Highlights
-
Lotus leaf-derived gradient hierarchical porous C/MoS2 morphology genetic composites nanocomposites were fabricated.
-
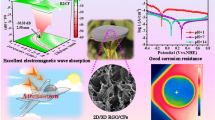
Excellent electromagnetic absorption performance was achieved with RLmin of − 50.1 dB and EBW of 6.0 GHz.
-
A brand-new dielectric sum-quotient model was proposed and corresponded well to the experimental results.
Abstract
Inspired by the nature, lotus leaf-derived gradient hierarchical porous C/MoS2 morphology genetic composites (GHPCM) were successfully fabricated through an in situ strategy. The biological microstructure of lotus leaf was well preserved after treatment. Different pores with gradient pore sizes ranging from 300 to 5 μm were hierarchically distributed in the composites. In addition, the surface states of lotus leaf resulted in the Janus-like morphologies of MoS2. The GHPCM exhibit excellent electromagnetic wave absorption performance, with the minimum reflection loss of − 50.1 dB at a thickness of 2.4 mm and the maximum effective bandwidth of 6.0 GHz at a thickness of 2.2 mm. The outstanding performance could be attributed to the synergy of conductive loss, polarization loss, and impedance matching. In particularly, we provided a brand-new dielectric sum-quotient model to analyze the electromagnetic performance of the non-magnetic material system. It suggests that the specific sum and quotient of permittivity are the key to keep reflection loss below − 10 dB within a certain frequency range. Furthermore, based on the concept of material genetic engineering, the dielectric constant could be taken into account to seek for suitable materials with designable electromagnetic absorption performance.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, with the gradual rise of 5G technology, more and more electronic devices rapidly appear in the world. These new-style products bring convenience to our life, and they also cause electromagnetic radiation problem, threating to our health [24], and Fe3O4 [25] to obtain excellent absorbing performance. However, there is still a lack of report on the composite of MoS2 and MGM.
Herein, we successfully prepared lotus leaf-derived GHPCM morphology genetic composites by an in situ method and followed by a carbonization process. A moderate heating rate makes lotus leaf to retain its original morphology after carbonization, including frontal papillae morphology. The hierarchical porous structure with gradient pore sizes and MoS2 with Janus morphologies are obtained in the MGM composites. More importantly, good electromagnetic performance was obtained in the MGM composites: a minimum RL of about − 50.1 dB at 13.24 GHz with thickness of 2.4 mm and the maximum effective bandwidth of 6.0 GHz from 11.6 to 17.6 GHz at thickness of 2.2 mm, covering the whole Ku band. Moreover, a dielectric sum-quotient model is put forward for the first time to analyze the absorption performance from a computational perspective. This work makes full use of the advantages of MGM and obtain a hierarchical porous morphology via a facile synthesis method, which is hard to achieve from traditional materials. The present results suggest that develo** highly efficient EMW absorbing materials from nature will be a renewable, eco-friendly, and feasible way in future.
2 Experimental Section
2.1 Materials
Lotus leaf were purchased from Yangqingtang (Jiangsu, China). Thiourea (NH2CSNH2) and ammonium molybdate tetrahydrate [(NH4)6Mo7O24·4H2O] were both obtained from Sinopharm Chemical Reagent Co., Ltd., Bei**g, China. All chemical reagents are of analytical grade and used were used as received without further purification. Deionized water obtained from a Milli-Q system was used all the time.
2.2 Preparation of GHPCM
The preparation of GHPCM is illustrated in Fig. 1, and the detail steps are as follows: First, the dried lotus leaves was cut into 2 × 2 cm2 square slices and washed with alcohol and deionized water for several times. Then, six pieces of lotus leaves slices, 1 g of thiourea, and 0.2 g ammonium molybdate tetrahydrate were added into 30 mL deionized water, following by vigorous stirring for 0.5 h. The mixed solution was transferred into a tetrafluoroethylene-lined stainless steel autoclave heated at 200 °C for 24 h to obtain hydrothermal C/MoS2. The resulting black slices were washed with deionized water via a suction filtration process, drying overnight at 60 °C. Finally, the dried powder was heated to X °C (X = 600, 700, 800) and held it for 1 h in Ar atmosphere at a rate of 1 °C min−1. After cooling to room temperature with furnace, the final GHPCM were denoted as LCMS-600, LCMS-700, and LCMS-800, respectively.
2.3 Preparation of Pure MoS2 and Pure Lotus Leaf Carbon
Pure MoS2 and lotus leaf carbon was also fabricated as a control group in order to study the influence of components on the absorption performance. 1 g of thiourea and 0.2 g ammonium molybdate tetrahydrate were added into 30 mL deionized water, following by vigorous stirring for 0.5 h. The mixed solution was transferred into a tetrafluoroethylene-lined stainless steel autoclave heated at 200 °C for 24 h to obtain pure MoS2, which was denoted as PMS. After the same hydrothermal treatment without adding thiourea and ammonium molybdate tetrahydrate, lotus leaves slices were heated to 700 °C and held it for 1 h in Ar atmosphere at a rate of 1 °C min−1. The final black Lotus leaves were denoted as PLC-700.
2.4 Characterizations
The X-ray diffraction (XRD) studies were carried out on DX-2700 X-ray diffractometer using Cu-Ka radiation (λ = 1.54 Å). The morphology was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The thermogravimetry (TG) and differential scanning calorimetry (DSC) curve was characterized using a Netzsch TG thermal gravimetric analyzer in a N2 atmosphere 30–800 °C. The differential thermal analysis (DTA) curve was characterized using a SDT Q600 in a N2 atmosphere 30–800 °C. ASAP2460 instrument was used to characterize the nitrogen adsorption and desorption isotherms, and the specific surface area was measured by the Langmuir method. Pore size distribution was deduced from the absorption isotherms by density functional theory. Raman spectrum was characterized by a cryogenic matrix isolated Raman spectroscopic system using a 532 nm laser. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific K-Alpha spectrometer. Electrical conductivities were detected by utilizing a standard four-probe station (HPS2524). Based on coaxial line theory, the EMW parameters of samples were measured over the 2–18 GHz range in a vector network analyzer (VNA, 3672B-S, Ceyear). Samples were prepared by uniformly mixing the products with paraffin at a mass fraction of 40 wt% and then compacted into a columnar ring of 7.00 mm outer diameter and 3.04 mm inner diameter. The final complex permeability and complex permittivity were determined from the experimental scattering parameters through the standard Nicolson-Ross and Weir theoretical calculations.
3 Results and Discussion
3.1 Characterization of GHPCM
The crystallographic structures and phase contents of the as-synthesized samples were investigated by XRD analysis shown in Fig. 2a. As for PMS, the peaks at 14.3°, 32.7°, 39.5°, and 60.1° are assigned to (002), (100), (103), and (008) crystal planes of 2H–MoS2 (JCPDS No. 37–1492) with hexagonal structure [24]. As for PLC, the peaks at 29.5° and 42.2° are assigned to (110) and (200) crystal planes of carbon (JCPDS No. 72–2091). As labeled in Fig. 2a, it can also be observed in the composites LCMS-600, LCMS-700, and LCMS-800, which infers the existence of crystalline phases for both 2H–MoS2 and carbon as well as the successful synthesis of the composites. According to Scherrer equation: D = kλ/Bcos θ, where D is the interplanar distance, k is the Scherrer constant, λ is the X-ray wavelength, B is the line broadening at half the maximum intensity, and θ is the scattering angle, the average size of MoS2 crystalline particles is about 2.84 nm. To further explore the composition and molecular structure of the samples fabricated at different temperatures, the results of Raman spectroscopy are shown in Figs. 2b and S1a. Three peaks at about 280, 360, and 408 cm−1, originating from E1g, E
12g
, and A1g Raman vibrational modes of 2H–MoS2, respectively, are detected in the composites LCMS-600, LCMS-700, and LCMS-800 [26]. In addition, two distinct peaks located at 1360 and 1580 cm−1 are the D and G bands of the carbon phase, respectively [27]. Generally speaking, the D band is correlated with the disorder or structure defects in the sp2-hybridized carbon atoms or amorphous carbon deposits, while the G band is associated with the in-plane vibrations of sp2 atoms in a 2D hexagonal graphitic lattice [
From Fig. 4a–c and S4a–c, visible distinction in MoS2 growth is displayed on two sides of lotus leaf. On the front side of the lotus leaf, MoS2 all existed in flower-like morphology. While on the back side, most part of MoS2 is flaked on the surface, and the remaining part exists in the form of hemisphere. The extraordinary growth mode of MoS2 allows GHPCM to show an anisotropic structure similar to Janus on the microscopic level. In view of the morphology and characteristics of two sides of lotus leaf, the specific formation mechanism we speculated is shown in Fig. 4d. At the initial stage of hydrothermal process, MoS2 sheets come into being first in the solvent. Due to the hydrophobicity of the front side and the hydrophilicity of the back side, the water solvent is more likely contacted with the back side, which makes the back side first covered with MoS2 sheets. Furthermore, the uneven surface of the front side which covers with papillae also limits the growth of MoS2 sheets. As the reaction progresses, the high temperature gradually destroys the structure of the hydrophobic surface. At the same time, MoS2 sheets in the solvent are assembled into flower-shaped MoS2, which are dispersed on the front side with the morphology presented in Fig. 4a. And the back side is against the flower-shaped MoS2 growth owing to the increased surface energy caused by previous MoS2 sheets.
The element analysis of GHPCM has been carried out by XPS to analyze the information on the surface electronic state and the composition of the samples. The XPS survey scan spectrum of the GHPCM in Fig. 5a indicated that the LCMS-700 consisted of element C, O, Mo, and S. The core-level spectra of C, Mo, and S are shown in Fig. 5b–d. As shown in Fig. 5b, the C 1s spectrum consisted of two peaks at ~ 285.8 and ~ 284.2 eV, which were related to C–O–C and C–C, respectively [25]. For Fig. 5c, the high-resolution spectrum of Mo could be deconvoluted into three peaks. The Mo4+ 3d3/2 (232.3 eV) and Mo4+ 3d5/2 (229.1 eV) peaks belong to the semiconducting 2H-phase MoS2. In the meantime, a satellite peak appears at 235.5 eV, which perhaps owe to the formation of Mo–S–C and Mo–C at the interface between MoS2 and carbon [20]. In addition, the peak at 226.2 eV is ascribed to S 2s [3]. So, PLC has higher conductive loss than pure MoS2 sample, resulting in a higher ε″ value. When the low conductivity MoS2 phases were grown on the surface of PLC, the conductivity of the originally flowing electrons is greatly reduced in the process of passing through these “obstacles.” As a result, the excess conductivity of MGM is tuned and a moderate permittivity is obtained at the same time. In addition to the conductive loss, the formation of interfacial polarization also plays a part of role. When MoS2 is added, the potential difference between the carbon and MoS2 causes charge accumulation at the interface under electromagnetic field and intensifies the polarization relaxation, which is helpful for dielectric loss [34]. To establish the relationship between interface structure and the permittivity, a Debye relaxation correction formula is described as Eqs. 1 and 2 [35]:
where A is defined as the interface factor, ω is the angular frequency, τ is viewed as the relaxation time, εrs and εr∞ are the static dielectric constant and limiting dielectric constant, respectively. According to previous reports, the higher value of A, the more interface polarization there are [35]. Figure 6d reveals the fitting curves of real part of permittivity, and the corresponding A values of PLC, PMS, and LCMS-700 are 0.01, 0.07, and 0.38, respectively. The increased A value of LCMS-700 represents that the combination MoS2 and carbon effectively improves the interface polarization. Although LCMS-700 is rich in interfacial polarization, it should be noted that the permittivity of LCMS-700 is still lower than that of PLC-700 because the proportion of interfacial polarization is much lower than the conductivity loss. Unlike the component group, dipole polarization is dominant in the carbonization temperature group, covering samples of LCMS-600, LCMS-700, and LCMS-800. Defect and functional groups on the surface of materials usually act as polarization center to bring about polarization relaxation under the impact of EMW field [36]. The intrinsic dipole moments of these centers cannot sum to zero, resulting in the dipole polarization. Once increasing the frequency of EMW field, the polarizability fails to maintain the original state, enhancing the dielectric loss [37]. According to the previous Raman results, the disordering degree of the GHPCM increases with the increased carbonization temperatures, suggesting that the material has more surface defects at higher carbonizing temperature. The dipole polarization dominated by these defects makes the permittivity of sample LCMS-600, LCMS-700, and LCMS-800 proportional to the temperature. A Cole–Cole semicircle model is used to better explain the polarization loss occurred in the EMW attenuation, which is described as Eq. 3 [38]:
Each semicircle in the ε′–ε″ curves stands for a polarization relaxation process (as shown in Fig. S5) [39]. The number of semicircles in the figure increases gradually with the increasing carbonization temperature, which represents that high temperature is conducive to the formation of dipole polarization process. To illustrate this point more intuitively, we quantitatively differentiate the conductance loss (\(\varepsilon_{\text{s}}^{{\prime \prime }}\)) and polarization loss (\(\varepsilon_{\text{p}}^{{\prime \prime }}\)) from the imaginary part of the permittivity, according to Eq. 4 [40]:
where εs, ε∞, σ, and τ represent the static dielectric constant, the dielectric constant at infinite frequency, electrical conductivity, and the polarization relaxation time, respectively. Electrical conductivities were detected by utilizing a standard four-probe station (Fig. S6a). Figure S6b, c illustrates the contributions of \(\varepsilon_{\text{c}}^{{\prime \prime }}\) and \(\varepsilon_{\text{p}}^{{\prime \prime }}\) of samples LCMS-600, LCMS-700, and LCMS-800. In most frequency range, the contribution of \(\varepsilon_{\text{p}}^{{\prime \prime }}\) is greater than that of \(\varepsilon_{\text{c}}^{{\prime \prime }}\) and increases with the increase in carbonization temperature, which is consistent with the Cole–Cole circle and the Raman results.
For further evaluating the EMW absorbing of the composites, the RL values at 0.5–5.0 mm thickness in the frequency range of 2–18 GHz are evaluated on the basis of transmission and Debye theory, which can be depicted as Eqs. 5 and 6 [41]:
where Z0 stands for the impedance of free space, Zin is the input impedance of the absorber, d is the thickness of the absorber, and c represents the velocity of light. Figure 7a–e displays the 3D RL curves of the samples at different thickness with 40% filling ratio. Generally speaking, the minimum RL value lower than − 10 dB is often considered as a suitable absorber for practical applications due to 90% of the EMW energy can be attenuated in this situation. From Fig. 7a, b, we can see that the minimum RL value of the PLC-700 is up to − 27.2 dB at 4.88 GHz when the thickness is 3.5 mm. And only a negligible part of the effective absorption region indicates the poor absorption performance of the PMS sample. From Fig. 7d–f, it can be observed that LCMS-700 and LCMS-800 show an improved EMW absorption performance because of the tunable conductive loss and enhanced polarization loss. The minimum RL value of LCMS-700 reaches − 50.1 dB at 13.24 GHz and the effective bandwidth is 5.80 GHz (from 10.52 to 16.32 GHz) with a thickness of only 2.4 mm. When the thickness reduces to 2.2 mm, the effective bandwidth is up to 6.04 GHz from 11.52 to 17.56 GHz, almost covering whole Ku band. If the carbonization temperature goes up to 800 °C, LCMS-800 has a minimum RL value of − 44.2 dB and the corresponding effective bandwidth exceeding − 10 dB is 4.1 GHz. Both the absorption intensity and effective bandwidth are lower than that of the LCMS-700 (Fig. S7). Due to the low carbonization temperature, LCMS-600 shows poor absorbing performance shown in Fig. 7c. Combined with previous analysis of EMW parameters, we speculate that the excessive high or excessive low permittivity count against getting good EMW absorption. To better illustrate this point, the RL values are codetermined by the attenuation constant and impedance matching [42]. The attenuation coefficient mainly measures the ability of EMW entering the material to be consumed by dielectric loss, as mentioned earlier. While the impedance matching refers to capacity that EMW can enter the material rather than being reflected on the surface. The attenuation coefficient (α) values of samples are calculated as Eq. 7, and relevant curves are displayed in Fig. 8a [43].
It can be found that the α of PLC-700 exhibits the highest value than that of others, which indicates that the PLC-700 possess the strongest attenuation ability. As the carbonization temperature increases from 600 to 800 °C, values of α increase from 20, 50, and 41 to 40, 218, and 233 for LCMS-600, LCMS-700, and LCMS-800 within 2–18 GHz, respectively, sharing the similar variation with permittivity. Figure 8b–f reveals a delta (Δ) model to evaluate the impedance matching of the absorbers and the relevant calculation formula is shown as Eq. 8 [44]:
where M and K parameter are associated with real part and imaginary part of εr and μr. The delta value between 0.4 and 0 suggests a satisfactory degree of impedance matching [45]. Clearly, the terrible impedance matching degree (Fig. 8c, d) for the LMS and LCMS-600 sample account for its poor EMW absorption performance, which should be attributed to the low permittivity. Although the overall impedance matching ability of PLC-700 is quite nice, the low Δ value region is mainly concentrated at low frequencies, where the attenuation coefficient is low. In this case, it is hard to get an appropriate absorption performance in the corresponding frequency band. Therefore, LCMS-700 and LCMS-800 with good impedance matching ability in the corresponding frequency band of high attenuation coefficient will naturally show outstanding absorbing performance. Figure 8g shows the corresponding relationship between RL, attenuation coefficient and impedance matching of LCMS-700 at 2.2 and 2.4 mm. The impedance matching is close to 0 at the lowest frequency of RL and the attenuation coefficient remains at a high value, which is consistent with the above analysis. Moreover, theoretical and experimental differences in matching thickness and frequency are also verified by the 1/4 wavelength formula: \(t_{m} = n\lambda /4 = nc/(4f_{m} \sqrt {\left| {\varepsilon_{\text{r}} } \right|\left| {\mu_{\text{r}} } \right|} )\), where n is positive odd number [46]. If the frequency and thickness at the lowest RL value compound this model, the reflected EMW are totally offset at the absorber–air interface due to the 180° phase difference between the incident and reflected EMW in the absorbent [47]. There is no doubt that the LCMS-700 is completely consistent with this model (Fig. S8). In addition, we also tested the absorbing performance of LCMS-700 at different temperature rising rates, as shown in Fig. S9. The EMW parameters of the materials show inconspicuous variation at different heating rates. With the increase in the heating rate, the reflection loss and effective band width of LCMS-700 decrease. Therefore, we consider that 1 °C/min is the most appropriate heating rate to obtain a high-performance absorbing material.
In recent years, few mathematical models and simulations have been applied in the analysis of absorbing materials to judge the consistency between the experimental results and theoretical results [48]. In this work, we set up a dielectric sum-quotient model for the first time from the perspective of mathematical calculation to assist the analysis of the cause of the wide bandwidth of the LCMS-700. In allusion to non-magnetic system, we assume that the real and imaginary parts of the permeability over full frequency band are 1 and 0, respectively. By adjusting the numbers of the real and imaginary parts of the permittivity, it can be observed that the final effective RL value (RL < −10) is influenced by the sum and quotient of two parts. Subsequently, εtotal is defined as the sum of the real and imaginary parts of the permittivity, and cot ε is the quotient of the real and imaginary parts. The relationships between εtotal, cot ε, thickness, frequency, and RL are established and expressed in Fig. 9a–d, and the relevant data are shown in the supplementary material (Table S2–S5). It can be concluded from the figures that with the increase of εtotal, the frequency band where the effective RL value (RL < −10) gradually moves to the low frequency and the effective RL value starts to appear at lower thickness. However, the effective RL band at the same thickness and cot ε are reduced. Combined with the electromagnetic parameters of LCMS-700, εtotal is close to 10 and cot ε is close to 2 at the frequency where RL is less than − 10. By matching this value to Fig. 9b, the effective frequency band of LCMS-700 is basically matched with the calculated results. The dielectric sum-quotient model not only verifies the authenticity of the experimental results, but also explains the reason why the material has a wide effective band from the perspective of calculation. In addition, this model will shed light on the designing consideration of the non-magnetic EMW absorbing material systems with different frequency bands and thickness in the future.
Finally, the mechanism of the excellent EMW absorption performance of LCMS-700 is shown schematically in Fig. 10. First of all, the interior hierarchical structures of lotus leaf can be regarded as the result of three layers, including huge-macropore layer, loose-packed macropores layer, and close-packed macropores layer. Due to porous structure of each layer, the EMW entering the material will be reflected back and attenuated in the pores with different sizes. Second, electrons in carbon could absorb EMW energy to migrate in surface/interlayer channels and then convert energy by colliding with the lattice. Moreover, below the percolation threshold, the samples are dispersed in paraffin to form a network of conductance. More electrons hop between different layers of carbon and network conductivity enhances, converting more EMW energy to heat energy. Third, capacitor-like structures would be generated at the interface of heterogeneous interface, which is also named as interfacial polarization. The addition of MoS2 introduces the interfacial polarization and modifies the dielectric constant of the samples, so that the impedance matching under high attenuation coefficient is improved. Fourth, numbers of functional groups and defects lead to the asymmetric charge distributions, resulting in the formation of dipoles. Increasing the concentration of dipole polarization makes LCMS-700 suffering more polarization relaxation process, which is beneficial to dielectric loss of the material. In the end, we compared the |RL|, effective bandwidth versus thickness for the MGM-based absorption materials published in the recently literature, as shown in Fig. 11 (details in Table S2) [49,50,51,52,53,54,55,56,57,58]. It clearly suggests that the outstanding EMW absorption performance of the lotus leaf-derived GHPCM morphology genetic composites (LCMS-700) makes it a potential candidate for practical application in EMW field.
4 Conclusion
In this study, morphology genetic C/MoS2 composites with gradient hierarchical porous structure were successfully fabricated by a facile in situ method. The morphological structures and the EMW absorption properties of the synthesized GHPCM samples were systematically studied. The obtained morphology genetic C/MoS2 composites have a variety of pore structures, giving full play to the peculiar and exquisite features of the morphology of the MGM. Besides, the papillae and hydrophilic–hydrophobic properties of lotus leaf resulted in the Janus microstructure of flower-like and sheet-like MoS2. The EMW absorption properties of the GHPCM could be conveniently tuned by the carbonization temperature. Remarkably, LCMS-700 sample achieved a strong reflection loss value of − 50.1 dB at the thickness of 2.4 mm and reaches an effective bandwidth of 6.0 GHz at a relatively thin thickness of 2.2 mm. The multistage pore structure in lotus leaf provides more channels for the reflection and attenuation of EMW. And the introduction of MoS2 not only enhances the interfacial polarization but also regulates the excessive dielectric of lotus leaf, which optimizes the impedance matching of composite. Particularly, a dielectric sum-quotient model is put forward based on the mathematical calculations to further verify the experimental results. This investigation provides a new paradigm for the development of pure dielectric loss EMW absorbing materials by taking advantages of the morphology genetic materials and sheds light on the designing consideration of the non-magnetic EMW absorbing material systems in the future.