Abstract
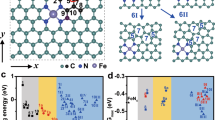
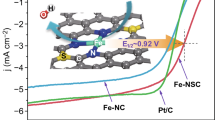
The design of non-noble metal heterogeneous catalyst with superior performance for selective hydrogenation or transfer hydrogenation of nitroarenes to amines is significant but challenging. Herein, a single-atom Fe supported by nitrogen-doped carbon (Fe1/N-C) catalyst is reported. The Fe1/N-C sample shows superior performances for the selective hydrogenation and transfer hydrogenation of nitrobenzene to aniline at different temperatures. Density functional theory (DFT) calculations show that the superior catalytic activity for the selective hydrogenation at lower temperatures could be attributed to the effective activation of the reactant and intermediates by the Fe1/N-C. Moreover, the excellent performance of Fe1/N-C for the selective transfer hydrogenation could be attributed to that the reaction energy barrier for dehydrogenation of isopropanol can be overcome by elevated temperatures.
摘要
设计性能优异的硝基化合物选择性加氢或转移加氢生成胺 类的非贵金属多相催化剂具有重要的意义, 但又具有很大的挑战 性. 本文报道了氮掺杂碳负载的单原子Fe催化剂(Fe1/N-C). 通过调 控温度, Fe1/N-C催化剂对硝基苯的选择性加氢和转移加氢均具有 良好的催化性能. DFT计算表明, Fe1/N-C在较低温度下能够很好 地活化反应物和中间体, 因此具有较高的选择性加氢活性. 此外, Fe1/N-C在较高温度下可以克服异丙醇脱氢反应的能量障碍, 因此 具有很好的转移加氢性能.
Similar content being viewed by others
References
Downing RS, Kunkeler PJ, van Bekkum H. Catalytic syntheses of aromatic amines. Catal Today, 1997, 37: 121–136
Blaser HU, Steiner H, Studer M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem, 2009, 1: 210–221
Corma A, Serna P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science, 2006, 313: 332–334
Wienhofer G, Sorribes I, Boddien A, et al. General and selective iron-catalyzed transfer hydrogenation of nitroarenes without base. J Am Chem Soc, 2011, 133: 12875–12879
Jagadeesh RV, Surkus AE, Junge H, et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science, 2013, 342: 1073–1076
Westerhaus FA, Jagadeesh RV, Wienhöfer G, et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat Chem, 2013, 5: 537–543
Li H, Cao C, Liu J, et al. Cobalt single atoms anchored on N-doped ultrathin carbon nanosheets for selective transfer hydrogenation of nitroarenes. Sci China Mater, 2019, 62: 1306–1314
You Y, Huang H, Mao K, et al. Tandem nanocatalyst design: Putting two step-reaction sites into one location towards enhanced hydrogen transfer reactions. Sci China Mater, 2019, 62: 1297–1305
Lin L, Yao S, Gao R, et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat Nanotechnol, 2019, 14: 354–361
Li X, Wang Z, Mao S, et al. Insight into the role of additives in catalytic synthesis of cyclohexylamine from nitrobenzene. Chin J Chem, 2018, 36: 1191–1196
Chen Y, Wang Z, Mao S, et al. Rational design of hydrogenation catalysts using nitrogen-doped porous carbon. Chin J Catal, 2019, 40: 971–979
Shimizu K, Miyamoto Y, Satsuma A. Size- and support-dependent silver cluster catalysis for chemoselective hydrogenation of nitroaromatics. J Catal, 2010, 270: 86–94
Jagadeesh RV, Wienhöfer G, Westerhaus FA, et al. Efficient and highly selective iron-catalyzed reduction of nitroarenes. Chem Commun, 2011, 47: 10972–10974
Nie R, Wang J, Wang L, et al. Platinum supported on reduced graphene oxide as a catalyst for hydrogenation of nitroarenes. Carbon, 2012, 50: 586–596
Zhang S, Chang CR, Huang ZQ, et al. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes. J Am Chem Soc, 2016, 138: 2629–2637
Fürstner A, Leitner A, Méndez M, et al. Iron-catalyzed cross-coupling reactions. J Am Chem Soc, 2002, 124: 13856–13863
Bolm C, Legros J, Le Paih J, et al. Iron-catalyzed reactions in organic synthesis. Chem Rev, 2004, 104: 6217–6254
Morris RH. Asymmetric hydrogenation, transfer hydrogenation and hydrosilylation of ketones catalyzed by iron complexes. Chem Soc Rev, 2009, 38: 2282–2291
Yang M, Allard LF, Flytzani-Stephanopoulos M. Atomically dispersed Au-(OH)x species bound on titania catalyze the low-temperature water-gas shift reaction. J Am Chem Soc, 2013, 135: 3768–3771
Liu J, Lucci FR, Yang M, et al. Tackling CO poisoning with singleatom alloy catalysts. J Am Chem Soc, 2016, 138: 6396–6399
Chen H, He S, Cao X, et al. Ru-cluster-modified Ni surface defects toward selective bond breaking between C-O and C-C. Chem Mater, 2016, 28: 4751–4761
Zhang H, Wei J, Dong J, et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework. Angew Chem Int Ed, 2016, 55: 14310–14314
Malta G, Kondrat SA, Freakley SJ, et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science, 2017, 355: 1399–1403
Huang F, Deng Y, Chen Y, et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene. J Am Chem Soc, 2018, 140: 13142–13146
Qin R, Liu P, Fu G, et al. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods, 2018, 2: 1700286
Li H, Wang L, Dai Y, et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat Nanotech, 2018, 13: 411–417
Abdel-Mageed AM, Rungtaweevoranit B, Parlinska-Wojtan M, et al. Highly active and stable single-atom Cu catalysts supported by a metal-organic framework. J Am Chem Soc, 2019, 141: 5201–5210
Cao L, Liu W, Luo Q, et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature, 2019, 565: 631–635
Sun T, Xu L, Wang D, et al. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res, 2019, 12: 2067–2080
Lee BH, Park S, Kim M, et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat Mater, 2019, 18: 620–626
Yan J, Kong L, Ji Y, et al. Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation. Nat Commun, 2019, 10: 2149
** W, Wang K, Shen Y, et al. Dynamic co-catalysis of Au single atoms and nanoporous Au for methane pyrolysis. Nat Commun, 2020, 11: 1919
Xu Q, Guo CX, Tian S, et al. Coordination structure dominated performance of single-atomic Pt catalyst for anti-Markovnikov hydroboration of alkenes. Sci China Mater, 2020, 63: 972–981
Gong YN, Jiao L, Qian Y, et al. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction. Angew Chem Int Ed, 2020, 59: 2705–2709
Li X, Rong H, Zhang J, et al. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res, 2020, 13: 1842–1855
Zhuang Z, Kang Q, Wang D, et al. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries. Nano Res, 2020, 13: 1856–1866
Deng D, Chen X, Yu L, et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci Adv, 2015, 1: e1500462
Wu P, Du P, Zhang H, et al. Graphyne-supported single Fe atom catalysts for CO oxidation. Phys Chem Chem Phys, 2015, 17: 1441–1449
Chen P, Zhou T, **ng L, et al. Atomically dispersed iron-nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew Chem Int Ed, 2017, 56: 610–614
Liu W, Zhang L, Liu X, et al. Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C-H bond. J Am Chem Soc, 2017, 139: 10790–10798
Fei H, Dong J, Feng Y, et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities. Nat Catal, 2018, 1: 63–72
Yang L, Cheng D, Xu H, et al. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc Natl Acad Sci USA, 2018, 115: 6626–6631
Wan X, Liu X, Li Y, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat Catal, 2019, 2: 259–268
Delley B. From molecules to solids with the DMol3 approach. J Chem Phys, 2000, 113: 7756–7764
Runge E, Gross EKU. Density-functional theory for time-dependent systems. Phys Rev Lett, 1984, 52: 997–1000
Ernzerhof M, Scuseria GE. Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional. J Chem Phys, 1999, 110: 5029–5036
Halgren TA, Lipscomb WN. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem Phys Lett, 1977, 49: 225–232
Mondal J, Kundu SK, Hung Ng WK, et al. Fabrication of ruthenium nanoparticles in porous organic polymers: Towards advanced heterogeneous catalytic nanoreactors. Chem Eur J, 2015, 21: 19016–19027
Ertl G, Grunze M, Weiss M. Chemisorption of N2 on an Fe(100) surface. J Vac Sci Technol, 1976, 13: 314–317
Benziger J. Reactions and reaction intermediates on iron surfaces I. Methanol, ethanol, and isopropanol on Fe(100). J Catal, 1980, 65: 36–48
Benziger J, Madix RJ. The effects of carbon, oxygen, sulfur and potassium adlayers on CO and H2 adsorption on Fe(100). Surf Sci, 1980, 94: 119–153
Luo Q, Pan Y, Guo P, et al. First-principles calculation of adsorption of shale gas on CaCO3 (100) surfaces. J Appl Biomater Funct Mater, 2017, 15: 45–51
Saraireh SA, Altarawneh M, Tarawneh MA. Adsorption and dissociation of the methanethiol (CH3SH) molecule on the Fe(100) surface. Surf Interface Anal, 2019, 52: 156–166
Hensley AJR, Collinge G, Wang Y, et al. Coverage-dependent adsorption of hydrogen on Fe(100): Determining catalytically relevant surface structures via lattice gas models. J Phys Chem C, 2020, 124: 7254–7266
Jenkins SJ. Aromatic adsorption on metals via first-principles density functional theory. Proc R Soc A, 2009, 465: 2949–2976
Honkela ML, Björk J, Persson M. Computational study of the adsorption and dissociation of phenol on Pt and Rh surfaces. Phys Chem Chem Phys, 2012, 14: 5849–5854
Mahata A, Rai RK, Choudhuri I, et al. Direct vs. indirect pathway for nitrobenzene reduction reaction on a Ni catalyst surface: A density functional study. Phys Chem Chem Phys, 2014, 16: 26365–26374
Morrissey C, He H. Silicene catalyzed reduction of nitrobenzene to aniline: A mechanistic study. Chem Phys Lett, 2018, 695: 228–234
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0702003), the National Natural Science Foundation of China (21890383, 21671117, 21871159 and 21901135), and the Science and Technology Key Project of Guangdong Province of China (2020B010188002). We thank the 1W1B station in Bei**g Synchrotron Radiation Facility.
Author information
Authors and Affiliations
Contributions
Tian S designed and performed the experiments, and analyzed the data; Hu M and He J conducted the density functional theory calculations; Xu Q and Zhu Y assisted to carry out the experiments; Gong W, Chen C, and Zhao H designed and performed the catalytic experiment; Chen W and Yang J helped with XAFS characterization and the corresponding data analysis; Liu Q assisted to analyze the data; Wang D and Li Y conceived the research project, and analyzed the results. All authors contributed to the general discussion and writing the paper.
Corresponding author
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Shubo Tian received his BSc (2013), MSc (2016), and PhD (2019) degrees from Hebei Normal University, University of the Chinese Academy of Sciences, and Tsinghua University, respectively. He is currently a postdoctor in the National University of Singapore. His research interests are focused on the syntheses and applications of isolated single-atom-site and cluster catalysts.
Min Hu received her BSc (2018) degree from Ludong University. She is currently studying for a master’s degree at the Institute for New Energy Materials and Low-Carbon Technology, Tian** University of Technology. Her main research directions are first-principles and molecular dynamics simulations.
Qi Xu received his BSc degree from the University of Science and Technology of China in 2017. Now, he is a PhD student under the supervision of Prof. Dingsheng Wang at Tsinghua University. His research interests mainly focus on the design and fabrication of single-atomic catalysts for heterogeneous catalysis.
Dingsheng Wang received his BSc degree from the Department of Chemistry and Physics, University of Science and Technology of China in 2004, and his PhD degree from the Department of Chemistry, Tsinghua University in 2009, under the supervision of Prof. Yadong Li. He did his postdoctoral research at the Department of Physics, Tsinghua University, with Prof. Shoushan Fan. He joined the faculty of the Department of Chemistry, Tsinghua University in 2012.
Supplementary Information
40843_2020_1443_MOESM1_ESM.pdf
Single-atom Fe with Fe1N3 structure showing superior performances for both hydrogenation and transfer hydrogenation of nitrobenzene
Rights and permissions
About this article
Cite this article
Tian, S., Hu, M., Xu, Q. et al. Single-atom Fe with Fe1N3 structure showing superior performances for both hydrogenation and transfer hydrogenation of nitrobenzene. Sci. China Mater. 64, 642–650 (2021). https://doi.org/10.1007/s40843-020-1443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1443-8