Abstract
Anaerobic digestion (AD) can remove the high organic load of molasses wastewater (MWW), but is ineffective in removing the characteristic dark brown colour caused by biorecalcitrant melanoidin. Ultraviolet (UV) photodegradation, can mineralise the biorecalcitrants leading to complete colour removal, but with a high-energy input and low organic load removal. This study investigated the plausibility of integrating the two processes for organic load and colour reductions. Desk scale AD and photodegradation processes were carried out in batch reactors. A hybrid photocatalyst consisting of titanium dioxide (TiO2) and zinc oxide (ZnO) was used for photocatalytic degradation. Biodegradation at thermophilic conditions in the bioreactor achieved high total organic carbon (TOC) and chemical oxygen demand (COD) reductions of 80 and 90%, respectively, but with an increased colour intensity. Contrastingly, UV photodegradation achieved a high colour reduction of 92% with an insignificant 6% TOC reduction, after 30 min of irradiation. During photodegradation, the mineralisation of the biorecalcitrant organic compounds led to the colour disappearance. The energy required for the TOC reduction was 40-fold higher than that required for the colour reduction in the same irradiation period, suggesting that the UV process was only suitable for colour reduction. Therefore, there is a possible synergy when the two processes are integrated, with AD preceding UV, where AD removes the high COD/TOC while UV removes the recalcitrant colour at a reduced cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol production from molasses is among the most polluting agro-based industries, generating a large amount of wastewater with high organic content (Satyawali and Balakrishnan 2008). Normally, for every litre of alcohol that is produced, about 15 litres of high-strength distillery effluent is generated (Wilkie et al. 2000; De Vrieze et al. 2014). In the distillation process, molasses is diluted and taken to a fermenter to produce bioethanol with the addition of yeast. The fermentation broth is filtered and then distilled to concentrate bioethanol. The final effluent after the distillation process is the molasses wastewater (Arimi et al. 2014; Chavan et al. 2006). Molasses wastewater (MWW) pollutes the aquatic ecosystem due to its dark brown colour, which cuts off light thereby preventing photosynthesis, leading to the death of aquatic plants. The high organic load [chemical oxygen demand (COD) of 80,000–100,000 mg/L] of the effluent leads to eutrophication, which further creates unwanted anaerobic conditions in the aquatic systems (Jai Shankar Pillai et al. 2012). MWW also affects agricultural crops in cases where it is directly disposed of into the soil by causing soil acidification (Navgire et al. 2012; De Vrieze et al. 2014). It is, therefore, of a necessity to effectively treat MWW for its high organic load and characteristic dark brown colour before discharge into receiving streams.
Anaerobic digestion (AD) has been employed conventionally for the treatment of wastes such as MWW due to its ability to degrade high strength wastewater with minimal sludge production and energy recovery in the form of biogas (Arnaiz et al. 2003). The AD process, however, is incapable of complete removal of biorecalcitrant organic compounds such as colour, causing melanoidin and phenolic compounds found in MWW. These compounds, especially the melanoidins, have a high inhibitory antimicrobial activity and are, therefore, not removed during AD (Chandra et al. 2008; Liu et al. 2013). Melanoidins, which constitutes 2% of the MWW, are polymeric in nature with high molecular weight (usually >5.0 kDa) making them anti-oxidative hence the recalcitrant nature (Liang et al. 2009; Liu et al. 2013). Moreover, in some instances, the melanoidin compounds undergo polymerisation during AD leading to the generation of AD effluents with a more intense dark colour (Mohana et al. 2009). The AD process, therefore, is only effective for organic load reduction with a minimal or negligible reduction in colour. Since AD alone cannot achieve effective MWW treatment, there is need to integrate it with other treatment methods such as advanced oxidation processes (AOPs), which can completely mineralize the recalcitrant organic pollutants and are considered as highly competitive water treatment technologies (Oller et al. 2011).
Photocatalytic degradation of MWW, an advanced oxidation treatment, has been reported to be effective for the complete removal of the biorecalcitrant melanoidins contained in MWW leading to the colour disappearance. This process, however, is ineffective for the removal of the high organic content of MWW and has, therefore, been recommended for application as a plausible post-treatment to AD (Otieno et al. 2016). Moreover, the widely used semiconductor oxide photocatalyst titanium (TiO2) and zinc (ZnO) despite having desirable properties such as low cost, chemical stability, and high resistance to photo-corrosion, still suffer from low quantum efficiency due to the rapid recombination of electron–hole pairs (Bechambi et al. 2015a; Jiang et al. 2013). The generated electrons react with water molecules leading to the production of highly reactive hydroxyl radicals (OH·), which initiate micropollutant degradation in addition to direct hole attack (Arany et al. 2014). To reduce the recombination rate and improve quantum yield, TiO2 and ZnO have been modified through do** with metals or non-metals or coupling with other semiconductors (Bechambi et al. 2015b; Otieno et al. 2017). The coupling of ZnO with TiO2 has gained interest recently as it results in the formation of a hybrid semiconductor (TiO2–ZnO) with superior photocatalytic activity and stability (Behzadnia et al. 2015). Jonidi-Jafari et al. (2015) developed a TiO2–ZnO hybrid semiconductor photocatalyst, which showed an improved activity in the photodegradation of Diazinon as compared to TiO2 and ZnO on their own. The improved activity was due to increased quantum efficiency resulting from reduced recombination rate of electron–hole pairs and extended range of light absorption.
The capability of AD in treating MWW for the high organic content and its inability to remediate the characteristic dark colour is widely known. However, it is of a necessity to gather information on the efficiency of employing AOPs such as photocatalytic degradation alongside AD, for colour and organic load elimination. Bench-scale and pilot-scale studies are important for information generation and development of technologies for new treatment processes. Such studies have been found to come in handy when investigating the possibility of combining two or more technologies for the effective treatment of wastewater (Oller et al. 2011). In this study, therefore, we report the separate treatment of MWW employing both AD and photocatalytic degradation using TiO2–ZnO hybrid, on a bench scale. Of special interest is the investigation of the plausibility of integrating the two processes for effective MWW treatment based on energy demand, and organic load and colour removal efficiencies.
Methods
Materials
Commercial molasses used for preparing MWW was outsourced from a local sugar milling company in Gauteng, South Africa (RSA) and kept at room temperature (25 °C). Cow dung used to prepare inoculum was obtained from a farm (Carletonville, RSA) and stored in a refrigerator at 4 °C before use, to minimise the decomposition of essential microbes. Zinc chloride (ZnCl2, 97%), sodium hydroxide (NaOH), and titanium (IV) oxide (TiO2, peroxide P25) used for synthesising the hybrid photocatalyst were sourced from Sigma-Aldrich, RSA.
Equipment
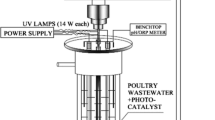
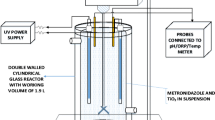
The desk-scale lab experimental setup (Fig. 1a), for AD consisted of an anaerobic batch reactor, a peristaltic pump, and a water bath. The batch reactor was a cylindrically shaped vessel with a total volume of 4 L and operational volume of 3.5 L. The bioreactor was sealed with an airtight lid at the top. Mixing was achieved by circulating the wastewater using a peristaltic pump. The AD experiments were maintained at thermophilic temperatures (55 °C) by means of the water bath. Mariotte water displacement method was used to collect the biogas produced. A batch photoreactor of 500 mL capacity with a 12 W UVC lamp, was sourced from Puritech, RSA, and used for photodegradation. The experimental setup for photodegradation consisted of a stirring plate, peristaltic pump, and a photoreactor (Fig. 1b).
Catalyst synthesis and characterization
The hybrid catalyst, TiO2–ZnO with TiO2 to ZnO ratio of 1:1 by weight was synthesised adopting a previous method employed by Jonidi-Jafari et al. (2015). In the method, ZnO was precipitated on the surface of commercial TiO2, from ZnCl2 and NaOH. The obtained hybrid was dried, calcined at 500 °C in a muffle furnace and characterised. The characterisations were carried out using scanning electron microscopy–energy-dispersive X-ray spectroscopy, SEM–EDX, (SEM-FEI Quanta 250 FEG ESEM with an integrated Oxford X-Max 20 EDX system), X-ray diffraction (XRD, Rigaku – Ultima IV, X-ray 40 kV, scan range 3°–90°), and a Jasco spectrofluorometer FP-8600 at an excitation wavelength of 425 nm.
Inoculum preparation and bioreactor operation
The inoculum was prepared by diluting the obtained cow dung using distilled water forming a slurry, which was then fed with glucose and nutrients to enhance the active microorganism population. The inoculum was thereafter incubated at thermophilic conditions in the water bath while adding MWW gradually, for acclimatisation, until a stable of pH of about 7 was obtained. Molasses wastewater (MWW) for AD, was prepared by diluting the commercial molasses obtaining a substrate of desired initial concentration. The characteristics of the commercial molasses, inoculum and the prepared MWW for AD are given in Table 1. To start the biodegradation experiment, the bioreactor was fed with the initially prepared inoculum and MWW. The mixture was diluted obtaining an initial total organic carbon concentration (TOC) and chemical oxygen demand (COD) of 3200 and 6600 mg/L, respectively. During digestion, samples were periodically collected from the bioreactor and analysed to investigate operating parameters such as COD, pH, TOC, biogas production, and nutrients reduction.
Photodegradation studies
For photodegradation purposes, the commercial molasses was again dissolved in distilled water to obtain a dark coloured MWW, but with minimal organic content (initial TOC concentration of 500 mg/L), as compared to the MWW used in AD, which had an initial TOC of 3200 mg/L. The synthesised catalyst was added to the prepared MWW at a catalyst loading of 1 g/L in the dark and continuously stirred for 1 h ensuring adsorption–desorption equilibrium. After adsorption, the solution was transferred to the photoreactor via a peristaltic pump for irradiation. Samples were taken at predetermined time intervals, subsequently filtered with 0.45 µm membrane filters and then analysed for colour and TOC. To monitor the mineralisation process during photodegradation, samples withdrawn at different time intervals were analysed for the presence of organic functional groups.
Analytical methods
Chemical oxygen demand (COD) analysis was carried out using closed reflux colorimetric method with potassium dichromate solution as oxidant and nano colour colorimeter. Colour analysis was determined from the absorbance at λ max of 475 nm using a UV–Vis spectrophotometer (T80+ UV/VIS Spectrometer, PG Instruments Ltd). TOC was determined from a TOC analyser (TELEDYNE Tekmar), while nutrient analysis was done using ion chromatography (IC, 882 Compact). The biogas composition was analysed using gas chromatography (Trace 1310 GC). Change in organic functional groups during photodegradation due to the mineralisation of the organic recalcitrants was determined from Fourier to transform infrared (PerkinElmer FT-IR/NIR).
Findings
Catalyst characterization
From the SEM image of TiO2–ZnO, Fig. 2, a possible hybridization of ZnO and TiO2 can be seen from the well dispersed smaller ZnO particles on the surface of the bigger TiO2 particles, similarly observed by Jonidi-Jafari et al. (2015). The presence of ZnO nanoparticles well distributed on the surface of TiO2 nanoparticles is of importance as it retards charge recombination. ZnO has a lower electron affinity to organic chromophores as compared with TiO2. The nanoparticles of ZnO are regarded as highly efficient charge traps, providing enough distributed surface traps, thereby reducing the charge recombination with minimal hindrance to organics-TiO2 electron injection (Mozaffari et al. 2015). The XRD patterns in Fig. 3a and photoluminescence (PL) spectra Fig. 3b show the crystalline structure and the electron–hole pairs recombination rate of the catalysts, respectively. In the XRD patterns, peaks observed for the ZnO curve represents hexagonal wurtzite ZnO (Zhang et al. 2012; Bechambi et al. 2015a, b). The XRD curve for TiO2 has peaks characteristic of anatase crystalline phase (Bel Hadjltaief et al. 2016). Liao et al. (2008) observed a similar curve for TiO2 with a dominant peak corresponding to 2θ = 25.3° in the pattern and attributed it to a dominant anatase phase.
XRD peak intensity is proportional to the content of corresponding crystallites and also the sharpness of the peak indicates higher crystallinity (Ohtani 2010; Bechambi et al. 2015a, b). The ZnO catalyst with sharp and intense XRD peaks was more crystalline than TiO2 that had less intense peaks. Coupling ZnO with TiO2 to form TiO2–ZnO hybrid altered the crystallinity of both catalysts while maintaining the phase composition as seen with the reduction in peak heights, but with the retention of number and position of peaks. In the TiO2–ZnO nanocomposite, both the characteristic peaks of wurtzite ZnO and anatase TiO2 were gradually decreased. The crystalline phase of the hybrid was more composed of ZnO than TiO2 as observed with the high number of peaks corresponding to ZnO. The XRD patterns thus indicate a successful preparation of TiO2–ZnO hybrid.
The PL spectra are generally used in the study of surface processes that involve electron–hole pairs of a catalyst. Electron–hole pair recombination resulting after a photocatalyst has been irradiated causes emission of photons, which results in photoluminescence. This behaviour is caused by the reverse radiative deactivation from the excited state (Xu et al. 2010). From the PL intensities in Fig. 3b, the hybrid TiO2–ZnO had the lowest intensity as compared to the pure ZnO and TiO2 thus indicating a reduced electron–hole pair recombination (Bechambi et al. 2015a, b). The reduced recombination is attributable to effective separation of electron/hole pairs in the hybrid system (Liu et al. 2010). In this system, transfer of electrons can take place from the conduction band (CB) of TiO2 to that of ZnO, while hole transfer takes place from the valence band of ZnO to that of TiO2 (Wang et al. 2013). This leads to charge separation efficiency, increased charged carriers lifetime and efficient transfer of interfacial charge to adsorbed substrates (Habib et al. 2013).
Anaerobic digestion
Effect of pH change on the production of biogas
Figure 4 represents the daily amount of biogas produced and pH variations during anaerobic digestion of MWW. From previous studies, it has been reported that an optimum pH range for the growth of methanogens lies within pH of 6.0–8.0 (Apollo et al. 2013; Acharya et al. 2008). The steady increase in pH over time is a sign of proficient degradation of the organic compounds into methane and carbon dioxide. The best degradation rate of organic matter with the highest biogas volume of 700 mL/day produced was attained at a pH level lower than 7.5. These findings are consistent with previous studies conducted by Li et al. (2011). The increase in pH may be attributed to the formation of ammonium nitrogen, which is generated during the degradation of proteins (Drosg 2013). According to Chen et al. (2008), methanogens have the lowest tolerance for ammonium nitrogen and are most likely to stop growing due to ammonia inhibition. The initial rapid increase in biogas production can be attributed to the readily available biodegradable organics in the feed, resulting in the high generation of methane during the initial stages of the process. The results also show that biogas production ceased on the 9th day at a substrate pH of 8.2. This may be due to the generation of the ammonium nitrogen and the depletion of the biodegradable organic substrate.
Cumulative biogas volume and biogas composition obtained during AD
Figure 5a, b shows the cumulative amount of biogas produced as a function of time and the biogas composition after 9 days. After 9 days of AD, a total volume of 1839 mL of biogas was produced. The average composition of the biogas obtained was 91.74 and 8.26% for methane and carbon dioxide, respectively.
TOC, COD, nutrient, and colour reductions during AD
The results obtained in this study show a significant difference in the percentage reduction of COD and TOC concentrations (Fig. 6). The reduction was due to the degradation and conversion of organic compounds into biogas (mainly CH4 and CO2). The achieved removal efficiencies of COD and TOC during the anaerobic treatment of MWW were 90 and 80%, respectively. It was reported by Ibn Abubakar and Ismail (2012), that bioconversion of organics into biogas is highly influenced by microbial activity within the biodigester. Therefore, factors such as biogas volume, production rate, and methane composition (shown in Figs. 4, 5a, b, respectively) are indicative of the readily available organics for chemical oxidation. The AD system is thus very effective in removing the high organic load of MWW with the recovery of bioenergy in form of biogas.
The reduction of nutrient concentration during the anaerobic treatment of MWW is shown in Fig. 7. The general decrease in nitrates and phosphates indicate their consumption by the anaerobic bacteria for microbial cell growth (Jackson-Moss and Duncan 1990). Alternatively, the concentration reduction of nitrate could have been due to the denitrification process in the bioreactor (Acharya et al. 2008). In the present study, the sulphate concentration reduced from 285.49 to 47.5 mg/L on the 9th day, resulting in a high sulphate removal rate. The high sulphate removal was also observed by Satyawali and Balakrishnan (2008) and was attributed to sulphate consumption by anaerobes as nutrients, and by sulphate reducing bacteria (SRB).
The colour intensity increased by 49% during the anaerobic process as depicted by the negative reduction in Fig. 8. The increased intensity could be due to the re-polymerization of the colour causing melanoidin compounds (Liang et al. 2009). Apollo et al. (2013) also observed similar results with a 13% increase in colour intensity during AD of MWW at mesophilic conditions. The high increase observed in this study is attributable to the thermophilic conditions employed, which offers conditions similar to those leading to the formation and polymerization of melanoidins (Satyawali and Balakrishnan 2008). It is noticeable that biological treatment of MWW with AD is highly effective in reducing the high organic load, but very poor in colour removal. In this case, anaerobically-treated molasses wastewater effluent cannot be directly discharged into receiving streams without undergoing further treatment. For this reason, AD should be combined with a post-treatment method for colour abatement.
Photodegradation studies
Colour reduction during photodegradation
Unlike the anaerobic digestion, which was poor in colour reduction, photodegradation was found to be very effective in colour reduction. The highest colour removal of 92% was achieved with the TiO2–ZnO hybrid as compared to TiO2 and ZnO which had 75 and 48% removals, respectively, after 30 min of irradiation, Fig. 9. The overall activity of the two semiconductors, when used in the hybrid, outweighed the sum activity of each signifying the realisation of a synergistic effect (Ohtani 2010). The hybrid photocatalyst had improved activity attributable to a reduced recombination rate of electron–hole pairs as observed in the PL analysis (see Fig. 3b). Coupling of ZnO and TiO2, therefore, resulted in a highly photoactive hybrid catalyst capable of removing MWW colour after a short irradiation time.
Energy demand and mineralisation process
Thirty minutes of UV light irradiation achieved colour reduction of 92%, and only 6% of TOC removal was realised, Fig. 10a. Again, apart from synergy in colour reduction, another synergy for TOC reduction was revealed. Thus, AD complements photodegradation in TOC reduction, while photodegrading aides in colour reduction. Removal efficiency for organic pollutants under UV photocatalytic processes can be determined from the energy requirement of the process. Energy efficiency of the photocatalytic processes can be evaluated using electrical energy per order (E o), which is defined as the required amount of electrical energy for the reduction of pollutant concentration in water. The E o is convenient for comparing the effectiveness of removal of various pollution parameters such as TOC and colour for the same wastewater. Also for the purposes of process optimisation and operation, the knowledge of electrical energy requirement for removal of contaminants is of great interest. The electrical energy efficiency of the batch photodegradation process was determined from electrical energy per order (E o) given by:
where P is the lamp power (kW), t is the duration of irradiation (minutes), V is the amount of wastewater degraded (L), C f and C i are the final and initial concentrations, respectively, (Jonidi-Jafari et al. 2015). It was observed from Fig. 10a, that after 60 min of irradiation, the colour removal of 92% only required 10.42 kWh/m3 of energy as compared to 452.83 kWh/m3 of energy required for the 6% TOC removal during the same period, further demonstrating the high-energy requirement for UV photo-reduction of TOC. Smaller values of are desirable as they signify efficient processes requiring less electrical energy in achieving the treatment goal (Shu et al. 2013).
The UV photodegradation process exhibited better and more efficient performance for the reduction of MWW colour as compared to the reduction of TOC. The colour causing melanoidin compounds were easily degraded under UV irradiation as compared to the more stable organic compounds responsible for TOC. Treatment of MWW using a UV photocatalyzed process for TOC reduction is, therefore, not feasible given the high electrical energy consumption resulting in high cost of operation. The higher the E o value of a pollutant parameter, the costlier and more difficult it will be to remove that parameter, relative to other parameters with lower E o values.
The FTIR spectra of MWW samples undergoing photodegradation at different time intervals (Fig. 10b), showed decreased peak intensities with increased irradiation time signifying mineralisation of the organic recalcitrant. After 60 min of irradiation, the peak intensities were reduced as compared to 30 min of irradiation, and upon continued irradiation, for another 60 min, the characteristic broad OH peak appearing at 3000 cm−1 was almost diminished. Even though after 60 min all the colour had been removed, the observed broad characteristic –OH peak indicated the presence of organic compounds, which required further irradiation for complete mineralisation. Colour disappearance, therefore, does not signify complete mineralisation as in most cases the colour causing melanoidin is first broken down to colourless intermediates such as salicylic acid. Because of the high-energy demand by the UVC lamp, a prolonged period of irradiation to achieve complete mineralisation at high initial TOC will result in high cost of operation. This further highlights the importance of employing photocatalysis alongside other treatment processes such as biodegradation, where the high TOC will be first removed before the rapid removal of the biorecalcitrant colour-causing melanoidin compounds through UV photodegradation.
Conclusion
Anaerobic digestion is very effective for the removal of the high organic load from MWW, but ineffective in removing the characteristic dark brown colour caused by biorecalcitrant melanoidins. UV photodegradation, on the other hand, can degrade the colour causing melanoidins leading to complete colour removal after a short duration of irradiation. However, the UV process is energy intensive and incapable of effective TOC reduction. For effective MWW treatment, these two processes can be integrated such that UV photodegradation is applied as a post-treatment to AD. In this system, the AD will first remove the high organic content followed by colour removal by the UV process. This integrated process, however, needs to be optimised to determine the best operational and combinational factors. Evaluating the overall energy demand of such a system is also important in determining whether the bioenergy produced from the AD process can be used to supplement the energy requirement of the integrated system.
References
Acharya BK, Mohana S, Madamwar D (2008) Anaerobic treatment of distillery spent wash—a study on upflow anaerobic fixed film bioreactor. Bioresour Technol 99:4621–4626. doi:10.1016/j.biortech.2007.06.060
Apollo S, Onyango MS, Ochieng A (2013) An integrated anaerobic digestion and UV photocatalytic treatment of distillery wastewater. J Hazard Mater 261:435–442. doi:10.1016/j.jhazmat.2013.06.058
Arany E, Láng J, Somogyvári D, Láng O, Alapi T, Ilisz I, Gajda-Schrantz K et al (2014) Vacuum ultraviolet photolysis of diclofenac and the effects of its treated aqueous solutions on the proliferation and migratory responses of Tetrahymena pyriformis. Sci Total Environ 468–469:996–1006. doi:10.1016/j.scitotenv.2013.09.019
Arimi MM, Zhang Y, Götz G, Kiriamiti K, Geißen SU (2014) Antimicrobial colorants in molasses distillery wastewater and their removal technologies. Int Biodeterior Biodegrad 87:34–43. doi:10.1016/j.ibiod.2013.11.002
Arnaiz C, Buffiere P, Elmaleh S, Lebrato J, Moletta R (2003) Anaerobic digestion of dairy wastewater by inverse fluidization: the inverse fluidized bed and the inverse turbulent bed reactors. Environ Technol 24:1431–1443. doi:10.1080/09593330309385687
Bechambi O, Najjar W, Sayadi S (2015a) The nonylphenol degradation under UV irradiation in the presence of Ag–ZnO nanorods: effect of parameters and degradation pathway. J Taiwan Inst Chem Eng. doi:10.1016/j.jtice.2015.11.017
Bechambi O, Sayadi S, Najjar W (2015b) Photocatalytic degradation of bisphenol A in the presence of C-doped ZnO: effect of operational parameters and photodegradation mechanism. J Ind Eng Chem 32:201–210. doi:10.1016/j.jiec.2015.08.017
Behzadnia A, Montazer M, Rad MM (2015) Simultaneous sonosynthesis and sonofabrication of N-doped ZnO/TiO2 core–shell nanocomposite on wool fabric: introducing various properties specially nano photo bleaching. Ultrason Sonochem 27:10–21. doi:10.1016/j.ultsonch.2015.04.017
Bel Hadjltaief H, Ben Zina M, Galvez ME, Da Costa P (2016) Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J Photochem Photobiol A Chem 315:25–33. doi:10.1016/j.jphotochem.2015.09.008
Chandra R, Naresh R, Rai V (2008) Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation 99:4648–4660. doi:10.1016/j.biortech.2007.09.057
Chavan MN, Kulkarni MV, Zope VP, Mahulikar PP (2006) Microbial degradation of melanoidins in distillery spent wash by an indigenous isolate. Indian J. Biotechnol 5:416–421
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Bioresour Technol 99:4044–4064. doi:10.1016/j.biortech.2007.01.057
De Vrieze J, Hennebel T, Van den Brande J, Bilad RM, Bruton TA, Vankelecom IFJ, Verstraete W, Boon N (2014) Anaerobic digestion of molasses by means of a vibrating and non-vibrating submerged anaerobic membrane bioreactor. Biomass Bioenergy 68:95–105. doi:10.1016/j.biombioe.2014.06.009
Drosg B (2013) Process monitoring in biogas plants. International Energy Agency Bioenergy, France. ISBN 978-1-910154-03-8
Habib A, Shahadat T, Bahadur NM, Ismail IMI, Mahmood AJ (2013) Synthesis and characterization of ZnO–TiO2 nanocomposites and their application as photocatalysts. Int Nano Lett 3:1–8. doi:10.1186/2228-5326-3-5
Ibn Abubakar BSU, Ismail N (2012) Anaerobic digestion of cow dung for biogas production. ARPN J Eng Appl Sci 7:169–172
Jackson-Moss C, Duncan J (1990) The effect of iron on anaerobic digestion. Biotechnol Lett 154:149–154. doi:10.3846/mla.2011.97
Jai Shankar Pillai HP, Ananth Nag B, Girish K, Puttaiah ET (2012) Biodegradation of solid waste molasses from Aranthangi sugar industry, Tamilnadu by using Aspergillus niger. Intern J Environ Sci 1:289–293
Jiang H, Yan P, Wang Q, Zang S, Li J, Wang Q (2013) High-performance Yb, N, P-tridoped anatase-TiO2 nano-photocatalyst with visible light response by sol-solvothermal method. Chem Eng J 215–216:348–357. doi:10.1016/j.cej.2012.10.082
Jonidi-Jafari A, Shirzad-Siboni M, Yang JK, Naimi-Joubani M, Farrokhi M (2015) Photocatalytic degradation of diazinon with illuminated ZnO–TiO2 composite. J Taiwan Inst Chem Eng 50:100–107. doi:10.1016/j.jtice.2014.12.020
Li A, Li X, Yu H (2011) Effect of the food-to-microorganism (F/M) ratio on the formation and size of aerobic sludge granules. Process Biochem 46:2269–2276. doi:10.1016/j.procbio.2011.09.007
Liang Z, Wang Y, Zhou Y, Liu H (2009) Coagulation removal of melanoidins from biologically treated molasses wastewater using ferric chloride. Chem Eng J 152:88–94. doi:10.1016/j.cej.2009.03.036
Liao DL, Badour CA, Liao BQ (2008) Preparation of nanosized TiO2/ZnO composite catalyst and its photocatalytic activity for degradation of methyl orange. J Photochem Photobiol A Chem 194:11–19. doi:10.1016/j.jphotochem.2007.07.008
Liu G, Wang L, Yang HG, Cheng HM, (Max) Lu GQ (2010) Titania-based photocatalysts—crystal growth, do** and heterostructuring. J Mater Chem 20:831–843. doi:10.1039/B909930A
Liu M, Zhu H, Dong B, Zheng Y, Yu S, Gao C (2013) Submerged nanofiltration of biologically treated molasses fermentation wastewater for the removal of melanoidins. Chem Eng J 223:388–394. doi:10.1016/j.cej.2013.02.107
Mohana S, Acharya BK, Madamwar D (2009) Distillery spent wash: treatment technologies and potential applications. J Hazard Mater 163:12–25. doi:10.1016/j.jhazmat.2008.06.079
Mozaffari S, Ranjbar M, Kouhestanian E, Salar Amoli H, Armanmehr M (2015) An investigation on the effect of electrodeposited nanostructured ZnO on the electron transfer process efficiency of TiO2 based DSSC. J Hazard Mater 40:310–318. doi:10.1016/j.tsf.2007.08.113
Navgire M, Yelwande A, Tayde D, Arbad B, Lande M (2012) Photodegradation of molasses by a MoO3–TiO2 nanocrystalline composite material. Chin J Catal 33:261–266
Ohtani B (2010) Photocatalysis A to Z—what we know and what we do not know in a scientific sense. J. Photochem Photobiol C Photochem Rev 11:157–178. doi:10.1016/j.jphotochemrev.2011.02.001
Oller I, Malato S, Sánchez-pérez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409:4141–4166. doi:10.1016/j.scitotenv.2010.08.061
Otieno BO, Apollo SO, Naidoo BE, Ochieng A (2016) Photodegradation of molasses wastewater using TiO2–ZnO nanohybrid photocatalyst supported on activated carbon. Chem Eng Commun 6445:1443–1454. doi:10.1080/00986445.2016.1201659
Otieno BO, Apollo SO, Naidoo BE, Ochieng A (2017) Photodecolorisation of melanoidins in vinasse with illuminated TiO2–ZnO/activated carbon composite. J Environ Sci Heal Part A. doi:10.1080/10934529.2017.1294963
Satyawali Y, Balakrishnan M (2008) Wastewater treatment in molasses-based alcohol distilleries for COD and color removal: a review. J Environ Manag 86:481–497. doi:10.1016/j.jenvman.2006.12.024
Shu Z, Bolton JR, Belosevic M, Gamal M, Din E (2013) Photodegradation of emerging micropollutants using the medium-pressure UV/H2O2 advanced oxidation process. Water Res 47:2881–2889. doi:10.1016/j.watres.2013.02.045
Wang X, Wu Z, Wang Y, Wang W, Wang X, Bu Y, Zhao J (2013) Adsorption–photodegradation of humic acid in water by using ZnO coupled TiO2/bamboo charcoal under visible light irradiation. J Hazard Mater 262:16–24. doi:10.1016/j.jhazmat.2013.08.037
Wilkie AC, Riedesel KJ, Owens JM (2000) Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 19:63–102. doi:10.1016/S0961-9534(00)00017-9
Xu Y, Zhuang Y, Fu X (2010) New insight for enhanced photocatalytic activity of TiO2 by do** carbon nanotubes: a case study on degradation of benzene and methyl orange. J Phys Chem C 114:2669–2676. doi:10.1021/jp909855p
Zhang P, Shao C, Li X, Zhang M, Zhang X, Sun Y, Liu Y (2012) In situ assembly of well-dispersed Au nanoparticles on TiO2/ZnO nanofibers: a three-way synergistic heterostructure with enhanced photocatalytic activity. J Hazard Mater 237–238:331–338. doi:10.1016/j.jhazmat.2012.08.054
Acknowledgements
This work was financially supported by Water Research Commission (WRC, Project no. K5/2388), South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Mabuza, J., Otieno, B., Apollo, S. et al. Investigating the synergy of integrated anaerobic digestion and photodegradation using hybrid photocatalyst for molasses wastewater treatment. Euro-Mediterr J Environ Integr 2, 17 (2017). https://doi.org/10.1007/s41207-017-0029-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-017-0029-6