Abstract
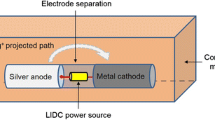
Implant-associated infections pose serious threats to patients in terms of morbidity, mortality, and medical costs. This paper focuses on the quantitative assessment of the antimicrobial efficacy of a silver-based prophylactic system for orthopaedic implant applications. The implant system is configured to induce controlled local administration of silver ions via low intensity direct current activation (1–14 µA). We developed a broth-based in vitro testing model to evaluate the effects of important design parameters on the antimicrobial efficacy of the system over a 48-h interval. The time-kill curves obtained with various parameter levels were analyzed through a longitudinal model. Five parameters were investigated independently through one-way factorial experiments (n = 12) against Staphylococcus aureus. In phase 1 of the study, we investigated the effect of cathode material on system performance. We also determined the linear structure of the longitudinal model based on the Akaike information criterion by fitting the empirical data to multiple candidate model structures. The results show that substituting the silver cathode with titanium does not weaken the antimicrobial efficacy of the system (p = 0.9946). In phase 2, a detailed analysis with four design parameters was conducted using the silver–titanium configuration. The performance of the system was found to be independent of the electrode separation distance (p = 0.9926) and the pulsating current frequency (p = 0.9956). However, the anode surface area (p < 0.0001) and the current intensity (p < 0.0001) influenced the antimicrobial efficacy in an interactive manner. Overall, this study characterizes the in vitro antimicrobial efficacy of the proposed system and provides a reference of design parameters for future product engineering.



Similar content being viewed by others
References
Lentino, J. R. (2003). Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clinical Practice, 36, 1157–1161.
Neut, D., van Horn, J. R., van Kooten, T. G., van der Mei, H. C., & Busscher, H. J. (2003). Detection of biomaterial-associated infections in orthopaedic joint implants. Clinical Orthopaedics and Related Research, 413, 261–268.
Shirwaiker, R. A., Samberg, M. E., Cohen, P. H., Wysk, R. A., & Monteiro-Riviere, N. A. (2013). Nanomaterials and synergistic low-intensity direct current (LIDC) stimulation technology for orthopedic implantable medical devices. WIREs Nanomedicine and Nanobiotechnology, 5, 191–204.
Smith, E. B., Wynne, R., Joshi, A., Liu, H., & Good, R. P. (2012). Is it time to include vancomycin for routine perioperative antibiotic prophylaxis in total joint arthroplasty patients? Journal of Arthroplasty, 27, 55–60.
Bini, S. A., Sidney, S., & Sorel, M. (2011). Slowing demand for total joint arthroplasty in a population of 3.2 million. Journal of Arthroplasty, 26, 124–128.
Darouiche, R. O. (2003). Antimicrobial approaches for preventing infections associated with surgical implants. Clinical Infectious Diseases, 36, 1284–1289.
Nanda, A., & Saravanan, M. (2009). Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine, 5, 452–456.
Loh, J. V., Percival, S. L., Woods, E. J., Williams, N. J., & Cochrane, C. A. (2009). Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. International Wound Journal, 6, 32–38.
Strohal, R., Schelling, M., Takacs, M., Jurecka, W., Gruber, U., & Offner, F. (2005). Nanocrystalline silver dressings as an efficient anti-MRSA barrier: A new solution to an increasing problem. Journal of Hospital Infection, 60, 226–230.
Jones, S. A., Bowler, P. G., Walker, M., & Parsons, D. (2004). Controlling wound bioburden with a novel silver-containing Hydrofiber® dressing. Wound Repair and Regeneration, 12, 288–294.
Pollini, M., Paladini, F., Catalano, M., Taurino, A., Licciulli, A., Maffezzoli, A., & Sannino, A. (2011). Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. Journal of Materials Science Materials in Medicine, 22, 2005–2012.
Masse, A., Bruno, A., Bosetti, M., Biasibetti, A., Cannas, M., & Gallinaro, P. (2000). Prevention of pin track infection in external fixation with silver coated pins: Clinical and microbiological results. Journal of Biomedical Materials Research, 53, 600–604.
Implantcast GmbH. (2011). MUTARS® (Modular Universal Tumor And Revision System). http://www.implantcast.info/index.php?option=com_content&view=category&layout=blog&id=1&Itemid=57&lang=en.
Hardes, J., Von Eiff, C., Streitbuerger, A., Balke, M., Budny, T., Henrichs, M., & Hauschild, G. (2010). Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. European Journal of Surgical Oncology, 101, 389–395.
Chen, W., Liu, Y., Courtney, H. S., Bettenga, M., Agrawal, C. M., Bumgardner, J. D., & Ong, J. (2006). In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials, 27, 5512–5517.
Chen, X., & Schluesener, H. (2008). Nanosilver: A nanoproduct in medical application. Toxicology Letters, 176, 1–12.
Feng, Q. L., Wu, J., Chen, G. Q., Cui, F. Z., Kim, T. N., & Kim, J. O. (2000). A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research Part A, 52, 662–668.
Holt, K. B., & Bard, A. J. (2005). Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry, 44, 13214–13223.
Jung, W. K., Koo, H. C., Kim, K. W., Shin, S., Kim, S. H., & Park, Y. H. (2008). Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Applied and Environment Microbiology, 74, 2171–2178.
Zhang, H., Wu, M., & Sen, A. (2012). Silver nanoparticle antimicrobials and related materials. In N. Cioffi & M. Rai (Eds.), Nano-antimicrobials: Progress and prospects (pp. 3–45). New York: Springer.
Ip, M., Lui, S. L., Poon, V. K. M., Lung, I., & Burd, A. (2006). Antimicrobial activities of silver dressings: An in vitro comparison. Journal of Medical Microbiology, 55, 59–63.
Castellano, J. J., Shafii, S. M., Ko, F., Donate, G., Wright, T. E., Mannari, R. J., et al. (2007). Comparative evaluation of silver-containing antimicrobial dressings and drugs. International Wound Journal, 4, 114–122.
Darouiche, R. O. (1999). Anti-infective efficacy of silver-coated medical prostheses. Clinical Infectious Diseases, 29, 1371–1377.
Spadaro, J. A., Berger, T. J., Barranco, S. D., Chapin, S. E., & Becker, R. O. (1974). Antibacterial effects of silver electrodes with weak direct current. Antimicrobial Agents and Chemotherapy, 6, 637–642.
Berger, T. J., Spadaro, J. A., Chapin, S. E., & Becker, R. O. (1976). Electrically generated silver ions: Quantitative effects on bacterial and mammalian cells. Antimicrobial Agents and Chemotherapy, 9, 357–358.
Fuller, T. A., Wysk, R. A., Charumani, C., Kennett, M., Sebastiennelli, W. J., Abrahams, R., et al. (2010). Develo** an engineered antimicrobial/prophylactic system using electrically activated bactericidal metals. Journal of Materials Science Materials in Medicine, 21, 2103–2114.
Shirwaiker, R. A., Wysk, R. A., Kariyawasam, S., Carrion, H., & Voigt, R. C. (2011). Micro-scale fabrication and characterization of a silver-polymer-based electrically activated antibacterial surface. Biofabrication, 3, 015003.
Samberg, M. E., Tan, Z., Monteiro-Riviere, N. A., Orndorff, P. E., & Shirwaiker, R. A. (2013). Biocompatibility analysis of an electrically-activated silver-based antibacterial surface system for medical device applications. Journal of Materials Science Materials in Medicine, 24, 755–760.
Chakravarti, A., Gangodawila, S., Long, M. J., Morris, N. S., Blacklock, A. R. E., & Stickler, D. J. (2005). An electrified catheter to resist encrustation by Proteus mirabilis biofilm. Journal of Urology, 174, 1129–1132.
Wysk, R. A., Sebastianelli, W. J., Shirwaiker, R. A., Bailey, G. M., Charumani, C., Kennett, M., et al. (2010). Prophylactic bactericidal orthopedic implants—Animal testing study. Journal of Biomedical Science and Engineering, 3, 917–926.
Atiyeh, B., Costagliola, M., Hayek, S., & Dibo, S. (2006). Effect of silver on burn wound infection control and healing: Review of the literature. Burns, 33, 139–148.
Drake, P. L., & Hazelwood, K. J. (2005). Exposure-related health effects of silver and silver compounds: A review. Annals of Occupational Hygiene, 49, 575–585.
AshaRani, P., Mun, G., Hande, M., & Valiyaveettil, S. (2009). Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano, 3, 279–290.
Arora, S., Jain, J., Rajwade, J., & Paknikar, K. (2008). Cellular responses induced by silver nanoparticles: In vitro studies. Toxicology Letters, 179, 93–100.
Braydich-Stolle, L., Hussain, S., Schlager, J., & Hofmann, M. (2005). In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicological Sciences, 88, 412–419.
Becker, R. O., & Spadaro, J. A. (1978). Treatment of orthopaedic infections with electrically generated silver ions. A preliminary report. Journal of Bone and Joint Surgery, American, 60, 871.
Webster, D. A., Spadaro, J. A., Becker, R. O., & Kramer, S. (1981). Silver anode treatment of chronic osteomyelitis. Clinical Orthopaedics and Related Research, 161, 105–114.
Rai, M., Yadav, A., & Gade, A. (2008). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27, 76–83.
Gosheger, G., Von Eiff, C., Hardes, J., Ahrens, H., Streitburger, A., & Buerger, H. (2004). Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials, 25, 5547–5556.
Albrektsson, T., & Johansson, C. (2001). Osteoinduction, osteoconduction and osseointegration. European Spine Journal, Suppl. 2, S96–S101.
Carlsson, L., Röstlund, T., Albrektsson, B., Albrektsson, T., & Brånemark, P. (1986). Osseointegration of titanium implants. Acta Orthopaedica Scandinavica, 57, 285.
Le Guéhennec, L., Soueidan, A., Layrolle, P., & Amouriq, Y. (2007). Surface treatments of titanium dental implants for rapid osseointegration. Dental Materials, 23, 844–854.
Tsvetkov, V. V. (1995). Corrosion-resistant titanium alloys. Pharmaceutical Chemistry Journal, 29, 564–566.
Trampuz, A., & Zimmerli, W. (2006). Antimicrobial agents in orthopaedic surgery: Prophylaxis and treatment. Drugs, 66, 1089.
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723.
Bosetti, M., Massè, A., Tobin, E., & Cannas, M. (2002). Silver coated materials for external fixation devices: In vitro biocompatibility and genotoxicity. Biomaterials, 23, 887–892.
Alt, V., Bechert, T., Steinrücke, P., Wagener, M., Seidel, P., Dingeldein, E., et al. (2004). An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials, 25, 4383–4391.
Das, K., Bose, S., Bandyopadhyay, A., Karandikar, B., & Gibbins, B. L. (2008). Implants, surface coatings for improvement of bone cell materials and antimicrobial activities of Ti. Journal of Biomedical Materials Research Part B, 87, 455–460.
Giglio, E. D., Cafagna, D., Cometa, S., Allegretta, A., Pedico, A., Giannossa, L. C., et al. (2013). An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Analytical and Bioanalytical Chemistry, 405, 805–816.
Tan, Z., Ganapathy, A., Orndorff, P. E., & Shirwaiker, R. A. (2015). Effects of cathode design parameters on in vitro antimicrobial efficacy of electrically-activated silver-based iontophoretic system. Journal of Materials Science Materials in Medicine, 26, 5382.
Acknowledgments
This work was supported by a research grant from NC State’s 2013 Research and Innovation and Seed Funding (RISF) program. The authors thank Ms. Patty Spears and Ms. Mitsu Suyemoto from NC State University’s College of Veterinary Medicine for their valuable and constructive suggestions during the antimicrobial efficacy testing experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, Z., Xu, G., Orndorff, P.E. et al. Effects of Electrically Activated Silver–Titanium Implant System Design Parameters on Time-Kill Curves Against Staphylococcus aureus . J. Med. Biol. Eng. 36, 325–333 (2016). https://doi.org/10.1007/s40846-016-0136-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-016-0136-x