Abstract
Well-defined atomically dispersed metal catalysts (or single-atom catalysts) have been widely studied to fundamentally understand their catalytic mechanisms, improve the catalytic efficiency, increase the abundance of active components, enhance the catalyst utilization, and develop cost-effective catalysts to effectively reduce the usage of noble metals. Such single-atom catalysts have relatively higher selectivity and catalytic activity with maximum atom utilization due to their unique characteristics of high metal dispersion and a low-coordination environment. However, freestanding single atoms are thermodynamically unstable, such that during synthesis and catalytic reactions, they inevitably tend to agglomerate to reduce the system energy associated with their large surface areas. Therefore, develo** innovative strategies to stabilize single-atom catalysts, including mass-separated soft landing, one-pot pyrolysis, co-precipitation, impregnation, atomic layer deposition, and organometallic complexation, is critically needed. Many types of supporting materials, including polymers, have been commonly used to stabilize single atoms in these fabrication techniques. Herein, we review the stabilization strategies of single-atom catalyst, including different synthesis methods, specific metals and carriers, specific catalytic reactions, and their advantages and disadvantages. In particular, this review focuses on the application of polymers in the synthesis and stabilization of single-atom catalysts, including their functions as carriers for metal single atoms, synthetic templates, encapsulation agents, and protection agents during the fabrication process. The technical challenges that are currently faced by single-atom catalysts are summarized, and perspectives related to future research directions including catalytic mechanisms, enhancement of the catalyst loading content, and large-scale implementation are proposed to realize their practical applications.
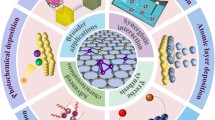
Graphical Abstract
Single-atom catalysts are characterized by high metal dispersibility, weak coordination environments, high catalytic activity and selectivity, and the highest atom utilization. However, due to the free energy of the large surface area, individual atoms are usually unstable and are prone to agglomeration during synthesis and catalytic reactions. Therefore, researchers have developed innovative strategies, such as soft sedimentation, one-pot pyrolysis, coprecipitation, impregnation, step reduction, atomic layer precipitation, and organometallic complexation, to stabilize single-atom catalysts in practical applications. This article summarizes the stabilization strategies for single-atom catalysts from the aspects of their synthesis methods, metal and support types, catalytic reaction types, and its advantages and disadvantages. The focus is on the application of polymers in the preparation and stabilization of single-atom catalysts, including metal single-atom carriers, synthetic templates, encapsulation agents, and the role of polymers as protection agents in the manufacturing process. The main feature of polymers and polymer-derived materials is that they usually contain abundant heteroatoms, such as N, that possess lone-pair electrons. These lone-pair electrons can anchor the single metal atom through strong coordination interactions. The coordination environment of the lone-pair electrons can facilitate the formation of single-atom catalysts because they can enlarge the average distance of a single precursor adsorbed on the polymer matrix. Polymers with nitrogen groups are favorable candidates for dispersing active single atoms by weakening the tendency of metal aggregation and redistributing the charge densities around single atoms to enhance the catalytic performance. This review provides a summary and analysis of the current technical challenges faced by single-atom catalysts and future research directions, such as the catalytic mechanism of single-atom catalysts, sufficiently high loading, and large-scale implementation.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The decline in available fossil fuels and the environmental pollution problems associated with their consumption have been considered as majors challenges to the sustainable development of human society [1, 2]. To mitigate these issues, many strategies have been explored, such as exploring clean and sustainable energy sources including solar, sea-wave, geothermal, wind, and hydropower energy to replace fossil fuels [3,4,5] and then develo** corresponding electrochemical energy technologies, such as batteries, supercapacitors, and brine electrolysis, to store the intermittent electrical energy generated from these sustainable sources [6,7,8,9,10,11]. Although electrochemical energy technologies have been proved to be efficient and practical, further improvement in their energy and power densities and lifetime is still in high demand for many applications such as electric vehicles [12]. To improve the performance of electrochemical energy devices, the pressing need is to develop high-performance electrode and electrolyte materials [13].
An electrode is always composed of an outstanding electrocatalytically stable and active electrocatalyst supported on an electrically conductive current collector. For example, in a low-temperature fuel cell or a metal–air battery, the cathode is normally coated with carbon-supported platinum (Pt) to catalyze the oxygen reduction reaction (ORR) [14,15,16,17,18]. This Pt-based electrocatalyst can accelerate the reduction from oxygen (O2) to water (H2O) (4-electron reduction pathway) and/or from oxygen to hydrogen peroxide (2-electron reduction pathway) in aqueous solutions [19,20,21]. The elemental steps of the ORR are usually presented in the following equations (where species with * denotes that this species is adsorbed to a catalyst active site) [22, 23].
Four-electron pathway:
Two-electron pathway:
Different electrocatalysts have been employed in electrochemical energy devices to catalyze the ORR, carbon dioxide reduction reaction (CO2RR), nitrogen reduction reaction (NRR), oxygen evolution reaction (OER), hydrogen oxidation reaction (HOR), and hydrogen evolution reaction (HER) [24,25,26,27,28,29,30,31,32,33,34,35,36]. The catalytic activity and stability of an electrocatalyst are strongly dependent on its morphology, structure, composition, conductivity, particle size, and specific surface area [92] also separated and encapsulated the metal precursor Fe(acac)3 using zeolitic imidazolate frameworks (ZIFs) as molecular-scale cages. The isolated single iron atoms were coordinated with four neighboring nitrogen atoms due to reduction of Fe(acac)3 by carbonization of the organic linker. Similarly, Ji et al. [93] separated precursors using ZIFs. In the synthesis, Ru3(CO)12 and precursors of ZIF-8 were mixed to encapsulate Ru3(CO)12 in ZIF-8 during ZIF-8 crystallization. Ru3 clusters were obtained after heat treatment. These clusters are uniformly stabilized through coordination with nitrogen species.
In addition, polymers can be used as surfactants to regulate the crystallization of the target catalyst. For example, He et al. [94] developed a surfactant-assisted MOF approach. An atomically dispersed Co-doped carbon was fabricated with a core–shell structure. The confinement effect mitigated the collapse of the internal microporous structures of ZIF-8 by suppressing the agglomeration of Co atomic sites during thermal activation. This unique confinement effect is attributed to the cohesive interactions of Co-doped ZIF-8 nanocrystals and selected surfactants. The hydrophilic groups in the surfactants coordinate with Zn2+ and Co2+ sites on the Co-ZIF-8 polyhedron surface. Because of the coordination effect, the crystal growth rate was slowed, and the crystal size, as well as the morphology of Co-ZIF-8 crystals, was controlled [95, 96].
Polymers containing nitrogen groups are favorable candidates for dispersing active single atoms without aggregation and influencing the charge distribution to improve the performance of catalysts. Heteroatoms with lone electron pairs, such as N, are usually abundant in polymers or their derivatives. These heteroatoms can anchor single metal atoms via strong coordination interactions. The lone electron pair coordination environment can enlarge the average adsorption distance between the single metal precursors and polymer matrix, effectively preventing agglomeration behavior during pyrolysis and favorably forming single-atom catalysts. In this regard, polymers with nitrogen-containing groups are widely used to prepare single-atom catalysts [97].
The research progress in metal single-atom catalysis has been summarized by several review papers, focusing on insights into the development of single-atom catalysts [98,99,100,101,102]. The main arguments focus on synthetic procedures, characterizations, reaction mechanisms, precise control of catalyst surface metal atomic structures, structure–performance relationships, applications, and challenges. In this review, the stabilization strategies for single-atom catalysts are reviewed in terms of synthesis methods along with a detailed analysis of their advantages and disadvantages. In particular, the application of polymers in the fabrication and stabilization of single-atom catalysts is discussed based on their functions as synthetic templates, carriers for metal single atoms, encapsulation agents, and protection agents during fabrication or catalysis. The employment of polymers with nitrogen-containing groups is emphasized regarding their specific functions in the capture of single atoms during fabrication and improving the conductivity of carbon-based carriers [103]. Finally, this review points out current challenges and potential applications in the field of monatomic catalysts, and future research directions are provided for the development of single-atom catalysts.
2 Single-Atom Catalysts
Single-atom catalysts, in which only isolated single-atom active centers are dispersed on support materials, are a new category of heterogeneous catalysts [78, 79, 104]. Single-atom catalysts have the properties of homogeneous catalysts, i.e., single active centers, but with easy separation characteristics. Therefore, single-atom catalysis represents a special type that bridges both heterogeneous and homogeneous catalysis [105, 106]. In general, single-atom catalysts are different from other forms of nanocatalysts (such as metal clusters) based on their novel metal–carrier interaction due to the absence of metal–metal interactions in the system, an unsaturated coordination environment, a high surface free energy, and quantum size effects [104]. Owing to the strong coupling between carriers and metal atoms, they have higher inherent catalytic selectivity and activity than conventional catalysts for the OER, ORR, and other reactions [107,108,109,110]. In this aspect, research on single-atom catalysts has progressed from monometallic single-atom catalysts [111,112,113,114,115,116] (Pt [117,118,119,120,121,122,123,124,125,126,127,128,129,130], Rh [169] (Au–Pd alloy [170], Cu–Pd alloy [171]), and nonmetallic single-atom catalysts (Si [172]). The involvement of secondary metal atoms can affect the steric and electronic environments of the primary metal atoms, which can separate the latter atoms and enhance the catalytic performance by synergistic action [172].
Although single-atom catalysts have great potential in practical applications in the ORR, OER, HER, and CO2RR, their developments are challenged by low single-atom loading, poor chemical/thermal stability, and the limitations of catalytic reactions, which require further development of novel fabrication methods, characterization techniques, and stabilization strategies. Normally, (1) the metal–metal interaction is stronger than the metal–carrier interaction in single metal catalyst, and individual atoms can move and combine easily with each other to form metal clusters and nanoparticles under practical reaction conditions [173]; (2) single atoms can also drive self-agglomeration and cluster formation because of the high surface free energy in the fabrication environments and during catalytic cycling, both of which can lead to catalyst deactivation. Therefore, the stabilization of single atoms represents the key task to achieve highly dispersed single-atom catalysts.
The core factor in stabilizing single-atom catalyst active species is to establish strong interactions between the single atoms and their carriers [174,175,176]. Such a strong interaction plays a dominant role: preventing isolated metal atoms from aggregating and producing single-atom metal catalysts with stability and good dispersity [80]. As identified, the interaction comes from the redistribution of electrons between the single atom and the carrier, which has a vital impact on the electronic structure and catalytic ability of the atom. Several stabilization techniques have been developed to enhance this interaction, thereby fabricating single-atom catalysts effectively and conveniently [177].
In most synthetic methods, polymers play important roles as carrier precursors, microstructure templates, inhibitors to limit the size of metallic particles, and protective agents for single-atom catalysts [178]. As recognized, the use of polymers as carriers is the most common approach because the structures and morphologies based on polymers can be adjusted through chemical or physical methods to achieve a high porosity, provide a high surface area, and impart chemical stability. Another critical function of polymer carriers is to anchor single atoms on their surface through coordination with metal atoms via their abundant functional groups. Lyu et al. [179] synthesized a single-atom catalyst (Fe–N–C) in which Fe single atoms were loaded on nitrogen-doped carbon through a Schiff base condensation reaction, as shown in Fig. 2a. This catalyst features a nitrogen-doped graphitic carbon matrix with a well-defined spherical morphology. The ultrahigh surface area of this matrix is 1 796 m2 g−1, which is decorated with single Fe atoms coordinated with nitrogen. The images of Cs-corrected HAADF-STEM (Fig. 2b, c) show nanospheres with a hollow structure decorated with scattered large bright spots, which represent homogeneously distributed Fe single atoms. Figure 2d presents the high-resolution image, which demonstrates that the spherical shell is graphite-type carbon, indicating that the carbon sphere is covered with few-layer graphene. In this case, the Fe-coordinated bis(imino)-pyridine ligand acts as both a carrier during synthesis and a precursor of nitrogen-doped carbon.
a Preparation of the Fe–N–C catalyst. b, c HAADF-STEM images of the Fe–N–C catalyst at different magnifications. d TEM images of the Fe–N–C catalyst. Reprinted with permission from Ref. [179]. Copyright © 2018, Elsevier Inc.
3 Stabilization of Single-Atom Catalysts
As identified, single-atom catalysts face the challenges of large-scale production and low stability in reactions [180,181,182,183,184,185]. Appropriate synthetic strategies are crucial for the stabilization of isolated single metal atoms to fabricate high-quality single-atom catalysts [186,187,211]. Cui et al. [212] prepared a nitrogen-doped carbon-carried Zn single-atom catalyst by a one-pot pyrolysis method, and the metal loading reached 1.56 wt.%. The authors believed that this catalyst represents the first Zn catalyst without Zn nanoparticles or clusters. Zhao et al. [213] successfully synthesized several Ni single-atom catalysts, of which the Ni loading reached 20.3 wt.%, supported on nitrogen-doped carbon nanotubes (N-CNTs), called NiSA-N-CNT. In particular, instead of being confined between the carbon layers, the single Ni atoms were combined with the layered carbon. Figure 5a shows a schematic diagram of single Ni atoms on CNTs formed by one-step pyrolysis, after which the layered Ni-g-C3N4 rolls up, forming a CNT structure. The length of bamboo-like CNTs can reach 5 μm, and their average diameters are 20–50 nm (Fig. 5b). The uniform distribution of Ni and N by elemental map** reveals that this catalyst has a typical tubular structure. Nanoparticles were not observed in this catalyst. The TOF of NiSA-N-CNT, 11.7 s−1, was higher than that of Ni nanoparticles (Ni–N–CNT) in the CO2RR at 0.55 V. These data demonstrate that single Ni atoms have high intrinsic catalytic activity (Fig. 5c). This TOF value was also better than those of Ni single-atom catalysts reported recently for the CO2RR, with TOF values of 1.46 and 4.11 s−1 [148, 214]. The higher TOF may be attributed to the fine-tuned coordination environment. The onset potentials of the NiSA-N-CNT catalyst for OER and ORR are 25 and 30 mV, respectively, which are both lower than that of Ni–N–CNT in alkaline solution (Fig. 5d). The activities of NiSA-N-CNT for the OER and ORR are lower than those of Ni–N–CNT, probably because the chemical and electronic environments of Ni single atoms and Ni nanoparticles are significantly different.
a The preparation of NiSA-N-CNT with a tubular structure. b Microscopy images of NiSA-N-CNT. c Faradaic efficiency and jCO at different applied potentials of Ni–N–CNT and NiSA-N-CNT-T for the CO2RR. d Linear sweep voltammetry curves of Ni–N–CNTs and NiSA-N-CNT-T for the OER and ORR. Reprinted with permission from Ref. [213]. Copyright © 2018, American Chemical Society. e Microscopy images of FeSA-G for the oxygen reduction reaction. f Linear sweep voltammetry curves of FeSA-G and Pt/C in acidic solution. g Electron transfer number and yield of H2O2 for the ORR. h Tafel slopes of FeSA-G and Pt/C for the ORR. i Linear sweep voltammetry curves of FeSA-G and Pt/C in acidic solution, which are compared with those of these catalysts after 5 000 cycles. Reprinted with permission from Ref. [219]. Copyright © 2019, WILEY–VCH
As identified, Fe single atoms easily agglomerate during the fabrication process, which limits the loading of Fe single atoms to within 1.9 wt.%–2.5 wt.% [215,216,217,218]. Cheng et al. [219] synthesized an Fe single-atom catalyst anchored on nitrogen-doped graphene (FeSA-G) by one-pot pyrolysis, where the loading of Fe atoms was 7.7 wt.%. Microscopy images (Fig. 5e) show the 2D graphene structure, which is formed by a porcine hemin precursor. HR-TEM images show only Fe single atoms on the graphene carriers. STEM with energy-dispersive spectroscopy (STEM-EDS) reveals that the N and Fe atoms are dispersed in the graphene sheets, with N and Fe distributed homogeneously. The AC-STEM results further confirm that Fe atoms are also atomically dispersed, even though Fe atoms have a high loading. Figure 5f–i shows that the half-wave potential and onset potential of FeSA-G for the ORR in an acid electrolyte are 0.804 and 0.950 V, respectively. This catalyst is stable, and compared to the Pt/C catalyst, it has much higher phosphate anion tolerance and the same catalytic performance. The stability test was carried out at 230 °C, and the results indicated that the FeSA-G cathode imparts the battery with stabler performance than the Pt/C cathode.
To fabricate single-atom catalysts by the one-pot pyrolysis technique, metal complexes are often used as precursors, and polymer monomers are employed as carrier precursors. The results all prove that the one-pot pyrolysis method is suitable for fabricating single-atom catalysts with high activity and loading. The addition of polymers as part of the synthesis precursors can significantly overcome the shortcomings of a low loading of single atoms. Notably, the high loading may be attributed to the metals doped in the carrier matrix during pyrolysis.
3.4 Co-precipitation Method
The co-precipitation method can be used to prepare supported metal catalysts with uniform dispersions of several active elements simultaneously. For example, two or more types of metal cations dissolved in precipitating agents can generate a precipitation reaction to achieve the molecular-level distribution of different elements through a co-precipitation process. The characteristics of catalysts fabricated by co-precipitation depend on many factors, including the pH of the base solution, aging time, temperature, effective mixing, order and speed of component solution addition, and the number of defects and distribution on the substrate.
To study the structural features of Pt species, Derevyannikova et al. [220] prepared a series of Pt/CeO2 catalysts with different Pt loadings from 1 wt.% to 30 wt.% by the co-precipitation method, and the high loading was attributed to the formation of nanoclusters. Their catalysts were either single atoms or PtOx clusters trapped by CeO2 defects and/or epitaxially attached on the CeO2 surface. Figure 6a displays TEM images of Pt/CeO2 (15 wt.%) and Pt/CeO2 (30 wt.%) samples calcined at 450 °C. In Pt/CeO2 (15 wt.%), both Pt clusters and their single atoms are distinctly observed in carrier defects and on the surface. In Pt/CeO2 (30 wt.%), only Pt clusters are observed. The reactivity of oxygen species of Pt–CeO2 was analyzed by temperature-programmed reduction (TPR) analysis. Figure 6b illustrates hydrogen consumption during TPR analysis. The hydrogen consumption was triggered at temperatures below 150 °C for all Pt–CeO2 samples. The reduction in Pt-containing species is responsible for the hydrogen consumption below 350 °C. Pt/CeO2 (1 wt.%) has a broad reduction peak at approximately 125 °C, which comes from the reduction of Pt single-atom ions. The increase in Pt loading promotes low-temperature reduction. Pt/CeO2 (5 wt.%) has two peaks at 15 and 45 °C, which correspond to the presence of nanoclusters and single atoms, respectively. The presence of Pt0 species accelerates the low-temperature reduction of Pt ionic species in Pt/CeO2 catalysts. The reduced species in Pt/CeO2 (15 wt.%) and Pt/CeO2 (30 wt.%) induce the exothermic and fast reduction of PtOx clusters, and the single-atom signals merge into that of the dominant cluster at 27 and 6 °C. Jan et al. [221] also successfully synthesized a Pt/CeO2 single-atom catalyst by a co-precipitation method. It was observed that the strong Pt–O–Ce bond in this single metal catalyst could yield sinter-resistant thermal stability. As presented in Fig. 6c, the STEM micrograph of the Pt/CeO2 composite shows that Pt nanoparticles are absent, while Pt is adsorbed on the ceria nanoparticles, forming well-dispersed single atoms. This catalyst can achieve a complete conversion for the CO shift reaction at 200 °C, as shown in Fig. 6d. The catalytic performance of the Pt single-atom catalyst is better than that of pure CeO2 and the other samples. This great performance may be attributed to two points: (1) the interaction between Pt and its nearby highly reducible O atom of CeO2 and (2) the preference of CO to adsorb on Pt rather than on the CeO2 free surface.
a HR-TEM images of the Pt/CeO2 (15 and 30 wt.%) samples calcined at 450 °C. b TPR-H2 profiles of the Pt–CeO2 catalysts heated at 450 °C, vol.% means volume percentage. Reprinted with permission from Ref. [220]. Copyright © 2018, American Chemical Society. c Schematic of the state of Pt nanoparticles after heat treatment at different temperatures. d STEM images of Pt/CeO2 showing Pt single atoms adsorbed on the ceria particles. e The conversion of CO oxidation, which is catalyzed by CeO2-base species reduced at different temperatures. Reprinted with permission from Ref. [221]. Copyright © 2019, The Royal Society of Chemistry. f Schematic representation of the synthesis mechanism of the reduced Cu-SiO2 catalyst. g HR-TEM images of the electron beam irradiation-aided Cu/SiO2 reduction. Reprinted with permission from Ref. [222]. Copyright © 2017, Elsevier Inc
To produce SiO2-supported metal single atoms with high loading in an atomically dispersed manner, Zhu et al. [222] anchored 15 wt.% atomically dispersed Cu on SiO2 through the formation of Cu–O–Si covalent bonds between Cu2+ and silanol (Si–OH) groups on SiO2 using a urea hydrolysis-assisted co-precipitation method, as illustrated in Fig. 6e. The TOF of the Cu/SiO2 catalyst is 127.8 h−1 for the low-temperature hydrogenation of 5-hydroxymethylfurfural (HMF). The HR-TEM images in Fig. 6f reveal that the reduced SiO2 traps the atomically dispersed Cu with the aid of electron beam exposure. The reduced Cu/SiO2 exhibits an ordered hexagonal mesoporous structure, and Cu nanoparticles cannot be detected directly (initial in Fig. 6g). The electron beam irradiation tests indicate that exposure to irradiation destroys the ordered mesoporous structure, forming a condensed matrix when the exposure time reaches 300 s. Cu atoms do not show obvious change or agglomeration. Highly dispersed Cu nanoparticles can be observed when the irradiation time is prolonged to 960 s. The tests show a stable coordination environment and high chemical bonding energy of Cu atoms in the catalysts.
In summary, co-precipitation is one of the most common methods widely applied to prepare single-atom catalysts because of its relatively simple procedure and high catalytic performance of the product. However, this method still has some disadvantages. For example, a highly diluted solution of the target metal must be used to avoid the agglomeration of individual atoms. In addition, at the interface area of the carrier, some metal atoms are anchored during co-precipitation. This is the reason why the metal loadings are apparently higher as observed with this method. These hidden metal single atoms do not participate in catalytic reactions because of the absence of effective contact.
3.5 Impregnation Method
For heterogeneous catalysts, one of the classic preparation methods is the impregnation method [97, 223]. In a typical synthesis procedure, the carrier is impregnated in a solution containing an active component (metal salt). The active component is gradually adsorbed on the carrier surface or restricted to the pore structure after the carrier contacts the solution. Then, the solution is filtered or dried to remove the excess solution. The obtained solid is dried, calcined, and activated to obtain the target catalyst. The metal dispersion depends on the characteristics of the anchoring sites, which are derived from precursor–carrier interactions.
To produce methyl acetate in a fixed-bed reactor with highly selective and high-yielding catalysts, Feng et al. [224] used the impregnation method to synthesize an Ir and La combined catalyst loaded on coconut shell activated carbon (AC) particles, i.e., Ir–La/AC (Ir loading of 1 wt.%). The precursors IrCl3 and La2O3 were dissolved in an aqueous mixture of hydrochloric acid (12% HCl) and AC of 20–40 mesh. HR-TEM images (Fig. 7a–c) show that nanoparticles with sizes of approximately 1–2 nm are uniformly dispersed in the fresh Ir–La/AC catalyst. The nanoparticles are not distinguishable after the catalytic reaction, except for the AC texture, indicating that the nanoparticles were dispersed on the carbon carrier during the reaction. Well-distributed Ir and La atoms on the AC surface can be observed in the HAADF-STEM image (Fig. 7d), suggesting that the active sites of the Ir–La/AC catalyst are in single-atom states. For heterogeneous methanol carbonylation, the space–time yield is 8 200 g kgcat−1 h−1 with the Ir–La/AC catalyst. This process can achieve a relatively high TOF, 2 200 h−1, with over 90% selectivity for methyl acetate. The catalyst shows high stability, as shown in Fig. 7e. For the catalytic reaction, the promoter La can stabilize the single atom of this catalyst, and the active sites are single Ir atoms. The possible mechanism is shown in Fig. 7f.
a HR-TEM images of the fresh Ir–La/AC catalyst, b corresponding particle size distribution, c spent Ir–La/AC catalyst, and d spent Ir–La/AC catalyst (the atom size in the red-dotted region is larger than that in the green-dotted region; red dots represent Ir atoms, and green dots represent La atoms). e Test of Ir/AC and Ir–La/AC for heterogeneous methanol carbonylation. f The mechanism of methanol carbonylation by the Ir–La/AC catalyst. Reprinted with permission from Ref. [224]. Copyright © 2019, American Chemical Society. HR-TEM images of g 0.3 wt.% Rh/ZrO2 and h 2 wt.% Rh/ZrO2. i The results of direct methane oxidation. The results of the recyclability test performed with the 0.3 wt.% Rh/ZrO2 catalyst. Yield of methane conversion to ethane. Reprinted with permission from Ref. [227]. Copyright © 2017, American Chemical Society
Although the impregnation method has become indispensable for preparing single-atom catalysts, it still faces the unavoidable disadvantage of low metal loading [106, 225, 226]. To overcome this challenge, Kwon et al. [227] prepared single-atom Rh catalysts (Rh/ZrO2) with loadings of 0.3 wt.% and 2 wt.%, respectively. The HR-TEM images indicate that Rh single atoms are the active species in 0.3 wt.% Rh/ZrO2 and that nanosized Rh clusters are the active materials in 2 wt.% Rh/ZrO2, as shown in Fig. 7g, h. Different coordination environments induce different catalytic performance. For example, for direct methane conversion, CO2 is produced only by Rh nanoparticles, while methanol or ethane is produced in the aqueous phase or gas phase by the single-atom Rh catalyst, as shown in Fig. 7i. For the stability test, the single-atom Rh catalysts were collected by centrifugation and washed with deionized water after one batch of reactions. The yield of the oxygenated products was quite similar even after five reaction–collection cycles. Methyl alcohol was not observed when the centrifuged supernatant without the solid catalyst was used under the same reaction conditions. Single-atomic Rh has been confirmed as an effective catalyst for the conversion of methane to methanol. Hai et al. [232]. Copyright © 2019, Wiley–VCH
The organometallic complex approach can prepare site-isolated metal single-atom catalysts by utilizing a molecular complex with a uniform structure. This method can accurately control the structure of the anchored complex and the number of metal atoms. A suitable ligand and strong interaction between the ligand and functional group are essential factors in maintaining the original properties of the molecular complex. For example, Liu et al. [231] used an organometallic complex (PhPCP)Pt–OH to prepare a Pt single-atom catalyst through the procedure shown in Fig. 8d. HR-TEM images reveal that 70% of the Pt species are single atoms, and no nanoparticles are observed, as shown in Fig. 8e. Figure 8f shows the CO adsorption/diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) results. For these Pt catalysts, the peak of single atoms is intenser than the peak of nanoparticles. In particular, the overall IR signal intensities of the overcoated samples are lower than those of the other samples due to the overcoats limiting accessibility to a portion of the Pt species. The metal species have comparable chemical environments in the bare and coated samples judging from their similar CO adsorption peak positions.
Sun et al. [232] encapsulated single Rh atoms within zeolites using [Rh(NH2CH2CH2NH2)3]Cl3 as a precursor accompanied by a ligand-protected direct hydrogen reduction, as shown in Fig. 8g. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) verifies that the Rh loading is 0.28 wt.% in all the samples. Scanning transmission electron microscopy observation and extended X-ray absorption analysis indicate that every individual Rh atom is trapped in the 5-membered ring and that the oxygen atom of the zeolite skeleton can stabilize these atoms. As shown in Fig. 8h, a few atomically dispersed Rh atoms can be observed in some regions of the zeolite framework by Cs-corrected STEM. Elemental map** analysis of the Rh catalyst further confirms the homogeneous distribution of Rh single atoms throughout the zeolite crystals (Fig. 8i). As shown in Fig. 8j, the Rh catalyst exhibits a superior H2 generation rate for ammonia borane (AB). The TOF value of AB hydrolysis is 432 \({\text{mol}}_{{\text{H}}_2}\) molRh−1 min−1 at 298 K. Most significantly, for the tandem hydrogenation of nitroarenes, zeolite-encaged Rh single-atom catalysts show superior efficiency. The H2 generation rates can be improved by increasing the reaction temperature. The TOF value can reach 1 829 min−1 with Rh@S-1-H at 313 K, with a calculated apparent activation energy of 75.5 kJ mol−1 for the catalyzed reaction. Furthermore, Rh@S-1-H possesses excellent recycling stability because the size of Rh species, crystallinity of zeolites, and the H2 generation rate do not show obvious changes after five AB hydrolysis cycles. Nitrobenzene is converted to aniline with more than 99% selectivity by such a Rh catalyst. This catalyst also has selectivity for different nitroarenes with reliable recycling stability. The authors also researched AB hydrolysis under nitroarene hydrogenation conditions with the aid of Rh@S-1-H. As shown in Fig. 8j, the involvement of nitrobenzene decreases the volume of generated H2 to 66 mL. AB hydrolysis and nitroarene hydrogenation follow the tandem catalytic process because nitrobenzene reduction consumes part of the AB hydrolysis-generated H2. The involvement of 4-nitroxylene does not influence H2 evolution, suggesting that zeolites prevent the Rh species from contacting with 4-nitroxylene in the tandem reaction.
Some researchers have explored transition metal single-atom catalysts with large-scale synthesis through a universal ligand-mediated method. Yang et al. [233] used the coordination ability between metals and nitrogen to synthesize transition metal single-atom catalysts by complexing and coordinating metal cations with 1,10-phenanthroline hydrate. The synthesis procedure is shown in Fig. 9a. Metal atoms are atomically dispersed on the carbon carrier, which is confirmed by HAADF-STEM, as shown in Fig. 9b. Single atoms of Mn, Cr, Co, Fe, Ni, Zn, and Cu on the carbon substrate are observed based on the high density of bright dots in the images. For Ru and Pt noble metal single-atom catalysts, the results are similar. From 0.7 to 1.5 V versus RHE, a wide potential range, Ni single atoms display excellent CO2RR performance, with high Faradaic efficiency to CO (FECO) exceeding 90%, as indicated in Fig. 9c. The CO2RR performance of these single-metal catalysts (M-SACs), with similar metal loadings of 2.5 wt.%–5.3 wt.%, was tested at 1.2 V versus RHE. For the CO2RR, Ni-SAC displays the highest activity among these M-SACs, while the curve of FECO shows that the value of FECO is related to the metal atomic number (Fig. 9c). The FECO increases with the use of Cr to Ni. When using Ni to Zn, the FECO decreases abruptly.
a Schematic of M-SAC synthesis. b Microscopy images induced by different metals. c CO2RR performance. LSV of Ni-SAC. FECO of Ni-SAC at different potentials. Stability tests for Ni-SAC. XANES spectra before and after CO2RR tests with Ni-SAC. FECO of Ni-SAC with different Ni loadings. FECO of M-SACs. Reprinted with permission from Ref. [233]. Copyright © 2019, Nature Publishing Group
As discussed above, due to the involvement of organometallic ligands, the organometallic complex approach has the advantage of obtaining single-atom catalysts with high metal loading. To maintain the structural characteristics of the molecular complexes, a strong interaction on the carrier surface between the functional groups and ligands is needed. This method uses precise molecular complexes to prepare isolated active center catalysts, which are ideal model catalysts to study catalytic mechanisms, by precisely controlling the structure of single-atom catalysts and the position and number of metal atoms. The organic precursors can be designed and synthesized to obtain the target structure and the related performance, such as by introducing nitrogen- or oxygen-containing groups. For example, Gao et al. [234] immobilized single Co atoms by a simple thermal treatment approach for photocatalytic CO2 conversion on partially oxidized graphene nanosheets. The nanosheets offer C/O functional groups as rigid ligands to catch Co single atoms. Abdel et al. [235] anchored Cu single atoms on the oxygen atoms of –OH/–OH2 species on UiO-66 (Zr) to cover the defect sites. The researchers obtained a Cu/UiO-66 (Zr) single-atom catalyst with an atomic ratio of Cu/Zr6 = 0.8. Razmjooei et al. [236] designed a Fe-based nitrogenous carbon single-atom catalyst with high micro- and mesoporosity as well as a high active site density, where Fe could be easily trapped with two nitrogen atoms and four oxygen atoms of EDTA. All these examples of single-atom catalysts highlight their flexibility in fabrication and design with different active metals.
3.7 Freestanding Single Metals, Clusters, Nanoparticles, or Dopants in Carriers
The selectivity and catalytic activity are determined by the single-atom coordination environment, depending on the fabrication technique and the number of loaded single atoms. The mass-separated soft-landing method and the ALD method are typical surface modulation techniques used to fabricate single-atom catalysts. The deposited metals react with the carriers in a very limited range through electronic interaction with limited spatial mass transfer. The freestanding single atoms can be stabilized on the carrier surface, and the metal do** effect should be quite rare in the matrix. A high deposition amount generates metal clusters, nanoparticles, and even films. Both methods can reflect the real loading amount of the single atoms on a specific surface. The difference is that the chemical bonds between the single atoms and the carriers may promote a higher loading amount in the ALD method. It should be noted that the deposition surface in ALD can have any morphology, which may induce a higher loading amount in complex three-dimensional frameworks. The loading amount in a specific area should still be very limited by the mass-separated soft-landing method because the chemical bonds energy is just due to electron exchange and not strong lattice confinement. This kind of dangling-like bonds cannot compete with the high surface free energy-induced agglomeration once the loading amount reaches its limit.
The active metal states are complex in catalysts fabricated with the one-pot pyrolysis method, co-precipitation method, impregnation method, and organometallic complex approach because the precursors of active metals are mixed with carrier precursors. Metal precursors can be adsorbed by the surface of carriers or carrier precursors. Some other metal precursors may participate in the formation of carriers and become dopants in the carriers. This portion of the metal will increase the apparent loading of metals if it cannot be distinguished from the freestanding and agglomerated metals. Furthermore, the reaction conditions cannot be controlled as precisely as those in the mass-separated soft-landing method and the ALD method. The formation of freestanding single atoms, nanoclusters, nanoparticles, and doped metals may occur simultaneously during these chemical-reaction-intensive fabrication methods. In-depth analysis of the coordination environments of the metals in single-atom catalysts is necessary to explore the spatial distribution and electronic structure. Clarifying the contribution of the actual portion of single atoms will benefit the theoretical exploration of single-atom catalysis. Relatively higher loadings can be detected in catalysts fabricated by the co-precipitation method, the one-pot pyrolysis method and the impregnation method. However, the metal species include single atoms, clusters, nanoparticles, and even dopants.