Abstract
Removal of inorganic soil pollutants (e.g. Sr2+) is considered necessary requirement to protect the environment and public health. So sugarcane bagasse biochar (SCBB) was examined as a biosorbent material for Sr2+. This was done through adsorption Stirred-batch technique including a kinetic experiment, at two concentrations (50 and 150 mg/l) and an adsorption isotherm experiment at five concentrations (20, 50, 100, 150, and 200 mg/l), by using SrCl2·6H2O. Moreover, an examination of the role of SCBB at three dosages (0.5, 1, 2%w/w) in reducing the bioavailability of strontium in polluted soil through pots experiment by using Raphanus sativus. Kinetic data revealed that equilibration time was 3 h and pseudo-second-order model was more represented in data at low and high concentrations where (R2 = 0.999 and R2 = 1), respectively. Thus, chemisorption governed the adsorption process for Sr2+removal by SCBB. Furthermore, Langmuir isotherm model (R2 = 0.99) described the adsorption data better, which indicated that a monolayer type of adsorption plays a vital role in the removal of Sr2+ by SCBB. Pots experiment revealed that SCBB could significantly reduce Sr2+ uptake by Raphanus sativus. The percentages of decrease in the shoot were 5.82, 18.17, and 26.80% for SCBB dosage 0.5, 1 and 2% w/w, respectively. The percentages of decrease in root were 17.20, 36.89, and 53.34% for SCBB dosage 0.5, 1 and 2% w/w, respectively. Specific surface area and surface functional groups of sugarcane bagasse play a vital role in the retention of strontium. Hence, biochar played an important role in the removal of Sr2+ from aqueous solution and reduced its uptake by plants in soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing the concentration of stable and radioactive strontium in soil and water is considered a big problem in standpoint of public health and environmental protection. Strontium is absorbed in human body as if it was calcium because the two elements are sufficiently similar chemically, and deposited preferentially in the bones and teeth of the human body and impaired bone growth in children (Cohen-Solal 2002). When strontium reaches our bodies it can replace calcium in bones or inhibit vitamin production, and it is related to leukemia, rickets and, renal diseases (Nielsen 2004). Besides, Steinhauser et al. (2013) demonstrated that Sr90 increased malignant diseases such as leukemia or skeleton cancer caused by damage to DNA in the cells. Because strontium and calcium are chemically similar; both elements are divalent and have close ionic radii, sequential extractions showed that, strontium like calcium in bonded to carbonate phase (Kamel 2010; Salem 2011; Dimovic et al. 2013). Additionally, since 90Sr bicarbonate is more soluble than calcium bicarbonate is, strontium in soil is more mobile than calcium (Kabata-Pendias and Mukherjee 2007).
Effects of stable strontium on plant genes are reported by Meena et al. (2013) who found abnormalities of chromosomal as chromosome breaks and chromosomal bridges at ana-and telophases in dividing cells in root tips. In addition, Sowa et al. (2014) reported that higher concentration of this element inhibited the growth of soybean seedlings. On the other hand, Burger and Lichtscheidl (2019) mentioned that adverse effects of the stable isotopes of strontium on plant development and growth are due to its negative impact on the uptake of some nutrients, especially calcium.
Naturally, strontium is found in the earth's crust as a mixture of the four stable isotopes (84Sr, 0.56%; 86Sr,9.87%;87Sr,7.04%; and88Sr,82.53%) (Gupta and Walther 2017). High levels of stable strontium can be found in the minerals celestite and strontianite as sulfate (SrSO4) and carbonate (SrCO3), respectively (Coudert 2015). Bentley (2006) and Capo et al. (1998) demonstrated that bedrock weathering releases strontium into the soils, surface water, and groundwater, where it becomes available for plant absorption and enters the food cycle. Natural levels of strontium in soil fluctuate widely, but the typical quantity is 0.2 mg per kilogram (kg) of soil (ATSDR 2004). Naturally occurring strontium is not radioactive (WHO 2010). Moreover, disposal of the wastes from some activities such as making ceramics and glass products, pyrotechnics, paint pigments, fluorescent lights, and medicines, in irrigation water and agricultural soil is considered an important source of strontium soil pollution (ATSDR 2004). In addition, Aberg (2001) demonstrated that strontium enters soil environment as impurities in super phosphate which represent another source of strontium, as well as the usage of road salt with marine origin caused an increase in the concentration of water-leachable strontium that can reach 30–126 ppm in soils near roads with considerable traffic. Thus, it becomes available for uptake by plants and enters the food chain. Furthermore, nuclear electricity generation is considered a new trend in some countries such as Egypt. This mechanism depends on using heat produced during nuclear fission to convert water to steam that runs turbines and thus generates electricity (Forsberg 2009). Radioactive strontium is one of the compounds that may be produced by nuclear industry in groundwater and soil at radioactive waste repositories (Fetter et al. 2017). In this context, it is important to demonstrate that Burger and Lichtscheidl (2019) stated that, chemical and biochemical behavior of stable strontium and radioactive strontium within plants and in soil solution are similar; so many experiments concerning uptake and distribution of radioactive strontium by plants were made with stable strontium.
Therefore, it is urgently necessary to find a suitable method to remediate strontium soil and water pollution. The most important chemical processes affecting the behavior and bioavailability of elements in soils are those concerned with the adsorption of elements from the liquid phase (Alloway 1995). Robin et al. (2015) and Nie et al. (2017) found that an ion exchange or a surface complexation described adsorption behavior of Sr2+ onto clay minerals, sediments, and soils. Adsorption procedures are thought to be more effective at removing radioactive species like Sr90 from aqueous solutions than electrocoagulation, ion exchange, and membrane processes because they do not require as much energy or advanced operational knowledge (Islam et al. 2018; Shin et al. 2021b). Furthermore, adsorption of Sr90depends on composition of the solid phase and its surface area, pH, and ionic strength (Wallace et al. 2012), which is in agreement with Kamel (2010) who stated that adsorption of strontium onto soil is strongly dependent on soil chemical composition. Similarly, Li et al. (2016) and Metwally et al. (2017) illustrated that chemical conditions and properties of adsorbent materials have an effect on sorption process of strontium. Activated carbon can be synthesized from agricultural wastes as a suitable biosorbent for pollutant remediation due to its effective surface properties that its pore structure and binding properties that permits attachment on the surface of the biosorbent (Oyekanmi et al. 2019, 2021). Due to its inexpensive cost, high specific surface area, and high pore volume, activated carbon has been recently used to eliminate radioactive species (Shin et al. 2021a, b). Nevertheless, Kılıc et al. (2013) demonstrated that, activated carbon needs to be regularly replaced, which means an increase in maintenance costs.
Biochar is an emerging adsorbent to many elements due to its plentiful functional groups and porous structures with high specific surface area (Li et al. 2019). The use of agricultural waste as a feedstock for the production of biochar can improve waste management practices and thus protect the environment (Karic et al. 2022). Biochar is a kind of environmentally friendly, economic, and renewable material, it is a carbon-rich pyrolysis product manufactured under no-oxygen conditions or oxygen-deficient at (300–700 °C) (Chaosheng et al. 2018). (Shin et al. 2021a, b) studied the adsorption of Sr2+ by spent coffee grounds (SCG) biochar, compared to powdered activated carbon (PAC), which has a much greater specific surface area (957.6 m2/g) and pore volume (0.676 cm3/g) than those of SCG biochar, he found that biochar showed higher maximum adsorption capacity than PAC. They argued that to the abundance of O-containing functional groups. Biochar could be prepared from pyrolysis of various types of feedstock as plant residue, animal litter, and sewage sludge (Srinivasan et al. 2015). Sugarcane bagasse (SCB) is a by-product of sugar production process; it is used in power generation and production of paper and fiberboard (Nakhla and El Haggar 2014). Sugarcane bagasse is the fibrous lignocellulosic residue of sugarcane (Iwuozor et al. 2021, 2022; Igwegbe et al. 2022). Biomasses such as sugarcane processing residues are widely used because they contain large amounts of 5 and 6 carbon sugars depending on the region (Avila et al. 2018). In addition to the fact that SCB is rich in carbon, it is inexpensive, and abundant, making it ideal for biochar production (Iwuozor et al. 2022). Producing of biochar by using sugarcane bagasse is a method to reuse this waste (Saleh and Hedia 2018) and has not been used yet for the removal of stable or radioactive strontium. on the other hand Azadi and Raisi (2021) mentioned that, sugarcane bagasse biochar (at pyrolysis temperature 600 °C) has high specific surface area 97.3 m2/g in comparison to its feed stock materials sugarcane bagasse 4.30 m2/g. The same result was observed by Moradi-Choghamarani et al. (2019) who stated that, SCB showed low adsorption capacity and pyrolysis has increased it and they argued that to the high specific surface area of SCB after pyrolysis. Salem et al. (2021) demonstrated that using sugarcane bagasse biochar could reduce the availability of heavy metals in polluted soil. On the other hand, Kashparov et al. (2005) clarified that oats cultivated on sandy soil accumulated several times more90Sr than plants grown on heavy loam because sandy soil is poor in organic matter and clay minerals, which can fix strontium in soil. Currently, there is still room to select the best in terms of sorption capacity and kinetics to remove Sr2+, and more adsorbents need to be tested and confirmed for their effectiveness to remove Sr2+ ions from aqueous solutions Therefore, this study examined the efficiency of sugarcane bagasse biochar in retention of strontium in aqueous solution and sandy soil.
The objectives of this study were to evaluate using sugarcane bagasse biochar as an adsorbent material to Sr2+in aqueous solutions and apply it in soil to remediate polluted soil and reduce strontium uptake by plant. In addition, to get rid of sugarcane bagasse waste in a beneficial way. These objectives were achieved through study the effect of sugarcane bagasse biochar on retention of Sr2+ at different aqueous solutions Sr2+ concentrations, understanding how rapidly reactions approach equilibrium as well as investigating reaction mechanisms. Furthermore, assess the effect of sugarcane bagasse biochar on strontium uptake by plant via pots experiment using polluted sandy soil, which is poor in organic matter and clay minerals.
Materials and Methods
Sugarcane Bagasse Biochar (Biosorbent) Preparation
Sugarcane bagasse (SCB) was collected from the local market. It was washed with tap water many times to remove any impurities. Moreover, it was washed three times with distilled water and dried at 80 °C for 24 h in the oven. The dried SCB was pyrolyzed using traditional method in which, the raw material was placed in a stainless steel net and then inside a well-sealed barrel of the pyrolysis unit, where it was burned in partially absence of oxygen for 2 h. The temperature was kept around 500 °C (El Gamal et al. 2017).
After naturally cooling biochar sample, it was washed with distilled water to remove excess impurities then it was oven-dried at 105 °C for 5.0 h. After cooling, biochar sample was crushed and sieved using 0.5-mm polypropylene sieve and stored in desiccator prior to adsorption experiments.
Characterization of Biochar
Scanning electron microscopy (SEM) was carried out to biochar sample before and after adsorption experiments to study surface morphologies using SEM Quanta FEG Unit, with accelerating voltage 30 k.v., (magnification 250 × up to 20,000 0061nd resolution for Gun.1 m). FTIR (Fourier transform infrared) was carried out to determine surface functional groups, which were determining by scanning SCBB with infrared rays in the range 400 – 4000 cm−1 using SHEMATZU infrared spectrophotometer model FT/IR5300, JASCO Corporation, Japan. Some Physicochemical characteristics of SCBB was determined, such as mass percentage of carbon (C), hydrogen (H), nitrogen (N) and sulfur by using a CHNS Elemental Analyzer(Vario type, El, elemental analyzer).The percentage of the ash content was calculated according to Lynch and Joseph (2010). SCB biochar's oxygen (O) fraction was estimated by subtract the ash, C, H, and N contents from their combined mass percentages. For evaluation the aromaticity and polarity of SCBB, H/C, O/C and (O + N)/C atomic ratios were calculated respectively. PH and EC of sugarcane bagasse biochar (SCBB) sample were determined at the ratio 1:20 w/v (biochar/water suspension). Its Average pore diameter and specific surface area were measured by the N2− BET method and total pore volume was determined. CEC was determined according to Song and Guo (2012).
Chemicals
SrCl2·6H2O and KCl were obtained from Sigma Aldrich Company (Germany). Hydrochloric acid, sodium hydroxide, nitric acid, sodium acetate, Ammonium acetate, Hydroxide amine hydrochloride, potassium hydroxide, sodium chloride, calcium chloride, ferric sulfate and ethanol were purchased from El-Gomhoria chemicals Co., Egypt.
Batch Experiments of Strontium Adsorption onto SCBB
Adsorption experiments were carried out by using a series of reaction vessels in a Stirred-Batch technique with a constant temperature water path Circulation Jacket at 25 °C and 400 rpm and in two replicates for each concentration. The experiments were carried out by mixing dried SCBB with aqueous solution of SrCl2.6 H2O in 1:200 w/v ratio (adsorbent dosage = 5 g/l) with background solution 0.01MKCl.
Adsorption kinetic experiments of Sr2+ by SCBB were carried out at two concentration (around 50 and 150 Sr2+ mg/l). During reaction, suspension was withdrawn with a polyethylene syringe from the reaction vessels after the desired contact time (10, 15, 30, 60,120, 180, 300, 420, 540, 720 and 1440 min).
At equilibration time obtained from kinetic experiment, Adsorption isotherm of Sr2+ on SCBB was carried out at five initial different concentration of Sr2+around (20, 50, 100, 150, and 200 mg/l) at 25 °C.
After all adsorption experiments, the suspended materials were centrifuged for 5 min then, filtered with Whatman 42 filter paper to separate SCBB from aqueous solutions. Immediately pH was measured in, aliquots of the supernatants then, raw and treated Sr solution were measured by inductively coupled argon plasma optical emission spectrometry (ICAP 6500 Duo, Termo Scientifc, England).
The adsorptive capacity of Sr2+ at time t is qt (mg/g) that was calculated from the difference between the initial and final concentrations of the metal in solution by using the following equation
where C0 and Ce (mg/l) are the concentrations of Sr2+ions in the raw and treated aqueous solutions, respectively. V (L) and M (g) are the sample volume and mass of SCBB.
For describing kinetic of Sr2+ sorption by SCBB three mathematical expressions were applied such as pseudo-first order, Elovich and pseudo-second order models in linear form (Zelentsov and Datsko 2017).
Pseudo-first-order model
where qe and qt (mg/g) are mounts of adsorbed Sr+2on to SCBB at equilibrium and at the selected time (t (h)) respectively. k1 (1/h) is the rate constant of pseudo first-order equation. K1 adsorption rate constant and qe were determined from the slope and intercept of the linear plot of ln (qe − qt) versus t.
Elovich equation
where qt (mg/g) is the mount of adsorbed Sr+2on to SCBB at the selected time α (g/mg·min) and β (mg/g·min) are constants that were determined from the slope (1/β) and intercept (1/β) ln (α/β) of the linear plot of qt versus ln t.
Pseudo-second order model
where qe and qt (mg/g) are mounts of adsorbed Sr+2on to SCBB at equilibrium and at the selected time (t (h)), respectively. k2 (g/mg.h) is the rate the constant of pseudo-second-order equation.
For describing Adsorption isotherm of Sr2+ on SCBB that was carried out at five initial different concentration of Sr2+ around, (20, 50, 100, 150, and 200 mg/l) Langmuir and Freundlich isotherm models were used (Cheng et al. 2012).
Freundlich isotherm model
where Ce (mg/l) is the concentration of the strontium ions at equilibrium, qe (mg/g) is the amount of adsorbed Sr+2 on to SCBB at equilibrium, KF (mg1−(1/n) L1/n/g) is the Freundlich adsorption constant and n (dimensionless) is the empirical constant describing sorption nonlinearity.
Langmuir equation
where Ce (mg/l) is the concentration of the strontium ions at equilibrium, qe (mg/g) is the amount of adsorbed Sr+2 on to SCBB at equilibrium, qmax (mg/g) is the maximum adsorption capacity of Sr+2 onto SCBB*** and KL (L/mg) is the Langmuir constant related to the adsorption energy.
The following equation was used to assess the favorability of the Sr2 + adsorption onto the SCBB (Liu et al. 2017):
where RL is the dimensionless constant separation factor, KL (L/mg) is the Langmuir constant** and C0 (mg/l) is the initial concentration of Sr+2.
-
RL = 1 indicates that the adsorption process is linear.
-
RL = 0 indicates that the adsorption process is irreversible.
-
RL > 1 indicates that the adsorption process is unfavorable.
-
RL < 1 indicates that the adsorption process is favorable > 0.
Planting Experiment
Soil Characterization
The soil used for the experiment was collected from Siwa oasis (an oasis found in Egypt between El Qattara Depression and Great Sand Sea in the Western Desert). Soil sample was collected at 0–30 cm depth within latitude 29° 10′ 0″ N and longitude 25° 30′ 0″ E.
Some chemical and physical properties of the soil sample were carried out according to Black (1965). PH was measured in 1:2.5 (w/v) soil: water suspension using Jenway pH-meter model 3305, soil salinity was measured (dS/m) in 1:2.5 soil: water suspension using Jenway conductivity meter model 4310. Cation exchange capacity (CEC) was measured using ammonium acetate method. Soil organic carbon (SOC) was determined by Walkley Black method (Black 1965). In addition, total carbonate equivalent was determined by Collin’s calcimeter. Particle size analysis of the fraction less than 2 mm was carried out using Pipette method (FAO 1970). Available concentration of strontium was extracted by Ammonium bicarbonate-DTPA method (Lindsay and Norvell 1978), and measured by inductively coupled argon plasma optical emission spectrometry (ICAP 6500 Duo, Thermo Scientific, England). While the total concentrations of strontium were determined in aqua regia according to Alloway (1995) and measured by inductively coupled argon plasma optical emission spectrometry (ICAP 6500 Duo, Thermo Scientific, England).
Planting Experiment
Pots experiment was conducted to ascertain the effect of SCBB on fixing Sr2+ on soil and reduce its uptake by plant. The study used radish plant (Raphanus sativus) as a bio indicator (Davies 1993; Hassan et al. 2018). The experiment was done during the crop** season of 2020–2021 in open field conditions. The soil properties used in this experiment are shown in Table 4. Three biochar treatments (0.5–1–2% w/w) in three replicates for each treatment. In addition to control (0%). Three kilograms of mixed soil were weighted in each pot. Five seeds were sown in each pot and water was added to bring the soil moisture to 75% of water holding capacity. After 40 days of sowing, the plant was harvested.
Soil and Plant Analysis
After harvesting, soil's available concentration of strontium was extracted by Ammonium bicarbonate-DTPA method and then, measured by using inductively coupled argon plasma optical emission spectrometry (ICAP 6500 Duo, Thermo Scientific, England). Plants were separated into shoots and roots and washed in tap and distilled water. After that, the shoot and root were oven dried for 48 h at 70 °C and ground in a stainless steel mill before digestion according to Jones (1989). Strontium content was measured using inductively coupled argon plasma optical emission spectrometry (ICAP 6500 Duo, Thermo Scientific, England).
Statistical Analysis
By using ANOVA test, the significance test was carried out; the least significant difference test (L.S.D) at 0.05 and 0.01 levels of probability according to Steel et al. (1997). Pearson's correlation coefficient was used to analyz**e the correlation between the measurements using PAST version 4.03-computer software (Hammer et al. 2020).
Results and Discussion
Characterization of Biosorbent (SCBB)
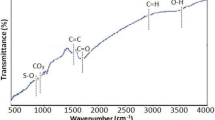
Table 1 illustrate some physicochemical characteristics of SCBB as SSA, CEC, PH, EC., C, H, N, O, ash contents, H/C, O/C and (O + N)/C atomic ratio. C (54.64%) and O (18.80%) are by far the most dominant elements, these results are in agreement with El-Damarawy et al. (2017). According to H/C, O/C and (O + N)/C atomic ratios of SCBB. The data showed that, SCBB has high level of carbonization and strong aromaticity that, means it has high biochemical stability where H/C < 0.6 (Cao and Harris 2010) and high polarity where it has high O/C (0.344) and (O + N)/C (0.351) atomic ratios (Chen et al. 2005). In addition, according to specific surface area (167.30 m2/g), total pore volume (0.13 cm3/g) and average pore diameter (2.83 nm); SCBB has high surface area and porous texture. Figure 1 shows Fourier-transform infrared spectra (FTIR) of SCBB, which clarify its functional group compositions. Ten radiation spectra in the range of 4000–400 cm−1 were obtained for SCB biochar sample. O–H stretching alcohol is detected in rage 3000–3750 (as in the spectra at frequency 3430.51 cm−1), in addition C=C, and C=O stretching carbonyl are detected in the range1500–2000 cm−1 as in 1602.90 cm−1 (Sahu et al 2010; Feng et al. 2017; Shin et al. 2021a, b). Triple bond C≡C region (2000–2500 cm−1), has been showed by peaks at 2351.30 cm−1 (Nandiyanto et al. 2019). In area, around 786.02 and 673.18 cm−1 C–H aromatic compound and alkyl bind were represented. In addition, it can be interpreted as Si–O–Si and Si–OH (siloxane and silanol) reactive groups (Saleh et al. 2014). These findings were consistent with the elemental composition results. The presence of these functional groups might interpret adsorption behavior of SCB biochar. Figure 2 shows scanning electron microscope (SEM) images that showed the surface morphology of SCBB before and after adsorption of Sr2+ to demonstrate the morphological changes on biochar surface. From the image (A) it is clear that biochar surface contains smooth surface and many pores and canals with uneven size (macro and meso porous structure), which were developed due to the thermal decomposition of SCB (Novotny et al. 2015). All these results might play an important role for better adsorption by SCBB. Image (B-1 and B-2) showed that there was difference in the surface morphology of biochar after adsorption. It is clear the presence of discrete aggregates on the biochar walls after Sr2+adsorption process. This was due to that Sr-related compound formed between strontium and sugarcane bagasse biochar (SCBB).
Kinetics of Strontium Adsorption
Kinetics experiments play an important role in understanding the adsorption mechanism and how rapidly reactions approach equilibrium. These experiments were performed at pH ranged between 6.23 and 6.94.
Effect of Contact Time on Sr+2 Adsorption
The contact time of kinetics adsorption, plays a major role in attaining equilibrium. Experiment was carried out during 10 min—24/h at two concentrations, high (around 150 Sr2+ mg/l) and low around (50 Sr2+mg/l). Data in Fig. 3 revealed that three hours were enough to achieve equilibrium after which no significant changes in the amount adsorbed were detected. By Evaluating the adsorption curves of Sr2+ on SCBB at two concentration, it is clear that the reaction followed two stages: (1) a rapid uptake or fast adsorption stage, which was within the first 60 min. This revealed the rapid diffusion of Sr2+ ions from the bulk solution to adsorption sites on the surface of SCBB. (2) The much slower adsorption stage representing the rate-limited time-dependent process that was within second two hours. Stage 1 might be attributed to instantaneous utilization of the most available adsorbing sites, while stage 2 might be attributed to the diffusion of ions into pores and micro channels of SCBB. These results assured by Imessaoudene et al. (2013) and Shin et al. (2021a, b) who demonstrated that adsorption of Sr2+ by biochar took place in two stages (fast and slow). FTIR data and physicochemical characteristics of SCBB might explain these results, for instant presence of–C = O, –OH, –C–H and C=C groups might interpret adsorption of Sr ions by SCB biochar because they played a vital role as active sorption sites for strontium ions (Sparks 1995). These results also are in accord with Inyang et al. (2010) who stated that –C=O–, –OH, and –C–H groups on SCB biochar can bind ions on it. In addition to Ding et al. (2014) who reported that the presence of oxygenated functional groups.onto SCB biochar were attributed to its high sorption capacity. Moreover Liang et al. (2020) who discovered that, O-containing functional groups were in charge of the biochar's ability to bind Sr2+. On the other hand, Table 1 illustrated physicochemical properties of SCBB that demonstrate high level of carbonization and polarity and strong aromaticity that play an important role in adsorption of Sr2+ by SCBB. These results are in agreement with Bogusz et al. (2015) who reported that the sorption of Sr2+ could be strongly influenced by physicochemical properties of the carbonaceous adsorbents.
Effect of Initial Concentration on Strontium Removal Efficiency
Figure 4 showed percentage of adsorption strontium that was plotted as a function of time in two concentrations. It explained the removal efficiencies of Sr2+ using SCBB as a biosorbent materials at high and low concentration. In general, the percentage of adsorbed Sr2+ increased with time until equilibration time (3 h) after that, no increment was observed. It was evident that the percentage of strontium removal was higher in high concentration than in low one at the same time, which might be explained by the accessibility of strontium ions at high concentration to available sorption sites. To put it in another way, at high concentration of Sr2+ the driving force increased and thus the active sites of adsorbent were surrounded by more strontium ions that enhance adsorption. Mohamed Zulfika et al. (2017) observed similar results and argued that to increase the efficient utilization of the adsorptive capacities of the adsorbent by high concentration due to greater driving force.
Application of Adsorption Kinetic Models
For describing kinetic of Sr2+ sorption by SCBB several mathematical expressions were applied such as pseudo-first order, Elovich and pseudo second order models in linear form (Zelentsov and Datsko 2017).
Pseudo-first-order Model
Pseudo-first-order model was shown at Fig. 5a for low and high concentration. It was clear that this graph gives a straight line with a low correlation coefficient for low and high conc. (R2 = 0.288 and R2 = 0.406) respectively, which indicated that Pseudo-first-order model could not represent adsorption of Sr2+ on SCBB Table 2.
Elovich Equation
Elovich equation was shown at Fig. 5b for low and high concentrations. In Table 2α and β are constants which have been used to estimate reaction rates, decrease in β and /or increase in α would increase reaction rate (Sparks 1995(. It was evident that this graph gives a straight line with a slightly high correlation coefficient for low and high conc. (R2 = 0.838 and R2 = 0.838) respectively, which indicated that Elovich equation was not efficiently explain adsorption of Sr2+ on SCBB but it presented a better correlation than pseudo first order model. Elovich kinetic model suggests a chemical reaction between the adsorbent and the adsorbate (Li et al. 2017). In this study α values was very high at high concentration compared to low concentration. However, β values were small in high and low concentrations that reveal the effective interaction between SCBB and Sr+2 (Pezoti et al. 2016).
Pseudo-second-order Model
Linear form of pseudo-second-order equation was used to describe the experimental data. It was found that the adsorption kinetics of strontium onto SCBB fits better with the pseudo-second-order models. The goodness of fits was compared using the coefficient of determination (R2) which has an extremely high value for low and high concentration (R2 = 0.999 and R2 = 1) respectively. In addition to the closeness of both observed and predicted values of qe at different time scales which, means that chemisorption governs the retention of Sr+2 by SCBB that include surface complexation and ion exchange between Sr2+ and sugarcane bagasse biochar (SCBB) Fig. 5d. These results are agreement with Shin et al. (2021a, b). The kinetic parameters for three used models of Sr2+ adsorption are shown in Table 2.
Adsorption Isotherm
Adsorption of Sr2+ onto SCBB was done at five initial conc. of Sr2+ (20, 50, 100, 150, and 200 mg/l), pH value of raw strontium solution was ranged between 6.3–6.5 and PH of treated solution was ranged between 6.4 and 6.8. Adsorption behavior was investigated using Langmuir and Freundlich isotherm models Fig. 6a, b and Table 3.
Adsorption of Sr2+ by SCBB was more represented by Langmuir isotherm model, where R2 = 0.993 compared to Freundlich model R2 = 0.972. Langmuir model suggested that adsorption of Sr2+ could be attributed to a monolayer type of adsorption on the surface of SCBB, this may be interpreted by the large surface area of SCBB and suggest that the surface of the SCBB (carbonaceous adsorbents) is homogenous (Kołodyńska et al. 2012). These results are in agreement with Jang et al. (2018) and Shin et al. (2021a, b).
By using Langmuir isotherm model RL, the adsorption affinity of Sr2+ for SCBB was assessed. The adsorption of Sr2+ onto SCB biochar was considered to be favorable since the RL values = 0.184–0.657 (Liu et al. 2017) Table 3.
Planting Experiment
Pots experiment was conducted to study the possibility of fixing strontium in the soil by SCBB and reducing the amount reach the plant.
Characterization of Soil
Characterization of soil used in this pot experiment was shown in Table 4. The data illustrated that the soil had low organic matter and high percentage of sand fraction thus; it has low cation exchange capacity. On the other hand, CaCO3 was 13.7, pH was 8.38 and EC was 3.03 ds/m. In addition to, total and chemical extractable of strontium element. The data illustrated that this soil is considered polluted with Sr according to ATSDR (2004) that clarified typical concentration is 0.2 mg per kilogram (kg) of soil.
Effect of Biochar Application on Available Strontium in Soil
Figure 7 showed the relation between percentage decrease in available strontium and SCBB treatments. The data illustrated that available Sr decrease with increasing SCBB treatments that was within pH value between 7.29 and 7.91. The percentage of decrease in available strontium was 5.26, 15.36, and 53.68% for SCBB treatments 0.5, 1, 2% w/w, respectively. This means that available form of strontium decreased by addition SCBB that might be due to the chemisorption between strontium and biochar (kinetic results), which is interpreted by the presence of different functional groups on biochar surface, which could facilitate strontium fixation and decrease its bioavailability in soil. Besides)in another meaning), these results might be due to increase the surface area, cation exchang capacity and organic matter of agricultural environment after biochar addition resulting in more metal binding sites and less mobile (Wang et al. 2020). These results are in agreement with Salem et al. (2021) who used SCBB for fixing elements like Zn, Cu, Cr and Ni in soil and found increase in heavy metal residual and organic fractions after addition biochar. In addition to Azadi and Raiesi (2021), who demonstrated success of using SCBB in immobilization of Cd and Pb in soil. On the other hand, similar results were obtained by Shin et al. (2021a) who used biochar derived from spent coffee grounds for removing Sr+2 from aqueous solution.
Effect of SCBB Treatments on Strontium Content in Radish Plant (Raphanus sativus)
Table 5 and Fig. 8 illustrated the amount of strontium in shoot and root in radish plant. The data showed that the amount of strontium in plant decreased after SCBB addition and there are significant differences between SCBB treatments compared with control. In general, the percentage of decrease was increased as biochar dosage increase. In shoot, the percentages of decrease were 5.82, 18.17 and 26.80% for SCBB dosage 0.5,1 and 2%, respectively. In root, the percentages of decrease were17.20, 36.89, and 53.34% for SCBB dosage 0.5, 1 and 2%, respectively. It was clearly that the decrease of strontium concentration in plant is directly proportional to its concentration in soil, which was due to, that SCBB can fix strontium in soil and thus decrease its bioavailability to plant. Table 6 showed that, there was a positive and significant correlation (p < 0.05 or 0.01) among strontium concentration in shoot and root and available after planting. On the other hand the concentration of strontium in shoot was more than in root this result is in agreement with Dresler et al. (2018) who reported that Strontium was accumulated preferentially in the leaves. This might be interpreted by the similarity between Sr+2and Ca+2 that can accumulate in a large amount in aboveground organs of plants (Kashparov et al. 2005; Sowa et al. 2014).
Conclusion
The study revealed that sugarcane bagasse biochar (SCBB) can be used for removal Sr2+ from aqueous solutions and reduce bioavailability of Sr2+ in sandy soil. The percentage of Sr2+ removal in aqueous solution was around 29% and 73% for low and high concentration, respectively. Pesudo-second-order model provided a good representation of the kinetic adsorption of Sr2+ by sugarcane bagasse biochar (SCBB) for low and high concentration (R2 = 0.999 and R2 = 1 respectively). That means chemisorption is the main process in the removal of Sr2+by using SCBB. On the other hand, Langmuir model could describe adsorption isotherm of Sr 2+ better (R2 = 0.99) than Frendlich model (R2 = 0.97) at different concentrations this might be interpreted that adsorption of Sr2+ by SCBB is monolayer adsorption. In soil, SCBB could significantly reduce bioavailability of Strontium and hence reduce its uptake by radish plant (Raphanus sativus). High specific surface area, porous structure of SCBB and various surface functional groups on SCBB explain how Sr2+ is removed from aqueous solutions and biochar ability to immobilize this element in soil. More studies are needed to decide the best type of biochar in removing strontium from aqueous solutions and its stabilization in soil.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Aberg GE (2001) Tracing pollution and its sources with isotopes. Water Air Soil Pollut 130:1577–1582
Agency for Toxic Substances and Disease Registry (ATSDR). April 2004
Alloway BJ (1995) Heavy metals in soils, 2nd edn. Blackie Academic and Professional, London
Avila PF, Forte MBS, Goldbeck R (2018) Biomass and bioenergy evaluation of the chemical composition of a mixture of sugarcane bagasse and straw after different pretreatments and their e ff ects on commercial enzyme combinations for the production of fermentable sugars. Biomass Bioenergy 116:180–188. https://doi.org/10.1016/j.biombioe.2018.06.015
Azadi N, Raiesi F (2021) Sugarcane bagasse biochar modulates metal and salinity stresses on microbial functions and enzyme activities in saline co-contaminated soils. Appl Soil Ecol 167:104043
Bentley RA (2006) Strontium isotopes from the earth to the archaeological skeleton: a review. J Archaeol Meth Theor 13:135–187. https://doi.org/10.1007/s10816-006-9009-x
Black CA (1965) Methods of soil analysis, part I. Physical and mineralogical properties. American Soc. Of Agronomy, Inc. Publisher, Madison
Bogusz A, Oleszczuk P, Dobrowolski R (2015) Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour Technol 196:540–549. https://doi.org/10.1016/j.biortech.2015.08.006
Burger A, Lichtscheidl I (2019) Strontium in the environment: review about reactions of plants towards stable and radioactive strontium isotopes. Sci Total Environ 653:1458–1512. https://doi.org/10.1016/j.scitotenv.2018.10.312
Cao X, Harris W (2010) Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour Technol 101:5222–5228. https://doi.org/10.1016/j.biortech.2010.02.052
Capo RC, Stewart BW, Chadwick OA (1998) Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma 82:197–225
Chaosheng Z, Liu L, Zhao M, Rong H, Xu Y (2018) The environmental characteristics and applications of biochar. Environ Sci Pollut Res 25:21525–21534
Chen BL, Johnson EJ, Chefetz B (2005) Sorption of polar and nonpolar aromatic organic contaminants by plant cuticular materials: role of polarity and accessibility. Environ Sci Technol 39:6138–6146. https://doi.org/10.1021/es050622q
Cheng Z, Gao Z, Ma W, Sun Q, Wang B, Wang X (2012) Preparation of magnetic Fe3O4 particles modified sawdust as the adsorbent to remove strontium ions. Chem Eng J 209:451–457. https://doi.org/10.1016/j.cej.2012.07.078
Cohen-Solal M (2002) Strontium overload and toxicity: Impact on renal osteodystrophy. Nephrol Dialysis Transplant 17(Suppl 2):30–34
Coudert FX (2015) Strontium’s scarlet sparkles. Nat Chem 7:940
Davies BE (1993) Radish as an indicator plant for derelict land: uptake of zinc at toxic concentrations. Commun Soil Sci Plant Anal 24:1883–1895
Dimovic S, Smiciklas I, Sljivic-Ivanovic M, Dojcinovic B (2013) Speciation of 90Sr and other metal cations in artificially contaminated soils: the influence of bone sorbent addition. J Soils Sediment 13:383–393
Ding W, Dong X, Ime IM, Gao B, Ma LQ (2014) Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105:68–74
Dresler S, Wójciak-Kosior M, Sowa I, Strzemski M, Sawicki J, Kováčik J, Blicharski T (2018) Effect of long-term strontium exposure on the content of phytoestrogens and allantoin in soybean. Int J Mol Sci 19:3864. https://doi.org/10.3390/ijms19123864
El-Damarawy YA, Saleh ME, Assad FF, Abdel Salam AA, Youssef RA (2017) Adsorption of lead onto a waste biomaterial-biochar. Nat Sci 15:154–164
El-Gamal E, Saleh M, Elsokkary I, Rashad M, Abd El-Latif M (2017) Comparison between properties of biochar produced by traditional and controlled pyrolysis. Alex Sci Exch J 38:412–425
FAO (1970) Physical and chemical methods of soil and water analysis. Soils Bull. No. 10. Rom, Italy
Feng D, Zhao Y, Zhang Y, Zhang Z, Zhang L, Gao J, Sun S (2017) Synergetic effects of biochar structure and AAEM species on reactivity of H2O-activated biochar from cyclone air gasification. Int J Hydrogen Energy 42:16045–16053. https://doi.org/10.1016/j.ijhydene.2017.05.153
Fetter CW, Boving T, Kreamer D (2017) Contaminant hydrogeology, 3rd edn. Prentice-Hall, Upper Saddle River
Forsberg CW (2009) Sustainability by combining nuclear, fossil, and renewable energy sources. Prog Nucl Energy 51(1):192–200. https://doi.org/10.1016/j.pnucene.2008.04.002
Gupta D, Walther C (2017) Behaviour of strontium in plants and the environment. Springer Switzerland, Cham
Hammer Ø, Harper DAT, Ryan PD (2020) Paleontological statistics software package. Palaeontol Electron 4:9–16
Hassan IA, Bell NB, Ashmore MR, Cotrozzi L, Haiba NS, Basahi JM, Summan A, Almeelbi T, Ismail IM (2018) Radish (Raphanus sativus L.) cultivar specific response to O3: patterns of biochemical and plant growth characteristics. Clean: Soil, Air, Water 46:1800124(1–9)
Igwegbe CA, Obiora-Okafo IA, Iwuozor KO, Ghosh S, Kurniawan SB, Rangabhashiyam S, Kanaoujiya R, Ighalo JO (2022) Treatment technologies for bakers’ yeast production wastewater. Environ Sci Pollut Res 29:11004–11026
Imessaoudene D, Hanini S, Bouzidi A (2013) Biosorption of strontium from aqueous solutions onto spent coffee grounds. J Radioanal Nucl Ch 298:893–902. https://doi.org/10.1007/s10967-013-2510-2
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman RZ (2010) Biochar from anaerobically digested sugarcane bagasse. Bioresour Technol 101:8868–8872
Islam MA, Morton DW, Johnson BB, Pramanik BK, Mainali B, Angove MJ (2018) Opportunities and constraints of using the innovative adsorbents for the removal of cobalt(II) from wastewater: a review. Environ Nanotechnol Monit Manag 10:435–456. https://doi.org/10.1016/j.enmm.2018.10.003
Iwuozor KO, Ighalo JO, Emenike EC, Ogunfowora LA, Igwegbe CA (2021) Adsorption of methyl orange: a review on adsorbent performance. Curr Res Green Sustain Chem 4:100179
Iwuozor KO, Emenike EC, Aniagor CO, Iwuchukwu FU, Ibitogbe EM, Okikiola BT, Omuku PE, Adeniyi AG (2022) Removal of pollutants from aqueous media using Cow dung-based adsorbents. Curr Res Green Sustain Chem 2022:100300
Jang J, Miran W, Divine SD, Nawaz M, Shahzad A, Woo SH, Lee DS (2018) Rice straw-based biochar beads for the removal of radioactive strontium from aqueous solution. Sci Total Environ 615:698–707. https://doi.org/10.1016/j.scitotenv.2017.10.023
Jones JB (1989) Plant tissue preparation for elemental assay in soil testing and plant analysis laboratory manual. Benton laboratories, Inc., Athens
Kabata-Pendias A, Mukherjee A (2007) Trace elements from soil to human. Springer, Berlin, p 550
Kamel NHM (2010) Adsorption models of 137Cs radionuclide and Sr(II) on some Egyptian soils. J Environ Radioact 101:297–303
Karic N, Maia AS, Teodorovic A, Atanasova N, Langergraber G, Crini G, Ribeiro AR, Đolic M (2022) Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in) organic pollutants in wastewater treatment. Chem Eng J Adv 9:100239
Kashparov VA, Lazarev NM, Polischuk SV (2005) Problems of agricultural radiology in Ukraine at the present stage. Agroecol J 3:31–41
Kılıc M, Kırbıyık Ҫ, Ҫepeliogullar O, Pütün AE (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862. https://doi.org/10.1016/j.apsusc.07.033
Kołodyńska D, Wnętrzak R, Leahy JJ, Hayes MHB, Kwapińskil W, Hubicki Z (2012) Kinetic and adsorptive characterization of biochar in metal ions removal. Chem Eng J 197:295–305. https://doi.org/10.1016/j.cej.2012.05.025
Li T, He F, Dai Y (2016) Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J Radioanal Nucl Chem 310:1139–1145
Li H, Hu J, Meng Y, Su J, Wang X (2017) An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci Total Environ 603–604:39–48
Li M, Liu H, Chen T, Dong C, Sun Y (2019) Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci Total Environ 651:1020–1028. https://doi.org/10.1016/j.scitotenv.2018.09.259
LiangJ LJ, Li X, Liu K, Wu L, Shan G (2020) The sorption behavior of CHA-type zeolite for removing radioactive strontium from aqueous solutions. Sep Purif Technol 230:115874
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. SSSAJ 42:421–428
Liu S, Huang B, Chai L, Liu Y, Zeng G, Wang X, Zeng W, Shang M, Deng J, Zhou Z (2017) Enhancement of As(V) adsorption from aqueous solution by a magnetic chitosan/biochar composite. RSC Adv 7(18):10891–10900. https://doi.org/10.1039/C6RA27341F
Lynch J, Joseph S (2010) Guidelines for the development and testing of pyrolysis plants to produce biochar. Ver. 1.1, 7
Meena D, Singh SK, Chaudari SK (2013) Effect of Sr2+ on mitotic activity and chromosomal behavior in root meristem of Allium cepa L. Int J Agric Environ Biotechnol 6:197–201
Metwally SS, Ghaly M, El-Sherief EA (2017) Physicochemical properties of synthetic nano-birnessite and its enhanced scavenging of Co2+ and Sr2+ ions from aqueous solutions. Mater Chem Phys 193:63–72
Mohamad Zulfika HBZ, Baini R, Zauzi NSA (2017) Effect of pH, dosage and concentration on the adsorption of Congo Red onto untreated and treated aluminium dross. In: IOP conference series: materials science and engineering, vol 205. The 2nd international conference on materials engineering and nanotechnology
Moradi-Choghamarani F, Moosavi AA, Sepaskhah AR, Baghernejad M (2019) Physico-hydraulic properties of sugarcane bagasse-derived biochar: the role of pyrolysis temperature. Cellulose 26:7125–7143
Nakhla DA, El Haggar S (2014) Environmentally blanced sugar cane industry. https://doi.org/10.1016/j.biortech.2014.08.108
Nandiyanto A, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscopy of organic materials. Indones J Sci Technol 4:97–118
Nie Z, Finck N, Heberling F, Pruessmann T, Liu C, Lützenkirchen J (2017) Adsorption of selenium and strontium on goethite: EXAFS study and surface complexation modeling of the ternary systems. Environ Sci Technol 51:3751–3758. https://doi.org/10.1021/acs.est.6b06104
Nielsen SP (2004) The biological role of strontium. Bone 35:583–588
Novotny EH, de Freitas-Maia CMB, de Carvalho MMT, Madari BE (2015) Biochar: pyrogenic carbon for agricultural use—a critical review. Rev Bras Ci Solo 39:321–344
Oyekanmi AA, Latiff AAA, Daudb Z, Mohamed RMS, Aziz NAA, Ismail N, Rafatullah M, Ahmad A, Hossain K (2019) Adsorption of pollutants from palm oil mill effluent using natural adsorbents: optimization and isotherm studies. Desalin Water Treat 169:181–190
Oyekanmi AA, Ahmad A, Setapar SHM, Alshammari MB, Jawaid M, Hanafiah MM, Abdul Khalil HPS, Vaseashta A (2021) Sustainable Durio zibethinus-derived biosorbents for Congo Red removal from aqueous solution: statistical optimization, isotherms and mechanism studies. Sustainability 13:23
Pezoti O, Cazetta AL, Bedin KC, Souza LS, Martins AC, Silva TL, Santos Junior OO, Visentainer JV, Almeida VC (2016) NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: kinetic, isotherm and thermodynamic studies. Chem Eng J 288:778–788
Robin V, Tertre E, Beaufort D, Regnault O, Sardini P, Descostes M (2015) Ion exchange reactions of major inorganic cations (H+, Na+, Ca2+, Mg2+ and K+) on beidellite: experimental results and new thermodynamic database. Toward a better prediction of contaminant mobility in natural environments. Appl Geochem 59:74–84. https://doi.org/10.1016/j.apgeochem.2015.03.016
Sahu JN, Acharya J, Meikap BC (2010) Optimization of production conditions for activated carbons from Tamarind wood by zinc chloride using response surface methodology. Bioresour Technol 101:1974–1982
Saleh ME, Hedia RMR (2018) Mg-modified sugarcane bagasse biochar for dual removal of ammonium and phosphate ions from aqueous solutions. Alex Sci Exch J 39(74–91):98–212
Saleh ME, Mahmoud AH, El-Refaey AA (2014) Removal of cadmium from aqueous solution by biochars derived from peanut hull and wheat straw. Adv Environ Biol 8:399–409
Salem LR, Saleh ME, Abu-Elnine DS (2021) Effect of biochar on chemical behavior and radish plant uptake of heavy metals grown in polluted soils. Alex Sci Exchange J 42(4):1054–1067
Salem LR (2011) Occurrence and chemical behavior of strontium in some calcareous and lacustrine soils, Egypt. Ph.D. Thesis, Fac. Agric., Alex. univ.
Shin J, Kwak J, Lee Y, Kim S, Son Ch, Cho K, Lee S, Park Y, Ren X, Chon K (2021a) Changes in adsorption mechanisms of radioactive barium, cobalt, and strontium ions using spent coffee waste biochars via alkaline chemical activation: enrichment effects of O-containing functional groups. Environ Resarch 199:111346
Shin J, Lee SH, Kim S, Ochir D, Park Y, Kim J, Lee YG, Chon K (2021b) Effects of physicochemical properties of biochar derived from spent coffee grounds and commercial activated carbon on adsorption behavior and mechanisms of strontium ions (Sr2+). Environ Sci Pollut Res 28:40623–40632. https://doi.org/10.1007/s11356-020-10095-6
Song W, Guo M (2012) Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J Anal Appl Pyrolysis 94:138–145
Sowa I, Wójciak-Kosior M, Strzemski M, Dresler S, Szwerc W, Blicharski T, Szymczak G (2014) Biofortification of soy (Glycine max (L.) Merr.) with strontium ions. Kocjan R J Agric Food Chem 62(23):5248–5252
Sparks DL (1995) Environmental soil chemistry. Academic Press, INC
Srinivasan P, Sarmah AK, Smernik R, Das O, Farid M, Gao WA (2015) Feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposite feedstock: production, characterization and potential applications. Sci Total Environ 512:495–505
Steel RGD, Torrie JH, Dickey DA (1997) Principles and rocedures of statistics: a biometrical approach, 3rd edn. McGraw Hill, New York
Steinhauser G, Schauer V, Shozugawa K (2013) Concentration of strontium-90 at selected hot spots in Japan. PLoS ONE 8:1–5
Wallace SH, Shaw S, Morris K, Small JS, Fuller AJ, Burke IT (2012) Effect of groundwater pH and ionic strength on strontium sorption in aquifer sediments: implications for 90Sr2+ mobility at contaminated nuclear sites. Appl Geochem 27:1482–1491
Wang Y, Liu Y, Zhan W, Zheng K, Wang J, Zhang C, Chen R (2020) Stabilizationof heavy metal-contaminated soils by biochar: challenges and recommendations. Sci Total Environ 729:139060
WHO (2010) Concise International Chemical Assessment Document 77: strontium and strontium compounds.
Zelentsov V, Datsko T (2017) Modelling of kinetics of fluorine adsorption onto modified diatomite. Chem Chem Eng Biotechnol Food Ind 18(1):085–095
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Science and Technology Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, L.R. Kinetics and Adsorption Isotherm of Strontium on Sugarcane Biochar and Its Application in Polluted Soil. Int J Environ Res 17, 42 (2023). https://doi.org/10.1007/s41742-023-00532-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-023-00532-y