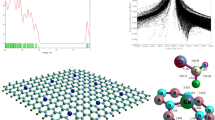
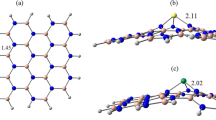
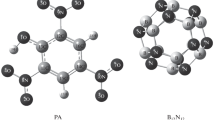
Abstract
Density functional techniques (DFT) were used to research the existence of intermolecular interactions between the gas molecule 1-Chloro-1,2,2,2-tetrafluoroethane (HCFC-124) and the single-walled boron nitride nanosheets doped with pristine, aluminum and galium (BNNS). PBE0, M06-2X, ωB97XD, and B3LYP-D3, functional calculations were applied for both isolated and complex structures to perform the optimization process. Along with split-valence triple-zeta basis sets of d-type and Cartesian-Gaussian polarization functions with 6-311G(d, p) basis set were performed in all. Electronic structure, total state density (DOS), natural bond orbital (NBO), quantum atom theory in molecules (QTAIM), and non-covalent interaction (NCI) analyses were investigated to study the intermolecular interaction of nanosheets with gas molecules. The results show that when the dopant atom was introduced to the BNNS, interactions at the HOMO–LUMO energy gap (HLG) were significantly altered. Eventually, optical properties are highly influenced by the mechanism of interaction in which, as a result of interaction with the proposed pristine nanosheets, the absorption spectrum (HCFC-124) receives a salient signal. This comparative study predicts that Al-doped BNNS is the most desirable material for designing a nanosensor that designs a sensitive nanosensor among all the absorbents.







Similar content being viewed by others
Data Availability
Yes available in manuscript
References
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Ai Z, Chang B, Xu C, Huang B, Wu Y, Hao X, Shao Y (2019) Interface engineering in the BNNS@ Ti3C2 intercalation structure for enhanced electrocatalytic hydrogen evolution. New J Chem 43:8613–8619. https://doi.org/10.1039/C9NJ01504C
Alkorta I, Trujillo C, Elguero J, Solimannejad M (2011) A theoretical study of the hydrogen bonding properties of H2BNH2: some considerations on the basis set superposition error issue. Comput Theor Chem 967:147–151
Azamat J, Khataee A, Joo SW (2016) Separation of copper and mercury as heavy metals from aqueous solution using functionalized boron nitride nanosheets: a theoretical study. J Mol Struct 1108:144–149
Baker J (1987) An algorithm for geometry optimization without analytical gradients. J Comput Chem 8:563–574
Bickelhaupt FM, Baerends EJ (2000) Kohn-Sham density functional theory: predicting and understanding chemistry. Rev Comput Chem 15:1–86
Binning R Jr, Curtiss L (1990) Compact contracted basis sets for third-row atoms: Ga–Kr. J Comput Chem 11:1206–1216
Blaudeau MPMJ-P, Curtiss LA, Radom L (1997) Extension of Gaussian-2 (G2) theory to molecules containing third-row atoms K and Ca. J Chem Phys 107:5016–5021
Bohórquez HJ, Boyd RJ, Matta CF (2011) Molecular model with quantum mechanical bonding information. J Phys Chem A 115:12991–12997
Bolton K (2003) A QM/MM study of HCl adsorption at ice surface defect sites. J Mol Struct (Thoechem) 632:145–156
Botas JA, Calleja G, Sánchez-Sánchez M, Orcajo MG (2010) Cobalt do** of the MOF-5 framework and its effect on gas-adsorption properties. Langmuir 26:5300–5303
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Bredas J-L (2014) Mind the gap! Mater Horiz 1:17–19
Bridgeman AJ, Cavigliasso G, Ireland LR, Rothery J (2001) The Mayer bond order as a tool in inorganic chemistry. J Chem Soc Dalton Transa 2095–2108
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632
Curtiss LA, McGrath MP, Blaudeau JP, Davis NE, Binning RC Jr, Radom L (1995) Extension of Gaussian-2 theory to molecules containing third-row atoms Ga–Kr. J Chem Phys 103:6104–6113
Dennington R, Keith TA, Millam JM (2016) GaussView, version 6.0.16. Semichem Inc., Shawnee Mission KS
Dorn RW, Ryan MJ, Kim T-H, Goh TW, Venkatesh A, Heintz PM, Zhou L, Huang W, Rossini AJ (2020) Identifying the molecular edge termination of exfoliated hexagonal boron nitride nanosheets with solid-state NMR spectroscopy and plane-wave DFT calculations. Chem Mater 32:3109–3121
Dos Santos R, Rivelino R, de Brito MF, Gueorguiev G, Kakanakova-Georgieva A (2015) Dopant species with Al–Si and N-Si bonding in the MOCVD of AlN implementing trimethylaluminum, ammonia and silane. J Phys D Appl Phys 48:295104
Esrafili MD (2018a) NO reduction by CO molecule over Si-doped boron nitride nanosheet: a dispersion-corrected DFT study. Chem Phys Lett 695:131–137
Esrafili MD (2018b) Epoxidation of ethylene over carbon and silicon-doped boron nitride sheets: a comparative DFT study. Solid State Commun 284:35–39
Esrafili MD, Asadollahi S (2018) A comparative DFT study on single-atom catalysis of CO oxidation over Al-and P-embedded hexagonal boron-nitride nanosheets. J Mol Graph Model 85:323–330
Esrafili MD, Saeidi N (2017) A DFT study on the healing of N-vacancy defects in boron nitride nanosheets and nanotubes by a methylene molecule. Int J Quantum Chem 117:e25450
Esrafili MD, Saeidi N (2018) Carbon-doped boron nitride nanosheet as a promising catalyst for N2O reduction by CO or SO2 molecule: a comparative DFT study. Appl Surf Sci 444:584–589
Esrafili MD, Saeidi N, Nematollahi P (2016) The healing of B-or N-vacancy defective BNNTs by using CO molecule: a DFT study. New J Chem 40:8024–8031
Esrafili MD, Mousavian P, Rad FA (2018) Adsorption of formamide over pristine and Al-doped boron nitride nanosheets: a dispersion-corrected DFT study. J Mol Graph Model 82:101–107
Esrafili MD, Asadollahi S, Heydari S (2019) A DFT study on NO reduction to N2O using Al-and P-doped hexagonal boron nitride nanosheets. J Mol Graph Model 89:41–49
Falin A, Cai Q, Santos EJ, Scullion D, Qian D, Zhang R, Yang Z, Huang S, Watanabe K, Taniguchi T (2017) Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat Commun 8:1–9
Foresman JB, Frisch A (1996) Exploring chemistry with electronic structure methods: a guide to using Gaussian (1996)
Foster AJ, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev. C.01. Wallingford, CT
Fu L, Chen G, Jiang N, Yu J, Lin C-T, Yu A (2016) In situ growth of metal nanoparticles on boron nitride nanosheets as highly efficient catalysts. J Mater Chem A 4:19107–19115
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874
Ghafur Rauf H, Majedi S, Abdulkareem Mahmood E, Sofi M (2019) Adsorption behavior of the Al-and Ga-doped B12N12 nanocages on COn (n = 1, 2) and HnX (n = 2, 3 and X = O, N): a comparative study. Chem Rev Lett 2:140–150
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys Chem Chem Phys 13:6670–6688
Grabowski SJ (2012) QTAIM characteristics of halogen bond and related interactions. J Phys Chem A 116:1838–1845
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the dam** function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Guerra V, Wan C, McNally T (2019) Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog Mater Sci 100:170–186
Hay PJ (1977) Gaussian basis sets for molecular calculations. The representation of 3 d orbitals in transition-metal atoms. J Chem Phys 66:4377–4384
Hjiri M, El Mir L, Leonardi S, Pistone A, Mavilia L, Neri G (2014) Al-doped ZnO for highly sensitive CO gas sensors. Sens Actuators B Chem 196:413–420
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864
Ibach H, Lüth H (1995) An Introduction to principles of materials science, solid state and physics. Springer, Berlin, p 244
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Kamel M, Morsali A, Raissi H, Mohammadifard K (2020) Theoretical insights into the intermolecular and mechanisms of covalent interaction of Flutamide drug with COOH and COCl functionalized carbon nanotubes: a DFT approach. Chem Rev Lett 3:23–37
Karimzadeh S, Safaei B, Jen TC (2020) Investigate the importance of mechanical properties of SWCNT on doxorubicin anti-cancer drug adsorption for medical application: a molecular dynamic study. J Mol Graph Model 101(2020):107745
Karimzadeh S, Safaei B, Jen TC (2021) Theorical investigation of adsorption mechanism of doxorubicin anticancer drug on the pristine and functionalized single-walled carbon nanotube surface as a drug delivery vehicle: a DFT study. J Mol Liq 322:114890
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140:A1133
Lei W, Zhang H, Wu Y, Zhang B, Liu D, Qin S, Liu Z, Liu L, Ma Y, Chen Y (2014) Oxygen-doped boron nitride nanosheets with excellent performance in hydrogen storage. Nano Energy 6:219–224
Li F, Asadi H (2020) DFT study of the effect of platinum on the H2 gas sensing performance of ZnO nanotube: explaining the experimental observations. J Mol Liq 113139
Li LH, Chen Y (2016) Atomically thin boron nitride: unique properties and applications. Adv Funct Mater 26:2594–2608
Li LH, **ng T, Chen Y, Jones R (2014) Boron nitride nanosheets for metal protection. Adv Mater Interfaces 1:1300132
Li H, Chen Z, Fang X, Tie D (2015) Absorption of NH3 on pristine and defected boron nitride nanosheets: a first principle study. Superlattices Microstruct 88:371–376
Li J-L, Yin J-H, Ji T, Feng Y, Liu Y-Y, Zhao H, Li Y-P, Zhu C-C, Yue D, Su B (2019) Microstructure evolution effect on high-temperature thermal conductivity of LDPE/BNNS investigated by in-situ SAXS. Mater Lett 234:74–78
Lin Y, Connell JW (2012) Advances in 2D boron nitride nanostructures: nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale 4:6908–6939
Lin S, Ye X, Johnson RS, Guo H (2013) First-principles investigations of metal (Cu, Ag, Au, Pt, Rh, Pd, Fe Co, and Ir) doped hexagonal boron nitride nanosheets: stability and catalysis of CO oxidation. J Phys Chem C 117:17319–17326
Lin S, Huang J, Gao X (2015) A Cu (111) supported h-BN nanosheet: a potential low-cost and high-performance catalyst for CO oxidation. Phys Chem Chem Phys 17:22097–22105
Liu S-B (2009) Conceptual density functional theory and some recent developments. Acta Phys Chim Sin 25:590–600
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Luo W, Wang Y, Hitz E, Lin Y, Yang B, Hu L (2017) Solution processed boron nitride nanosheets: synthesis, assemblies and emerging applications. Adv Funct Mater 27:1701450
Ma F, Li Z-R, Zhou Z-J, Wu D, Li Y, Wang Y-F, Li Z-S (2010) Modulated nonlinear optical responses and charge transfer transition in endohedral fullerene dimers Na@C60C60@F with n-fold covalent bond (n = 1, 2, 5, and 6) and long range ion bond. J Phys Chem C 114:11242–11247
Matta CF (2006) Hydrogen–hydrogen bonding: the non-electrostatic limit of closed-shell interaction between two hydro. In: hydrogen bonding—new insights. Springer, Berlin, pp 337–375
Mayer I (1983) Charge, bond order and valence in the AB initio SCF theory. Chem Phys Lett 97:270–274
Mayer I (2012) Improved definition of bond orders for correlated wave functions. Chem Phys Lett 544:83–86
McGrath MP, Radom L (1991) Extension of Gaussian-1 (G1) theory to bromine-containing molecules. J Chem Phys 94:511–516
Mohammadi MD, Abdullah HY (2020a) The adsorption of chlorofluoromethane on pristine, and Al-and Ga-doped boron nitride nanosheets: a DFT, NBO, and QTAIM study. J Mol Model 26:1–15
Mohammadi MD, Abdullah HY (2020b) Theoretical study of the adsorption of amantadine on pristine, Al-, Ga-, P-, and As-doped boron nitride nanosheets: a PBC-DFT, NBO, and QTAIM study. Theoret Chem Acc 139:1–17
Mohammadi MD, Abdullah HY (2020c) The Adsorption of Chlorofluoromethane on Pristine, Al, Ga, P, and As-doped Boron Nitride Nanotubes: A PBC-DFT, NBO, and QTAIM Study. ChemistrySelect 5:12115–12124
Mohammadi MD, Abdullah HY (2021a) The adsorption of bromochlorodifluoromethane on pristine and Ge-doped silicon carbide nanotube: a PBC-DFT, NBO, and QTAIM study. Struct Chem 32:481–494
Mohammadi MD, Abdullah HY (2021b) The adsorption of bromochlorodifluoromethane on pristine, Al, Ga, P, and As-doped boron nitride nanotubes: a study involving PBC-DFT, NBO analysis, and QTAIM. Comput Theor Chem 1193:113047
Mohammadi MD, Hamzehloo M (2018) The adsorption of bromomethane onto the exterior surface of aluminum nitride, boron nitride, carbon, and silicon carbide nanotubes: a PBC-DFT NBO, and QTAIM study. Comput Theor Chem 1144:26–37
Mohammadi MD, Salih IH, Abdullah HY (2020a) An ultimate Investigation on the adsorption of amantadine on pristine and decorated fullerenes C59X (X = Si, Ge, B, Al, Ga, N, P, and As): a DFT NBO, and QTAIM study. J Comput Biophys Chem 20:23–29
Mohammadi MD, Salih IH, Abdullah HY (2020b) The adsorption of chlorofluoromethane on pristine and Ge-doped silicon carbide nanotube: a PBC-DFT, NBO, and QTAIM study. Mol Simul 46:1405–1416
Moladoust R, Esrafili MD, Hosseinian A, Alkorta I, Vessally E (2019) Adsorption sensitivity of pristine and Al-or Si-doped boron nitride nanoflake to COCl2: a DFT study. Mol Phys 117:626–634
Mulliken RS (1955) Electronic population analysis on LCAO–MO molecular wave functions. I. J Chem Phys 23:1833–1840
O’boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845
Nemati-Kande E, Abbasi M, Mohammadi MD (2018) DFT, QTAIM and NBO investigation of the interaction of rare gases with pristine and decorated boron nitride nanotube. ChemistrySelect 3:9833–9840
Nemati-Kande E, Abbasi M, Mohammadi MD (2019) Feasibility of pristine and decorated AlN and SiC nanotubes in sensing of noble gases: a DFT study. ChemistrySelect 4:2453–2462
Nemati-Kande E, Abbasi M, Mohammadi MD (2020) DFT studies on the interactions of pristine, Al and Ga-doped boron nitride nanosheets with CH3X (X = F, Cl and Br). J Mol Struct 1199:126962
Omidirad R, Azizi K (2019) DFT study of charge-controlled mechanism of water molecule dissociation on vacancy defected boron nitride nanosheets. J Mol Graph Model 93:107448
Perdew JP, Ernzerhof M, Burke K (1996a) Rationale for mixing exact exchange with density functional approximations. J Chem Phys 105:9982–9985
Perdew JP, Burke K, Ernzerhof M (1996b) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Pople JA, Gill PM, Johnson BG (1992) Kohn–Sham density-functional theory within a finite basis set. Chem Phys Lett 199:557–560
Raghavachari K, Trucks GW (1989) Highly correlated systems. Excitation energies of first row transition metals Sc–Cu. J Chem Phys 91:1062–1065
Raghavachari JSBK, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. 20. Basis set for correlated wave-functions. J Chem Phys 72:650–654
Russo TV, Martin RL, Hay PJ (1994) Density functional calculations on first-row transition metals. J Chem Phys 101:7729–7737
Saha D, Deng S (2009) Hydrogen adsorption on ordered mesoporous carbons doped with Pd, Pt, Ni, and Ru. Langmuir 25:12550–12560
Sizova OV, Skripnikov LV, Sokolov AY (2008) Symmetry decomposition of quantum chemical bond orders. J Mol Struct (Thoechem) 870:1–9
Srinivasu K, Ghosh SK (2012) Transition metal decorated porphyrin-like porous fullerene: promising materials for molecular hydrogen adsorption. J Phys Chem C 116:25184–25189
Wang L-Q, Baer DR, Engelhard MH, Shultz AN (1995) The adsorption of liquid and vapor water on TiO2 (110) surfaces: the role of defects. Surf Sci 344:237–250
Weinhold F (2012) Discovering chemistry with natural bond orbitals. Wiley, Hoboken
Weinhold F, Landis CR (2001) Natural bond orbitals and extensions of localized bonding concepts. Chem Educ Res Pract 2:91–104
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Wilke K, Breuer H (1999) The influence of transition metal do** on the physical and photocatalytic properties of titania. J Photochem Photobiol A 121:49–53
Wu Z, Li M, Howe J, Meyer HM III, Overbury SH (2010) Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 26:16595–16606
Yang X, Guo Y, Han Y, Li Y, Ma T, Chen M, Kong J, Zhu J, Gu J (2019) Significant improvement of thermal conductivities for BNNS/PVA composite films via electrospinning followed by hot-pressing technology. Compos B Eng 175:107070
Yildirim T, Íñiguez J, Ciraci S (2005) Molecular and dissociative adsorption of multiple hydrogen molecules on transition metal decorated C60. Phys Rev B 72:153403
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc 120:215–241
Acknowledgements
I would like to thank the Solid-State Theory Group at the Physics Department at the Universita‘ degli Studi di Milano-Italy for providing computational facilities.
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Contributions
Mohsen Doust Mohammadi was involved in writing original draft, formal analysis and visualization. Hewa Y. Abdullah was involved in supervision, investigation and project administration. A. Suvitha was involved in review & editing, and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed Consent to Participate
All authors agreed.
Rights and permissions
About this article
Cite this article
Mohammadi, M.D., Abdullah, H.Y. & Suvitha, A. The Adsorption of 1-Chloro-1,2,2,2-Tetrafluoroethane Onto the Pristine, Al-, and Ga-Doped Boron Nitride Nanosheet. Iran J Sci Technol Trans Sci 45, 1287–1300 (2021). https://doi.org/10.1007/s40995-021-01117-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-021-01117-0