Abstract
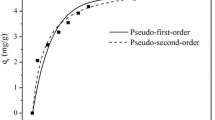
The main source of drinking and cooking water for the people living in rural and semi-urban areas of Assam—a Northeastern province of India is groundwater. However, the groundwater is having iron (Fe+2) and fluoride (F−) concentrations much higher than permissible limit of drinking water. The people use different variants of indigenous household iron filters to remove iron from the groundwater, but these filters are ineffective for removal of fluoride. Thus, the present research work explores the potential of pineapple juice extracted residue—a renewable modified agricultural waste (MPJER), for the remediation of fluoride from groundwater. The FTIR, Raman, Proximate, BET surface area, pore size, pHPZC, and SEM analyses were carried out to characterize and interpret the adsorption mechanism of fluoride onto MPJER. The adsorption was carried out using groundwater spiked with fluoride ions. The adsorption of fluoride achieved equilibrium after 120 min with a 0.9 g/L adsorbent dosage. The Langmuir model described the equilibrium data with monolayer adsorption capacity of 7.06 mg/g at 303 K. Kinetically, fluoride adsorption took place by obeying the pseudo-second-order kinetic model with intra-particle diffusion as the rate determining step. The fluoride loaded adsorbent could be efficiently regenerated using 0.05 M NaOH. The low cost associated in the preparation of MPJER encourages its utilization as a potential absorbent for the remediation of fluoride contaminated groundwater. The application of MPJER may improve the indigenous household iron filters for the removal of fluoride as well.
Highlights
-
The developed adsorbent, MPJER is low cost and renewable. It is highly efficient for the adsorption of fluoride from aqueous media. The adsorption process achieves equilibrium at 2 h with a dose of 0.9 g/L at natural pH condition.
-
The monolayer adsorption capacity of MPJER is7.06 mg/g.
-
Mechanism of adsorption showed the involvement of H-bonding and intra-particle diffusion.
-
81% desorption of fluoride was achieved using 0.05 M NaOH.
-
Low cost associated for the preparation of the MPJER justified its utilization in the removal of fluoride from wastewater.














Similar content being viewed by others
Data Availability
All data are fully available without restriction.
References
Abe I, Iwasaki S, Tokimoto T, Kawasaki N, Nakamura T, Tanada S (2004) Adsorption of fluoride ions onto carbonaceous materials. J Colloid Interface Sci 275(1):35–39
Ahamad KU, Jawed M (2007) Role of wooden charcoal in indigenous household iron filters used in Assam (India). Asian J Water Environ Pollut 5:23–28
Ahmed MdJK, Ahmaruzzaman M, Reza RA (2014) Lignocellulosic-derived modified agricultural waste: development, characterization and implementation in sequestering pyridine from aqueous solutions. J Colloid Interface Sci 428:222–234
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Møller KN, Sarr M, Johnson CA (2008) Statistical modeling of global geogenic fluoride contamination in ground waters. Environ Sci Technol 42:3662–3668
ASTM-D1762-84 (1984) Annual book of ASTM standard D1762-84. pp 292–293.
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36(6):433–487
Azbar N, Turkman A (2000) Defluorination in drinking waters. Water Sci Technol 42:403–407
Banks D, Reimann C, Røyset O, Skarphagen H, Saether OM (1995) Natural concentrations of major and trace elements in some Norwegian bedrock groundwater. Appl Geochem 10:1–16
Bharath G, Alhseinat E, Ponpandian P, Khan MA, Siddiqui MR, Ahmed F, Alsharaeh EH (2017) Development of adsorption and electrosorption techniques for removal of organic and inorganic pollutants from wastewater using novel magnetite/porous graphene-based nanocomposites. Sep Purif Technol 188:206–218
Bharath G, Hai A, Rambabu K, Ahmed F, Haidyrah AS, Ahmad N, Hasan SW, Banat F (2021) Hybrid capacitive deionization of NaCl and toxic heavy metal ions using faradic electrodes of silver nanospheres decorated pomegranate peel-derived activated carbon. Environ Res 197:111110
Biswas K, Debnath S, Ghosh UC (2010) Physicochemical aspects on fluoride adsorption for removal from water by synthetic hydrous iron(III)-chromium (III) mixed oxide. Sep Sci Technol 45:472–485
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multi molecular layers. J Am Chem Soc 60:309–319
Costodes VCT, Fauduet H, Porte C, Ho YS (2005) Removal of lead (II) ions from synthetic and real effluents using immobilized Pinussylvestris sawdust: adsorption on a fixed-bed column. J Hazard Mater 123:135–144
Czarnowski W, Wrzesniowska K, Krechniak J (1996) Fluoride in drinking water and human urine in Northern and Central Poland. Sci Total Environ 191:177–184
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412
Garaga RJ (2015) Evaluation of filter media of traditional filter units of Assam and performance monitoring of filter unit under field conditions. M. Tech Thesis, Department of Civil Engineering, Indian Institute of Technology Guwahati, India
Goel J, Kadirvelu K, Rajagopal C, Garg VK (2016) Removal of lead (II) by adsorption using treated granular activated carbon: batch and column studies. J Hazard Mater 125:211–220
Hussain D, Khan SA, Khan TA (2021) Fabrication and characterization of mesoporous guar gum/NiWO4 nanocomposite for efficient adsorption of phloxine B and crystal violet from aqueous solution and evaluation of its antioxidant activity. Colloids Interface Sci Commun 44:100488
Hussain D, Siddiqui MF, Shirazi Z, Khan TA (2022) Evaluation of adsorptive and photocatalytic degradation properties of FeWO4/polypyrrole nanocomposite for rose Bengal and alizarin red S from liquid phase: modeling of adsorption isotherms and kinetics data. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.13822
Kalyani G, Rao GB, Saradhi BV, Kuma YP (2009) Biosorption isotherms of fluoride from aqueous solution on Ulva fasciata sp.—a waste material. Int J Appl Environ Sci 4:173–182
Kamble SP, Jagtap S, Labhsetwar NK, Thakare D, Godfrey S, Devotta S, Rayalu SS (2007) Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem Eng J 129:173–180
Khan TA, Khan EA, Shahjahan (2016) Adsorptive uptake of basic dyes from aqueous solution by novel brown linseed deoiled cake activated carbon: equilibrium isotherms and dynamics. J Environ Chem Eng 4(3):3084–3095
Khan EA, Shahjahan, Khan TA (2018) Adsorption of methyl red on activated carbon derived from custard apple (Annona squamosa) fruit shell: equilibrium isotherm and kinetic studies. J Mol Liq 249:1195–1211
Khan TA, Sidqui MF, Abbasi N, Alharthi SS (2022a) Adsorptive decolouration of anionic dye from water by goat drop** activated carbon prepared via microwave-assisted H3PO4 activation: process optimization using response surface methodology, isotherm and kinetics modelling. Biomass Convers Biorefin
Khan TA, Nouman M, Dua D, Khan, Khan SA, Alharthi SS (2022b) Adsorptive scavenging of cationic dyes from aquatic phase by H3PO4 activated Indian jujube (Ziziphus mauritiana) seeds based activated carbon: isotherm, kinetics, and thermodynamic study. J Saudi Chem Soc 26:101417
Klokov SV, Locative ES, Golubina EV, Malenkov KI, Levanov AV, Chernyak SA, Likholobov VA (2016) Effective Pd/C catalyst for chloro benzene and hexachloro-benzene hydride chlorination by direct pyrolysis of sawdust impregnated with palladium nitrate. Catal Commun 77:37–41
Liang LV, He J, Wei M, Evans DG, Duan X (2006) Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–Co3 layered double hydroxides. J Hazard Mater B113:19–128
Linsen BG (1970) Physical and chemical aspects of adsorbents and catalyst. Academic Press, London
Liu Z, Khan TA, Azharullslam M, Tabrez U (2022) A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour Technol 354:127168
Malolan R, Jayaraman RS, Adithya S, Arun J, Gopinath KP, Rajan PSS, Nasif O, Kim W, Govarthanan M (2021) Anaerobic digestate water for Chlorella pyrenoidosa cultivation and employed as co-substrate with cow dung and chicken manure for methane and hydrogen production: a closed loop approach. Chemosphere 266:128963
Mennakshi, Matheshari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater B137:456–463
Mohapatra M, Rout K, Singh P, Anand S, Layek S, Verma HC, Mishra BK (2011) Fluoride adsorption studies on mixed-phase nano iron oxides prepared by surfactant mediation-precipitation technique. J Hazard Mater 186:1751–1757
Raichur AM, Basu JM (2001) Adsorption of fluoride onto mixed rare earth oxides. Sep Purif Technol 24:121–127
Rathore VK, Dohare DK, Mondal P (2016) Competitive adsorption between arsenic and fluoride from binary mixture on chemically treated laterite. J Environ Chem Eng 4:2417–2430
Reza RA, Ahmed MJK, Sil AK, Ahmaruzzaman M (2014) A non-conventional adsorbent for the removal of clofibric acid from aqueous phase. Sep Sci Technol 491:592–1603
Saha S (1993) Treatment of aqueous effluent for fluoride removal. Water Res 27:1347–1350
Sharma R, Shah S, Mahanta C (2005) Hydrogeochemical study of groundwater fluoride contamination: a case study from Guwahati city India. Asian J Water Environ Pollut 2:47–54
Shortt WE (1937) Endemic fluorosis in Nellore District, South India. Indian Medical Gazette, 72–396
Siddiqui FA, Khan SA, Hussain D, Tabrez U, Ahamad I, Tasneem F, Khan TA (2022) A sugarcane bagasse carbon-based composite material to decolor and reduce bacterial loads in waste water from textile industry. Ind Crops Prod 176:114301
Singh G, Kumar B, Sen PK, Maunder J (1999) Removal of fluoride from spent pot liner leach ate using ion exchange. Water Environ Res 71:36–42
Sujanaa MG, Mishrab A, Acharyaa BC (2013) Hydrous ferric oxide doped alginate beads for fluoride removal: adsorption kinetics and equilibrium studies. Appl Surf Sci 270:767–776
Sundaram CS, Viswanathan N, Meenakshi S (2008) Uptake of fluoride by nano-hydroxyapatite/chitosan, a bioinorganic composite. Bioresour Technol 99:8226–8230
Tein C (1994) Adsorption calculations and modelling. Butterworth-Heinemann, Boston
Thakre JD, Jagtap S, Bansiwal A, Labhsetwar N, Rayalu S (2010) Synthesis of La-incorporated chitosan beads for fluoride removal from water. J Fluor Chem 131:373–377
Vijaya Y, Krishnaiah A (2009) Sorptive response profile of chitosan coated silica in the defluoridation of aqueous solution. e-J Chem 6:713–724
Viswanathan N, Meenakshi S (2008) Selective sorption of fluoride using Fe(III) loaded carboxylated chitosan beads. J Fluor Chem 129:503–509
Viswanathan N, Sundaram CS, Meenakshi S (2009) Removal of fluoride from aqueous solution using protonated chitosan beads. J Hazard Mater 161:423–430
Viswanathana N, Meenakshib S (2010) Development of chitosan supported zirconium (IV) tungstophosphate composite for fluoride removal. J Hazard Mater 176:459–465
Wang LFM, Huang JZ (1995) Outline of control practice of endemic fluorosis in China. Soc Sci Med 41:1191–1195
WHO (2006) Guidelines for drinking-water quality [electronic resource]: incorporating first addendum, pp 375–377
Yamuna M, Kamaraj M (2016) Pineapple peel waste activated carbon as an adsorbent for the effective removal of methylene blue dye from aqueous solution. Int J Chem Tech Res 9(5):544–550
Yang J, Qiu K (2010) Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chem Eng J 165:209–217
Yao R, Meng F, Zhang L, Ma D, Wang M (2009) Defluoridation of water using neodymium-modified chitosan. J Hazard Mater 165:454–460
Author information
Authors and Affiliations
Contributions
RAR data curation, writing—original draft preparation, methodology and software, review and revision. MA supervision, concept of study, critical review, commentary and revision, and investigation.
Corresponding author
Rights and permissions
About this article
Cite this article
Reza, R.A., Ahmaruzzaman, M. Remediation of Fluoride from Groundwater Using Modified Pineapple Juice Extracted Residue. Int J Environ Res 16, 52 (2022). https://doi.org/10.1007/s41742-022-00426-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-022-00426-5