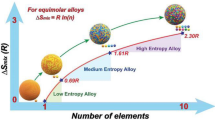
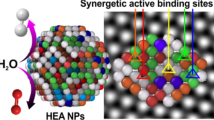
Abstract
High entropy alloys (HEAs), which can incorporate five or more constituents into a single phase stably, have received considerable attention in recent years. The composition/structure complexity and adjustability endow them with a huge design space to adjust electronic structure, geometric configuration as well as catalytic activity through constructing reaction active sites with optimal binding energies of different reaction intermediates. This paper reviews the recent progress on the preparation methods, characterization techniques, electrocatalytic applications and functional mechanisms of HEAs-based electrocatalysts for hydrogen evolution, oxygen evolution and oxygen reduction reactions. The synthesis approaches for HEAs from bottom-up (high-energy ball milling, cryo-milling, melt-spinning and dealloying) to top-down strategies (carbothermal shock, sputtering deposition and solvothermal) and the corresponding materials characterizations are discussed and analyzed. By summarizing and analyzing the electrocatalytic performance of HEAs for diverse electrocatalytic reactions in water electrolysis cells, metal-air batteries and fuel cells, the basic principle of their designs and the relevant mechanisms are discussed. The technical challenges and prospects of HEAs-based electrocatalysts are also summarized with the proposed further research directions. This review can provide a beneficial theoretical reserve and experimental guidance for develo** high performance electrocatalytic materials via the paradigm of high entropy.
Graphical Abstract




















Similar content being viewed by others
Data Availability
Yes.
References
Zheng, Y., Wang, J.C., Yu, B., et al.: A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): advanced materials and technology. Chem. Soc. Rev. 46, 1427–1463 (2017). https://doi.org/10.1039/c6cs00403b
Wu, H.M., Feng, C.Q., Zhang, L., et al.: Non-noble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochem. Energy Rev. 4, 473–507 (2021). https://doi.org/10.1007/s41918-020-00086-z
Haider, R., Wen, Y., Ma, Z.F., et al.: High temperature proton exchange membrane fuel cells: progress in advanced materials and key technologies. Chem. Soc. Rev. 50, 1138–1187 (2021). https://doi.org/10.1039/d0cs00296h
Yang, D., Tan, H.T., Rui, X.H., et al.: Electrode materials for rechargeable zinc-ion and zinc-air batteries: current status and future perspectives. Electrochem. Energy Rev. 2, 395–427 (2019). https://doi.org/10.1007/s41918-019-00035-5
Leow, W.R., Lum, Y., Ozden, A., et al.: Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020). https://doi.org/10.1126/science.aaz8459
Pan, J., Xu, Y.Y., Yang, H., et al.: Advanced architectures and relatives of air electrodes in Zn-air batteries. Adv. Sci. 5, 1700691 (2018). https://doi.org/10.1002/advs.201700691
Yang, D.J., Zhang, L.J., Yan, X.C., et al.: Recent progress in oxygen electrocatalysts for zinc-air batteries. Small Methods 1, 1700209 (2017). https://doi.org/10.1002/smtd.201700209
Zhang, L., Doyle-Davis, K., Sun, X.L.: Pt-based electrocatalysts with high atom utilization efficiency: from nanostructures to single atoms. Energy Environ. Sci. 12, 492–517 (2019). https://doi.org/10.1039/c8ee02939c
Wang, D., **n, H.L., Hovden, R., et al.: Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 12, 81–87 (2013). https://doi.org/10.1038/nmat3458
Huang, X.Q., Zhao, Z.P., Cao, L., et al.: High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015). https://doi.org/10.1126/science.aaa8765
Zhao, X., Chen, S., Fang, Z.C., et al.: Octahedral Pd@Pt1.8Ni core-shell nanocrystals with ultrathin PtNi alloy shells as active catalysts for oxygen reduction reaction. J. Am. Chem. Soc. 137, 2804–2807 (2015). https://doi.org/10.1021/ja511596c
Li, M., Zhao, Z., Cheng, T., et al.: Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016). https://doi.org/10.1126/science.aaf9050
Jiang, K.Z., Zhao, D.D., Guo, S.J., et al.: Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 3, e1601705 (2017). https://doi.org/10.1126/sciadv.1601705
Chong, L., Wen, J., Kubal, J., et al.: Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018). https://doi.org/10.1126/science.aau0630
Tian, X., Zhao, X., Su, Y.Q., et al.: Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 366, 850–856 (2019). https://doi.org/10.1126/science.aaw7493
Escudero-Escribano, M., Malacrida, P., Hansen, M.H., et al.: Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016). https://doi.org/10.1126/science.aad8892
Choi, S.I., Lee, S.U., Kim, W.Y., et al.: Composition-controlled PtCo alloy nanocubes with tuned electrocatalytic activity for oxygen reduction. ACS Appl. Mater. Interfaces 4, 6228–6234 (2012). https://doi.org/10.1021/am301824w
Zhang, C., Hwang, S.Y., Trout, A., et al.: Solid-state chemistry-enabled scalable production of octahedral Pt-Ni alloy electrocatalyst for oxygen reduction reaction. J Am. Chem. Soc. 136, 7805–7808 (2014). https://doi.org/10.1021/ja501293x
Oezaslan, M., Hasché, F., Strasser, P.: PtCu3, PtCu and Pt3Cu alloy nanoparticle electrocatalysts for oxygen reduction reaction in alkaline and acidic media. J. Electrochem. Soc. 159, B444–B454 (2012). https://doi.org/10.1149/2.106204jes
Sun, S.H., Murray, C.B., Weller, D., et al.: Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287, 1989–1992 (2000). https://doi.org/10.1126/science.287.5460.1989
Murty, B.S., Yeh, J.W., Ranganathan, S.: High-entropy alloys: basic concepts. In: Murty, B.S., Yeh, J.W., Ranganathan, S. (eds.) High Entropy Alloys, pp. 13–35. Butterworth-Heinemann, Boston (2014)
Greer, A.L.: Confusion by design. Nature 366, 303–304 (1993). https://doi.org/10.1038/366303a0
Cantor, B., Chang, I.T.H., Knight, P., et al.: Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 375, 213–218 (2004). https://doi.org/10.1016/j.msea.2003.10.257
Yeh, J.W., Chen, S.K., Lin, S.J., et al.: Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 6, 299–303 (2004). https://doi.org/10.1002/adem.200300567
George, E.P., Raabe, D., Ritchie, R.O.: High-entropy alloys. Nat. Rev. Mater. 4, 515–534 (2019). https://doi.org/10.1038/s41578-019-0121-4
Yeh, J.W., Lin, S.J., Chin, T.S., et al.: Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 35, 2533–2536 (2004). https://doi.org/10.1007/s11661-006-0234-4
Qiu, X.W., Zhang, Y.P., He, L., et al.: Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloys Compd. 549, 195–199 (2013). https://doi.org/10.1016/j.jallcom.2012.09.091
Lu, Y., Dong, Y., Guo, S., et al.: A promising new class of high-temperature alloys: eutectic high-entropy alloys. Sci. Rep. 4, 6200 (2014). https://doi.org/10.1038/srep06200
Wu, J.M., Lin, S.J., Yeh, J.W., et al.: Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 261, 513–519 (2006). https://doi.org/10.1016/j.wear.2005.12.008
Samal, S., Mohanty, S., Misra, A.K., et al.: Mechanical behavior of novel suction cast Ti-Cu-Fe-Co-Ni high entropy alloys. Mater. Sci. Forum 790–791, 503–508 (2014). https://doi.org/10.4028/www.scientific.net/msf.790-791.503
Gludovatz, B., Hohenwarter, A., Catoor, D., et al.: A fracture-resistant high-entropy alloy for cryogenic applications. Science 345, 1153–1158 (2014). https://doi.org/10.1126/science.1254581
Li, H.N., Zhu, H., Zhang, S.G., et al.: Nano high-entropy materials: synthesis strategies and catalytic applications. Small Struct. 1, 2070004 (2020). https://doi.org/10.1002/sstr.202070004
**n, Y., Li, S.H., Qian, Y.Y., et al.: High-entropy alloys as a platform for catalysis: progress, challenges, and opportunities. ACS Catal. 10, 11280–11306 (2020). https://doi.org/10.1021/acscatal.0c03617
Ostovari Moghaddam, A., Trofimov, E.A.: Toward expanding the realm of high entropy materials to platinum group metals: a review. J. Alloys Compd. 851, 156838 (2021). https://doi.org/10.1016/j.jallcom.2020.156838
Yang, Y., Luo, M.C., Zhang, W.Y., et al.: Metal surface and interface energy electrocatalysis: fundamentals, performance engineering, and opportunities. Chem 4, 2054–2083 (2018). https://doi.org/10.1016/j.chempr.2018.05.019
Shao, Q., Wang, P.T., Huang, X.Q.: Opportunities and challenges of interface engineering in bimetallic nanostructure for enhanced electrocatalysis. Adv. Funct. Mater. 29, 1806419 (2019). https://doi.org/10.1002/adfm.201806419
Luo, M.C., Guo, S.J.: Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017). https://doi.org/10.1038/natrevmats.2017.59
**a, Z., Guo, S.: Strain engineering of metal-based nanomaterials for energy electrocatalysis. Chem. Soc. Rev. 48, 3265–3278 (2019). https://doi.org/10.1039/c8cs00846a
**e, C., Niu, Z., Kim, D., et al.: Surface and interface control in nanoparticle catalysis. Chem. Rev. 120, 1184–1249 (2020). https://doi.org/10.1021/acs.chemrev.9b00220
Tomboc, G.M., Kwon, T., Joo, J., et al.: High entropy alloy electrocatalysts: a critical assessment of fabrication and performance. J. Mater. Chem. A 8, 14844–14862 (2020). https://doi.org/10.1039/d0ta05176d
Xue, Q., Bai, X.Y., Zhao, Y., et al.: Au core-PtAu alloy shell nanowires for formic acid electrolysis. J. Energy Chem. 65, 94–102 (2022). https://doi.org/10.1016/j.jechem.2021.05.034
Dai, W.J., Lu, T., Pan, Y.: Novel and promising electrocatalyst for oxygen evolution reaction based on MnFeCoNi high entropy alloy. J. Power Sources 430, 104–111 (2019). https://doi.org/10.1016/j.jpowsour.2019.05.030
Torralba, J.M., Venkatesh Kumarán, S.: Development of competitive high-entropy alloys using commodity powders. Mater. Lett. 301, 130202 (2021). https://doi.org/10.1016/j.matlet.2021.130202
Fang, G., Gao, J.J., Lv, J., et al.: Multi-component nanoporous alloy/(oxy) hydroxide for bifunctional oxygen electrocatalysis and rechargeable Zn-air batteries. Appl. Catal. B Environ. 268, 118431 (2020). https://doi.org/10.1016/j.apcatb.2019.118431
Qiu, H.J., Fang, G., Gao, J.J., et al.: Noble metal-free nanoporous high-entropy alloys as highly efficient electrocatalysts for oxygen evolution reaction. ACS Mater. Lett. 1, 526–533 (2019). https://doi.org/10.1021/acsmaterialslett.9b00414
Al Bacha, S., Pighin, S.A., Urretavizcaya, G., et al.: Effect of ball milling strategy (milling device for scaling-up) on the hydrolysis performance of Mg alloy waste. Int. J. Hydrog. Energy 45, 20883–20893 (2020). https://doi.org/10.1016/j.ijhydene.2020.05.214
Uemoto, Y., Kondo, K., Niwa, T.: Cryo-milling with spherical crystalline cellulose beads: a contamination-free and safety conscious technology. Eur. J. Pharm. Sci. 143, 105175 (2020). https://doi.org/10.1016/j.ejps.2019.105175
Ma, P.Y., Zhao, M.M., Zhang, L., et al.: Self- supported high-entropy alloy electrocatalyst for highly efficient H2 evolution in acid condition. J. Materiomics 6, 736–742 (2020). https://doi.org/10.1016/j.jmat.2020.06.001
Chen, H., Lin, W.W., Zhang, Z.H., et al.: Mechanochemical synthesis of high entropy oxide materials under ambient conditions: dispersion of catalysts via entropy maximization. ACS Mater. Lett. 1, 83–88 (2019). https://doi.org/10.1021/acsmaterialslett.9b00064
Chen, Y.: Solid-state formation of carbon nanotubes. In: Dai, L. (ed.) Carbon Nanotechnology, pp. 53–80. Elsevier, Amsterdam (2006)
Hemantkumar, J., Mayur, H., Patil, C.K., et al.: A review on cryogenic grinding. Int. J. Eng. Sci. 7, 420–423 (2017)
Nellaiappan, S., Katiyar, N.K., Kumar, R., et al.: High-entropy alloys as catalysts for the CO2 and CO reduction reactions: experimental realization. ACS Catal. 10, 3658–3663 (2020). https://doi.org/10.1021/acscatal.9b04302
Laplanche, G., Horst, O., Otto, F., et al.: Microstructural evolution of a CoCrFeMnNi high-entropy alloy after swaging and annealing. J. Alloys Compd. 647, 548–557 (2015). https://doi.org/10.1016/j.jallcom.2015.05.129
Dong, Y., Duan, S.G., Huang, X., et al.: Excellent strength-ductility synergy in as-cast Al0.6CoCrFeNi2Mo0.08V0.04 high-entropy alloy at room and cryogenic temperatures. Mater. Lett. 294, 129778 (2021). https://doi.org/10.1016/j.matlet.2021.129778
Bae, J.W., Park, J.M., Moon, J., et al.: Effect of μ-precipitates on the microstructure and mechanical properties of non-equiatomic CoCrFeNiMo medium-entropy alloys. J. Alloys Compd. 781, 75–83 (2019). https://doi.org/10.1016/j.jallcom.2018.12.040
Chen, X.H., **e, W.Y., Zhu, J., et al.: Influences of Ti additions on the microstructure and tensile properties of AlCoCrFeNi2.1 eutectic high entropy alloy. Intermetallics 128, 107024 (2021). https://doi.org/10.1016/j.intermet.2020.107024
Yi, J.J., Tang, S., Xu, M.Q., et al.: A novel Al0.5CrCuNiV 3d transition metal high-entropy alloy: phase analysis, microstructure and compressive properties. J. Alloys Compd. 846, 156466 (2020). https://doi.org/10.1016/j.jallcom.2020.156466
Niu, Z.Z., **e, Y., Axinte, E., et al.: Development and characterization of novel Ni-rich high-entropy alloys. J. Alloys Compd. 846, 156342 (2020). https://doi.org/10.1016/j.jallcom.2020.156342
Park, K.B., Park, J.Y., Kim, Y.D., et al.: Spark plasma sintering behavior of TaNbHfZrTi high-entropy alloy powder synthesized by hydrogenation-dehydrogenation reaction. Intermetallics 130, 107077 (2021). https://doi.org/10.1016/j.intermet.2020.107077
Liu, Q., Wang, G.F., Sui, X.C., et al.: Ultra-fine grain TixVNbMoTa refractory high-entropy alloys with superior mechanical properties fabricated by powder metallurgy. J. Alloys Compd. 865, 158592 (2021). https://doi.org/10.1016/j.jallcom.2020.158592
Zhou, J., Liao, H.C., Chen, H., et al.: Carbon-alloyed Fe35Mn10Cr20Ni35 high entropy alloy synthesized by mechanical alloying plus spark plasma sintering. J. Alloys Compd. 859, 157851 (2021). https://doi.org/10.1016/j.jallcom.2020.157851
Qiu, H.J., Fang, G., Wen, Y.R., et al.: Nanoporous high-entropy alloys for highly stable and efficient catalysts. J. Mater. Chem. A 7, 6499–6506 (2019). https://doi.org/10.1039/c9ta00505f
**, Z.Y., Lv, J., Jia, H.L., et al.: Nanoporous Al-Ni-Co-Ir-Mo high-entropy alloy for record-high water splitting activity in acidic environments. Small 15, 1904180 (2019). https://doi.org/10.1002/smll.201904180
**, Z.Y., Lyu, J., Zhao, Y.L., et al.: Rugged high-entropy alloy nanowires with in situ formed surface spinel oxide as highly stable electrocatalyst in Zn-air batteries. ACS Mater. Lett. 2, 1698–1706 (2020). https://doi.org/10.1021/acsmaterialslett.0c00434
Yang, L.Z., Li, Y.Y., Wang, Z.F., et al.: Nanoporous quasi-high-entropy alloy microspheres. Met. Basel 9, 345 (2019). https://doi.org/10.3390/met9030345
Peng, H.L., **e, Y., **e, Z.C., et al.: Large-scale and facile synthesis of a porous high-entropy alloy CrMnFeCoNi as an efficient catalyst. J. Mater. Chem. A 8, 18318–18326 (2020). https://doi.org/10.1039/D0TA04940A
Ding, Z.Y., Bian, J.J., Shuang, S., et al.: High entropy intermetallic-oxide core-shell nanostructure as superb oxygen evolution reaction catalyst. Adv. Sustain. Syst. 4, 1900105 (2020). https://doi.org/10.1002/adsu.201900105
Koh, S., Strasser, P.: Electrocatalysis on bimetallic surfaces: modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Am. Chem. Soc. 129, 12624–12625 (2007). https://doi.org/10.1021/ja0742784
Wang, D., Yu, Y., **n, H.L., et al.: Tuning oxygen reduction reaction activity via controllable dealloying: a model study of ordered Cu3Pt/C intermetallic nanocatalysts. Nano Lett. 12, 5230–5238 (2012). https://doi.org/10.1021/nl302404g
Li, X., Chen, Q., McCue, I., et al.: Dealloying of noble-metal alloy nanoparticles. Nano Lett. 14, 2569–2577 (2014). https://doi.org/10.1021/nl500377g
Li, G.G., Lin, Y., Wang, H.: Residual silver remarkably enhances electrocatalytic activity and durability of dealloyed gold nanosponge particles. Nano Lett. 16, 7248–7253 (2016). https://doi.org/10.1021/acs.nanolett.6b03685
Li, G.G., Villarreal, E., Zhang, Q.F., et al.: Controlled dealloying of alloy nanoparticles toward optimization of electrocatalysis on spongy metallic nanoframes. ACS Appl. Mater. Interfaces 8, 23920–23931 (2016). https://doi.org/10.1021/acsami.6b07309
Pavlišič, A., Jovanovič, P., Šelih, V.S., et al.: Atomically resolved dealloying of structurally ordered Pt nanoalloy as an oxygen reduction reaction electrocatalyst. ACS Catal. 6, 5530–5534 (2016). https://doi.org/10.1021/acscatal.6b00557
Strasser, P., Kühl, S.: Dealloyed Pt-based core-shell oxygen reduction electrocatalysts. Nano Energy 29, 166–177 (2016). https://doi.org/10.1016/j.nanoen.2016.04.047
Oezaslan, M., Heggen, M., Strasser, P.: Size-dependent morphology of dealloyed bimetallic catalysts: linking the nano to the macro scale. J. Am. Chem. Soc. 134, 514–524 (2012). https://doi.org/10.1021/ja2088162
Erlebacher, J., Aziz, M.J., Karma, A., et al.: Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001). https://doi.org/10.1038/35068529
Gan, L., Heggen, M., O’Malley, R., et al.: Understanding and controlling nanoporosity formation for improving the stability of bimetallic fuel cell catalysts. Nano Lett. 13, 1131–1138 (2013). https://doi.org/10.1021/nl304488q
Ortega, S., Ibáñez, M., Liu, Y., et al.: Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 46, 3510–3528 (2017). https://doi.org/10.1039/c6cs00567e
Nugroho, F.A.A., Iandolo, B., Wagner, J.B., et al.: Bottom-up nanofabrication of supported noble metal alloy nanoparticle arrays for plasmonics. ACS Nano 10, 2871–2879 (2016). https://doi.org/10.1021/acsnano.5b08057
Yu, H.D., Regulacio, M.D., Ye, E.Y., et al.: Chemical routes to top-down nanofabrication. Chem. Soc. Rev. 42, 6006–6018 (2013). https://doi.org/10.1039/c3cs60113g
Junka, R., Laurencin, C.T., et al.: Introduction to regenerative engineering. In: Narayan, R. (ed.) Encyclopedia of Biomedical Engineering, pp. 624–630. Elsevier, Amsterdam (2019)
Yao, Y., Huang, Z., **e, P., et al.: Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018). https://doi.org/10.1126/science.aan5412
**e, P., Yao, Y., Huang, Z., et al.: Highly efficient decomposition of ammonia using high-entropy alloy catalysts. Nat. Commun. 10, 4011 (2019). https://doi.org/10.1038/s41467-019-11848-9
Xu, X., Du, Y.K., Wang, C.H., et al.: High-entropy alloy nanoparticles on aligned electronspun carbon nanofibers for supercapacitors. J. Alloys Compd. 822, 153642 (2020). https://doi.org/10.1016/j.jallcom.2020.153642
Peters, T.A., Stange, M., Bredesen, R.: Fabrication of palladium-based membranes by magnetron sputtering. In: Koumanakos, A., Kakaras, E. (eds.) Palladium Membrane Technology for Hydrogen Production. Carbon Capture and Other Applications, pp. 25–41. Woodhead Publishing, Cambridge (2015)
Zhang, N., Feng, X., Rao, D., et al.: Lattice oxygen activation enabled by high-valence metal sites for enhanced water oxidation. Nat. Commun. 11, 4066 (2020). https://doi.org/10.1038/s41467-020-17934-7
Löffler, T., Meyer, H., Savan, A., et al.: Discovery of a multinary noble metal-free oxygen reduction catalyst. Adv. Energy Mater. 8, 1802269 (2018). https://doi.org/10.1002/aenm.201802269
Löffler, T., Savan, A., Meyer, H., et al.: Design of complex solid-solution electrocatalysts by correlating configuration, adsorption energy distribution patterns, and activity curves. Angew. Chem. Int. Ed. 59, 5844–5850 (2020). https://doi.org/10.1002/anie.201914666
Rao, B.G., Mukherjee, D., Reddy, B.M.: Novel approaches for preparation of nanoparticles. In: Ficai, D., Grumezescu, A.M. (eds.) Nanostructures for Novel Therapy, pp. 1–36. Elsevier, Amsterdam (2017)
Ud Din, M.A., Saleem, F., Ni, B., et al.: Porous tetrametallic PtCuBiMn nanosheets with a high catalytic activity and methanol tolerance limit for oxygen reduction reactions. Adv. Mater. 29, 1604994 (2017). https://doi.org/10.1002/adma.201604994
Mahmood, A., **e, N., Ud Din, M.A., et al.: Shape controlled synthesis of porous tetrametallic PtAgBiCo nanoplates as highly active and methanol-tolerant electrocatalyst for oxygen reduction reaction. Chem. Sci. 8, 4292–4298 (2017). https://doi.org/10.1039/c7sc00318h
Li, H., Han, Y., Zhao, H., et al.: Fast site-to-site electron transfer of high-entropy alloy nanocatalyst driving redox electrocatalysis. Nat. Commun. 11, 5437 (2020). https://doi.org/10.1038/s41467-020-19277-9
Kusada, K., Kitagawa, H.: A route for phase control in metal nanoparticles: a potential strategy to create advanced materials. Adv. Mater. 28, 1129–1142 (2016). https://doi.org/10.1002/adma.201502881
Wu, D., Kusada, K., Yamamoto, T., et al.: On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles. Chem. Sci. 11, 12731–12736 (2020). https://doi.org/10.1039/d0sc02351e
Wu, D., Kusada, K., Yamamoto, T., et al.: Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 142, 13833–13838 (2020). https://doi.org/10.1021/jacs.0c04807
Jones, H.: Introduction to solid state physics by C. Kittel. Acta Crystallogr. 10, 390–390 (1957). https://doi.org/10.1107/s0365110x57001280
Takeuchi, A., Inoue, A.: Quantitative evaluation of critical cooling rate for metallic glasses. Mater. Sci. Eng. A Struct. 304–306, 446–451 (2001). https://doi.org/10.1016/S0921-5093(00)01446-5
Yang, X., Zhang, Y.: Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 132, 233–238 (2012). https://doi.org/10.1016/j.matchemphys.2011.11.021
Wang, X.F., Zhang, Y., Qiao, Y., et al.: Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 15, 357–362 (2007). https://doi.org/10.1016/j.intermet.2006.08.005
Zhang, K.B., Fu, Z.Y., Zhang, J.Y., et al.: Characterization of nanocrystalline CoCrFeNiTiAl high-entropy solid solution processed by mechanical alloying. J. Alloys Compd. 495, 33–38 (2010). https://doi.org/10.1016/j.jallcom.2009.12.010
Zhou, Y.J., Zhang, Y., Kim, T.N., et al.: Microstructure characterizations and strengthening mechanism of multi-principal component AlCoCrFeNiTi0.5 solid solution alloy with excellent mechanical properties. Mater. Lett. 62, 2673–2676 (2008). https://doi.org/10.1016/j.matlet.2008.01.011
Zhou, Y.J., Zhang, Y., Wang, F.J., et al.: Phase transformation induced by lattice distortion in multiprincipal component CoCrFeNiCuxAl1–x solid-solution alloys. Appl. Phys. Lett. 92, 241917 (2008). https://doi.org/10.1063/1.2938690
Yao, Y., Liu, Z., **e, P., et al.: Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 6, eaaz0510 (2020). https://doi.org/10.1126/sciadv.aaz0510
Lu, Z.L., Chen, Z.W., Singh, C.V.: Neural network-assisted development of high-entropy alloy catalysts: decoupling ligand and coordination effects. Matter 3, 1318–1333 (2020). https://doi.org/10.1016/j.matt.2020.07.029
Zhang, D., Shi, Y., Zhao, H., et al.: The facile oil-phase synthesis of a multi-site synergistic high-entropy alloy to promote the alkaline hydrogen evolution reaction. J. Mater. Chem. A 9, 889–893 (2021). https://doi.org/10.1039/d0ta10574k
Guo, S., Ng, C., Lu, J., et al.: Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 109, 103505 (2011). https://doi.org/10.1063/1.3587228
Hodoroaba, V.D.: Energy-dispersive X-Ray spectroscopy. In: Hodoroaba, V.D., Unger, W.E.S., Shard, A.G. (eds.) Characterization of Nanoparticles, pp. 397–417. Elsevier, Amsterdam (2020)
Valković, V.: Measurements of radioactivity. In: Valković, V. (ed.) The Environment, pp. 117–258. Elsevier Science, Amsterdam (2000)
Chen, Y.F., Zhan, X., Bueno, S.L.A., et al.: Synthesis of monodisperse high entropy alloy nanocatalysts from core@shell nanoparticles. Nanoscale Horiz. 6, 231–237 (2021). https://doi.org/10.1039/d0nh00656d
Iwashita, N.: X-Ray powder diffraction. In: Inagaki, M., Kang, F. (eds.) Materials Science and Engineering of Carbon, pp. 7–25. Butterworth-Heinemann, Oxford (2016)
Song, B., Yang, Y., Yang, T.T., et al.: Revealing high-temperature reduction dynamics of high-entropy alloy nanoparticles via in situ transmission electron microscopy. Nano Lett. 21, 1742–1748 (2021). https://doi.org/10.1021/acs.nanolett.0c04572
Luo, M.C., Zhao, Z.L., Zhang, Y.L., et al.: PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019). https://doi.org/10.1038/s41586-019-1603-7
Hart, D.: Hydrogen, end uses and economics. In: Cleveland, C.J. (ed.) Encyclopedia of Energy, pp. 231–239. Elsevier, New York (2004)
Dubouis, N., Grimaud, A.: The hydrogen evolution reaction: from material to interfacial descriptors. Chem. Sci. 10, 9165–9181 (2019). https://doi.org/10.1039/c9sc03831k
Li, Y., Wang, H., **e, L., et al.: MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). https://doi.org/10.1021/ja201269b
Liu, M.M., Zhang, Z.H., Okejiri, F., et al.: Entropy-maximized synthesis of multimetallic nanoparticle catalysts via a ultrasonication-assisted wet chemistry method under ambient conditions. Adv. Mater. Interfaces 6, 1900015 (2019). https://doi.org/10.1002/admi.201900015
Li, L.G., Wang, P.T., Shao, Q., et al.: Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. (2021). https://doi.org/10.1002/adma.202004243
Moriau, L., Bele, M., Marinko, Ž, et al.: Effect of the morphology of the high-surface-area support on the performance of the oxygen-evolution reaction for iridium nanoparticles. ACS Catal. 11, 670–681 (2021). https://doi.org/10.1021/acscatal.0c04741
Lee, J., Kumar, A., Yang, T., et al.: Stabilizing the OOH* intermediate via pre-adsorbed surface oxygen of a single Ru atom-bimetallic alloy for ultralow overpotential oxygen generation. Energy Environ. Sci. 13, 5152–5164 (2020). https://doi.org/10.1039/d0ee03183f
Zhao, Z.L., Wang, Q., Huang, X., et al.: Boosting the oxygen evolution reaction using defect-rich ultra-thin ruthenium oxide nanosheets in acidic media. Energy Environ. Sci. 13, 5143–5151 (2020). https://doi.org/10.1039/d0ee01960g
Tsai, F.T., Deng, Y.T., Pao, C.W., et al.: The HER/OER mechanistic study of an FeCoNi-based electrocatalyst for alkaline water splitting. J. Mater. Chem. A 8, 9939–9950 (2020). https://doi.org/10.1039/d0ta01877e
Urbain, F., Du, R.F., Tang, P.Y., et al.: Upscaling high activity oxygen evolution catalysts based on CoFe2O4 nanoparticles supported on nickel foam for power-to-gas electrochemical conversion with energy efficiencies above 80%. Appl. Catal. B Environ. 259, 118055 (2019). https://doi.org/10.1016/j.apcatb.2019.118055
Wu, Y.Z., Meng, Y.N., Hou, J.G., et al.: Orienting active crystal planes of new class lacunaris Fe2PO5 polyhedrons for robust water oxidation in alkaline and neutral media. Adv. Funct. Mater. 28, 1801397 (2018). https://doi.org/10.1002/adfm.201801397
Xu, K.L., Song, F., Gu, J., et al.: Solvent-induced surface hydroxylation of a layered perovskite Sr3FeCoO7–δ for enhanced oxygen evolution catalysis. J. Mater. Chem. A 6, 14240–14245 (2018). https://doi.org/10.1039/c8ta04976a
Favaro, M., Drisdell, W.S., Marcus, M.A., et al.: An operando investigation of (Ni–Fe–Co–Ce)Ox system as highly efficient electrocatalyst for oxygen evolution reaction. ACS Catal. 7, 1248–1258 (2017). https://doi.org/10.1021/acscatal.6b03126
Zheng, X.J., Cao, X.C., Zeng, K., et al.: A self-jet vapor-phase growth of 3D FeNi@NCNT clusters as efficient oxygen electrocatalysts for zinc-air batteries. Small 17, 2006183 (2021). https://doi.org/10.1002/smll.202006183
Yan, Y., Liu, C.Y., Jian, H.W., et al.: Substitutionally dispersed high-oxidation CoOx clusters in the lattice of rutile TiO2 triggering efficient Co-Ti cooperative catalytic centers for oxygen evolution reactions. Adv. Funct. Mater. 31, 2009610 (2021). https://doi.org/10.1002/adfm.202009610
He, Y.Q., Liu, X.H., Yan, A.L., et al.: Hybrid nanostructures of bimetallic NiCo nitride/N-doped reduced graphene oxide as efficient bifunctional electrocatalysts for rechargeable Zn-air batteries. ACS Sustain. Chem. Eng. 7, 19612–19620 (2019). https://doi.org/10.1021/acssuschemeng.9b04703
Li, Y.J., Sun, Y.J., Qin, Y.N., et al.: Recent advances on water-splitting electrocatalysis mediated by noble-metal-based nanostructured materials. Adv. Energy Mater. 10, 1903120 (2020). https://doi.org/10.1002/aenm.201903120
Ma, P.Y., Zhang, S.C., Zhang, M.T., et al.: Hydroxylated high-entropy alloy as highly efficient catalyst for electrochemical oxygen evolution reaction. Sci. China Mater. 63, 2613–2619 (2020). https://doi.org/10.1007/s40843-020-1461-2
Lim, D.H., Wilcox, J.: Mechanisms of the oxygen reduction reaction on defective graphene-supported Pt nanoparticles from first-principles. J. Phys. Chem. C 116, 3653–3660 (2012). https://doi.org/10.1021/jp210796e
Nørskov, J.K., Rossmeisl, J., Logadottir, A., et al.: Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004). https://doi.org/10.1021/jp047349j
Wang, K., Huang, J.H., Chen, H.X., et al.: Recent advances in electrochemical 2e oxygen reduction reaction for on-site hydrogen peroxide production and beyond. Chem. Commun. 56, 12109–12121 (2020). https://doi.org/10.1039/d0cc05156j
Li, T.Y., Yao, Y.G., Ko, B.H., et al.: Carbon-supported high-entropy oxide nanoparticles as stable electrocatalysts for oxygen reduction reactions. Adv. Funct. Mater. 31, 2010561 (2021). https://doi.org/10.1002/adfm.202010561
Huang, K., Peng, D.D., Yao, Z.X., et al.: Cathodic plasma driven self-assembly of HEAs dendrites by pure single FCC FeCoNiMnCu nanoparticles as high efficient electrocatalysts for OER. Chem. Eng. J. 425, 131533 (2021). https://doi.org/10.1016/j.cej.2021.131533
Nandan, R., Rekha, M.Y., Devi, H.R., et al.: High-entropy alloys for water oxidation: a new class of electrocatalysts to look out for. Chem. Commun. 57, 611–614 (2021). https://doi.org/10.1039/d0cc06485h
Funding
Authors would like to thank the financial support of the Training Program of the Major Research Plan of the National Natural Science Foundation of China (92061124), the National Natural Science Foundation of China (21975292, 21978331, 21905311, 21776176), Guangdong Province Nature Science Foundation (2020A1515010343, 2021A1515010167, 2022A1515011196), Tip-top Scientific and Technical Innovative Youth Talents of Guangdong Special Support Program (No. 2016TQ03N322).
Author information
Authors and Affiliations
Contributions
No any statement need to be additionally declared.
Corresponding authors
Ethics declarations
Conflict of interest
There is no any conflict or competing interest.
Rights and permissions
About this article
Cite this article
Wang, K., Huang, J., Chen, H. et al. Recent Progress in High Entropy Alloys for Electrocatalysts. Electrochem. Energy Rev. 5 (Suppl 1), 17 (2022). https://doi.org/10.1007/s41918-022-00144-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41918-022-00144-8