work have been compared with exisiting literature as shown in Table 1.
Figure 7b shows the cyclic performance of electrode material over 1000 cycles. Cyclic retention of 82.4% was obtained even after 1000 cycles profoundly suggesting MoO3 nanorods as a right candidate for supercapacitor.
4 Conclusions
A facile hydrothermal method was adopted to synthesize Molybdenum Oxide nanorods. The preliminary studies showed that the obtained nanoparticles were 1D nanorods. The electrochemical studies exhibited excellent specific capacitance of 411 F g−1 in a three-electrode aqueous electrolyte configuration within a potential window of − 0.2 V to 1 V (vs. Ag/AgCl) at a scan rate 5 mV/s of whereas the galvanometric studies resulted in capacitance of 389 F g−1 at 1 m Ag−1 current density. The synthesized molybdenum oxide nanorods showed excellent cyclic stability with 82.4% capacitance retention even after 1000 cycles. These findings suggest that the Molybdenum Oxide nanostructures can be employed as a promising electrode in supercapacitor devices.
References
Chen SM, Ramachandran R, Mani V, Saraswathi R (2014) Recent advancements in electrode materials for the high-performance electrochemical supercapacitors: a review. Int J Electrochem Sci 9:4072–4085
Wang X, Lu X, Liu B, Chen D, Tong Y, Shen G (2014) Flexible energy-storage devices: design consideration and recent progress. Adv Mater 26:4763–4782
Choi HJ, Jung SM, Seo JM, Chang DW, Dai L, Baek JB (2012) Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 1:534–551
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828
Hatui G, Nayak GC, Udayabhanu G, Mishra YK, Pathak DD (2017) Template-free single pot synthesis of SnS2@Cu2O/reduced graphene oxide nanoflowers for high-performance supercapacitors. New J Chem 41:2702–2716
Zhi M, **ang C, Li J, Li M, Wu N (2013) Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review. Nanoscale 5:72–88
Anwar AW, Majeed A, Iqbal N, Ullah W, Shuaib A, Ilyas U, Rafique HM (2015) Specific capacitance and cyclic stability of graphene-based metal/metal oxide nanocomposites: a review. J Mater Sci Technol 31:699–707
Cottineau T, Toupin M, Delahaye T, Brousse T, Bélanger D (2006) Nanostructured transition metal oxides for aqueous hybrid electrochemical supercapacitors. Appl Phys A 82:599–606
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sour 196:1–12
Wang H, Lin J, Shen ZX (2016) Polyaniline (PANI) based electrode materials for energy storage and conversion. J Sci Adv Mater Devices 1:225–255
Deng W, Ji X, Chen Q, Banks CE (2011) Electrochemical capacitors utilizing transition metal oxides: an update of recent developments. RSC Adv 1:1171–1178
Mastragostino M, Arbizzani C, Soavi F (2002) Conducting polymers as electrode materials in supercapacitors. Solid State Ion 148:493–498
Moussa M, El-Kady MF, Zhao Z, Majewski P, Ma J (2016) Recent progress and performance evaluation for polyaniline/graphene nanocomposites as supercapacitor electrodes. Nanotechnology 27:442001
Chithambararaj A, Bose AC (2011) Investigation on structural, thermal, optical and sensing properties of meta-stable hexagonal MoO3 nanocrystals of one-dimensional structure. Beilstein J Nanotechnol 2:585
Maheswari N, Muralidharan G (2017) Controlled synthesis of nanostructured molybdenum oxide electrodes for high-performance supercapacitor devices. Appl Surf Sci 416:461–469
Wu D, Shen R, Yang R, Ji W, Jiang M, Ding W, Peng L (2017) Mixed molybdenum oxides with superior performances as an advanced anode material for lithium-ion batteries. Sci Rep 7:44697
Chiang TH, Ho PY, Chiu SY, Chao AC (2015) Synthesis, characterization and photocatalytic activity of α–MoO3 particles utilizing different polyol monomers under visible light irradiation. J Alloy Compd 651:106–113
Yao B, Huang L, Zhang J, Gao X, Wu J, Cheng Y, Zhou J (2016) Flexible transparent molybdenum trioxide nanopaper for energy storage. Adv Mater 28:6353–6358
Kim WS, Kim HC, Hong SH (2010) Gas sensing properties of MoO3 nanoparticles synthesized by solvothermal method. J Nanoparticle Res 12:1889–1896
Khandare L, Terdale SS, Late DJ (2016) Ultra-fast α-MoO3 nanorod-based humidity sensor. Adv Device Mater 2:15–22
Zilberberg K, Gharbi H, Behrendt A, Trost S, Riedl T (2012) Low-temperature, solution-processed MoOx for efficient and stable organic solar cells. ACS Appl Mater Interfaces 4:1164–1168
Zhou E, Wang C, Zhao Q, Li Z, Shao M, Deng X, Xu X (2016) Facile synthesis of MoO2 nanoparticles as high-performance supercapacitor electrodes and photocatalysts. Ceram Int 42:2198–2203
Nagabhushana GP, Samrat D, Chandrappa GT (2014) α-MoO3 nanoparticles: solution combustion synthesis, photocatalytic and electrochemical properties. RSC Adv 4:56784–56790
Parviz D, Kazemeini M, Rashidi AM, Jozani KJ (2010) Synthesis and characterization of MoO3 nanostructures by solution combustion method employing morphology and size control. J Nanoparticle Res 12:1509–1521
Dong W, Dunn B (1998) Sol–gel synthesis and characterization of molybdenum oxide gels. J Noncryst Solids 225:135–140
Manteghain M, Tari F, Bozorgi B (2015) Microwave-assisted synthesis of molybdenum oxide nanoparticles. J Particle Sci Technol 1:121–127
Fang L, Shu Y, Wang A, Zhang T (2007) Green synthesis and characterization of anisotropic uniform single-crystal α-MoO3 nanostructures. J Phys Chem C 111:2401–2408
Sen UK, Mitra S (2014) Synthesis of molybdenum oxides and their electrochemical properties against Li. Energy Proc 54:740–747
Wang S, Zhang Y, Ma X, Wang W, Li X, Zhang Z, Qian Y (2005) Hydrothermal route to single-crystalline α-MoO3 nanobelts and hierarchical structures. Solid State Commun 136:283–287
Li G, Jiang L, Pang S, Peng H, Zhang Z (2006) Molybdenum trioxide nanostructures: the evolution from helical nanosheets to cross like nanoflowers to nanobelts. J Phys Chem B 110:24472–24475
Mai L, Yang F, ZhaoY X, Xu L, Hu B, Liu H (2011) Molybdenum oxide nanowires: synthesis & properties. Mater Today 14:346–353
Muthamizh S, Suresh R, Giribabu K, Manigandan R, Kumar SP, Munusamy S, Narayanan V (2015) Molybdenum oxide nanocubes: synthesis and characterizations. AIP Conf Proc 1665:050155
Bai S, Chen S, Tian Y, Luo R, Li D, Chen A (2012) Hydrothermal synthesis of α-MoO3 nanorods for NO2 detection. Int J Nanosci 11:1240044
Miao F, Wu W, Li Q, Miao R, Tao B (2017) Fabrication and application of molybdenum trioxide nanostructured materials for electrochemical capacitors. Int J Electrochem Sci 12:12060–12073
Wang Y, Zhu Y, **ng Z, Qian Y (2013) Hydrothermal synthesis of α-MoO3 and the influence of later heat treatment on its electrochemical properties. Int J Electrochem Sci 8:9851–9857
Shakir I, Shahid M, Rana UA, Warsi MF (2014) In situ hydrogenation of molybdenum oxide nanowires for enhanced supercapacitors. RSC Adv 4:8741–8745
Lou XW, Zeng HC (2002) Hydrothermal synthesis of α-MoO3 nanorods via acidification of ammonium heptamolybdate tetrahydrate. Chem Mater 14(11):4781–4789
Toyoda T, Nakanishi H, Endo S, Irie T (1985) Fundamental absorption edge in the semiconductor CdInGaS4 at high temperatures. J Phys D Appl Phys 18(4):747
**a T, Li Q, Liu X, Meng J, Cao X (2006) Morphology-controllable synthesis and characterization of single-crystal molybdenum trioxide. J Phys Chem B 110(5):2006–2012
Cheng L, Shao M, Wang X, Hu H (2009) Single-crystalline molybdenum trioxide nanoribbons: photocatalytic, photoconductive, and electrochemical properties. Chem Eur J 15(10):2310–2316
Klinbumrung A, Thongtem T, Thongtem S (2012) Characterization of orthorhombic α-MoO3 microplates produced by a microwave plasma process. J Nanomater 2012:10
Huang L, Gao X, Dong Q, Hu Z, **ao X, Li T, Ling Z (2015) HxMoO3 − y nanobelts with seawater as electrolyte for high-performance pseudocapacitors and desalination devices. J Mater Chem A 3(33):17217–17223
Vattikuti SP, Nagajyothi PC, Reddy PAK, Kumar MK, Shim J, Byon C (2018) Tiny MoO3 nanocrystals self-assembled on folded molybdenum disulfide nanosheets via a hydrothermal method for supercapacitor. Mater Res Lett 6(8):432–441
Khalate SA, Kate RS, Pathan HM, Deokate RJ (2017) Structural and electrochemical properties of spray deposited molybdenum trioxide (αMoO3) thin films. J Solid State Electrochem 21:2737–2746
Icaza JC, Guduru RK (2017) Characterization of α-MoO3 anode aqueous beryllium sulfate for supercapacitors. J Alloy Compd 726:453–459
Prakash NG, Dhananjaya M, Narayana AL, Shaik DP, Rosaiah P, Hussain OM (2018) High performance one dimensional α-MoO3 nanorods for supercapacitor applications. Ceram Int 44(8):9967–9975
Acknowledgements
The author would like to express his appreciation to Centre for Nano Science and Technology, Institute of Science and Technology, Jawaharlal Nehru Technological University Hyderabad and Nanospan India Pvt. Ltd. for the facilities in their laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reddy, R.K.K., Kailasa, S., Rani, B.G. et al. Hydrothermal approached 1-D molybdenum oxide nanostructures for high-performance supercapacitor application. SN Appl. Sci. 1, 1365 (2019). https://doi.org/10.1007/s42452-019-1295-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1295-5