Abstract
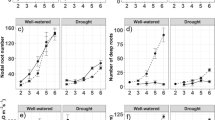
Six wheat genotypes with contrasting rooting depth and root system size at anthesis stage as initially identified from PVC pipe method were further evaluated for root system architecture (RSA) by agar gel plate and trench profile wall methods. Wheat genotypes C-306, HI-1500 and RAJ-3765 showed deeper rooting in pipe, narrower seminal root angle by agar gel plate method and higher root number density in the trench profile wall. Genotype HI1500 showed maximum total root length in pipe and highest root length density (RLD) in trench profile. Whereas, the genotypes EC-573549 and HD-2824 showed relatively shallow rooting depth in pipe, wider seminal root angle and a relatively lower root number density in the trench profile wall. Correlations were observed among the diverse RSA parameters measured from different methods. Finally, the response of photosynthesis and yield parameters in these genotypes were evaluated under terminal drought stress in field. It was tested whether deeper RSA (as in HI1500, C-306 and HI-1500) performed better under terminal drought stress than the shallower RSA genotypes (EC-573549 and HD-2824). Higher root number density in soil profile was associated with a lesser decline in leaf photosynthesis rate under drought. Deeper rooting genotypes showed lower Stress Susceptibility Index (SSI) for photosynthesis parameters compared to the shallow rooting types. Highest photosynthesis rate, stomatal conductance and transpiration rate maintained in HI1500 in drought stress was associated with its deeper and largest root system (RLD), however its 1000 grain weight was susceptible (SSI > 1). The genotype Drysdale with a higher intrinsic water use efficiency showed more tolerance for 1000 grain weight. In general, higher terminal stress tolerance for yield (SSI < 1) in C-306, RAJ-3765 and HI1500 was associated with deeper rooting.




Similar content being viewed by others
References
Arai-Sanoh, Y., Takai, T., Yoshinaga, S., Nakano, H., Kojima, M., Sakakibara, H., Kondo, M., & Uga, Y. (2014). Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Scientific Reports, 4(1), 1–6.
Araki, H., & Iijima, M. (2001). Deep rooting in winter wheat: Rooting nodes of deep roots in two cultivars with deep and shallow root systems. Plant Production Science, 4(3), 215–219.
Arsenault, J. L., Poulcur, S., Messier, C., & Guay, R. (1995). WinRHlZO™, a root-measuring system with a unique overlap correction method. HortScience, 30(4), 906D – 906.
Barrs, H. D., & Weatherley, P. E. (1962). A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Australian Journal of Biological Sciences, 15, 413–428.
Bengough, A. G., Gordon, D. C., Al-Menaie, H., Ellis, R. P., Allan, D., Keith, R., Thomas, W. T. B., & Forster, B. P. (2004). Gel observation chamber for rapid screening of root traits in cereal seedlings. Plant and Soil, 262(1), 63–70.
Böhm, W. (1979). Root parameters and their measurement. In W. Böhm (Ed.), Methods of studying root systems (pp. 125–138). Berlin, Heidelberg: Springer.
Bucksch, A., Burridge, J., York, L. M., Das, A., Nord, E., Weitz, J. S., & Lynch, J. P. (2014). Image-based high-throughput field phenoty** of crop roots. Plant Physiology, 166(2), 470–486.
Comas, L., Becker, S., Cruz, V. M. V., Byrne, P. F., & Dierig, D. A. (2013). Root traits contributing to plant productivity under drought. Frontiers in Plant Science, 4, 442.
Condon, A. G., Richards, R. A., Rebetzke, G. J., & Farquhar, G. D. (2002). Improving intrinsic water-use efficiency and crop yield. Crop Science, 42, 122–131.
Figueroa-Bustos, V., Palta, J. A., Chen, Y., Stefanova, K., & Siddique, K. H. (2020). Wheat cultivars with contrasting root system size responded differently to terminal drought. Frontiers in Plant Science, 11, 1285.
Fischer, R. A., & Maurer, O. R. (1976). Crop temperature modification and yield potential in a dwarf spring wheat 1. Crop Science, 16(6), 855–859.
Gao, Y., & Lynch, J. P. (2016). Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). Journal of Experimental Botany, 67(15), 4545–4557.
Griffiths, M., Delory, B. M., Jawahir, V., Wong, K. M., Cody Bagnall, G., Dowd, T. G., Nusinow, D. A., Miller, A. J., & Topp, C. N. (2022). Optimization of root traits to provide enhanced ecosystem services in agricultural systems: A focus on cover crops. Plant, Cell & Environment,. https://doi.org/10.1111/pce.14247
Gupta, A., Singh, C., Kumar, V., Tyagi, B. S., Tiwari, V., Chatrath, R. & Singh, G. P. (2018). Wheat varieties notified in India since 1965. ICAR- Indian Institute of Wheat & Barley Research, Karnal- 132001, India, 101.
El Hassouni, K., Alahmad, S., Belkadi, B., Filali-Maltouf, A., Hickey, L. T., & Bassi, F. M. (2018). Root system architecture and its association with yield under different water regimes in durum wheat. Crop Science, 58(6), 2331–2346.
Jain, N., Singh, G. P., Yadav, R., Pandey, R., Ramya, P., Shine, M. B., Pandey, V. C., Rai, N., Jha, J., & Prabhu, K. V. (2014). Root trait characteristics and genotypic response in wheat under different water regimes. Cereal Research Communications, 42(3), 426–438.
King, J., Gay, A., Sylvester-Bradley, R. O., Bingham, I. A., Foulkes, J., Gregory, P., & Robinson, D. (2003). Modelling cereal root systems for water and nitrogen capture: towards an economicoptimum. Annals of Botany, 91(3), 383–90.
Kitomi, Y., Hanzawa, E., Kuya, N., Inoue, H., Hara, N., Kawai, S., Kanno, N., Endo, M., Sugimoto, K., Yamazaki, T., & Sakamoto, S. (2020). Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proceedings of the National Academy of Sciences, 117(35), 21242–21250.
Lewis, J. M., & Reynolds, M. (2022). The future of climate resilience in wheat. http://foresight.cgiar.org; doi.org/https://doi.org/10.31235/osf.io/hvd4e.
Lynch, J. P. (2013). Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Annals of Botany, 112(2), 347–357.
Manschadi, A. M., Christopher, J., deVoil, P., & Hammer, G. L. (2006). The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology, 33(9), 823–837.
Manschadi, A. M., Hammer, G. L., Christopher, J. T., & Devoil, P. (2008). Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant and Soil, 303(1), 115–129.
Metselaar, K., Pinheiro, E. A. R., & de Jong van Lier, Q. (2019). Mathematical description of rooting profiles of agricultural crops and its effect on transpiration prediction by a hydrological model. Soil Systems, 3(3), 44.
Miyazaki, A., & Arita, N. (2020). Deep rooting development and growth in upland rice NERICA induced by subsurface irrigation. Plant Production Science, 23(2), 211–219.
Monteith, J. L. (1986). How do crops manipulate water supply and demand? Philosophical Transactions of the Royal Society of London Series a, Mathematical and Physical Sciences, 316(1537), 245–259.
Nakamoto, T., & Oyanagi, A. (1994). The direction of growth of seminal roots of Triticum aestivum L. and experimental modification thereof. Annals of Botany, 73(4), 363–367.
Ober, E. S., Alahmad, S., Cockram, J., Forestan, C., Hickey, L. T., Kant, J., Maccaferri, M., Marr, E., Milner, M., Pinto, F., Rambla, C., Reynolds, M., Salvi, S., Sciara, G., Snowdon, R. J., Thomelin, P., Tuberosa, R., Uauy, C., Voss-Fels, K. P., … Watt, M. (2021). Wheat root systems as a breeding target for climate resilience. Theoretical and Applied Genetics, 134, 1645–1662.
Palta, J. A., Chen, X., Milroy, S. P., Rebetzke, G. J., Dreccer, M. F., & Watt, M. (2011). Large root systems: Are they useful in adapting wheat to dry environments? Functional Plant Biology, 38(5), 347–354.
Passioura, J. B. (1983). Roots and drought resistance. In J. F. Stone & W. O. Willis (Eds.), Developments in agricultural and managed forest ecology (Vol. 12, pp. 265–280). Elsevier.
Poorter, H., Bühler, J., van Dusschoten, D., Climent, J., & Postma, J. A. (2012). Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology, 39(11), 839–850.
Puttamadanayaka, S., Balaramaiah, M., Biradar, S., Parmeshwarappa, S. V., Sinha, N., Prasad, S. S., Mishra, P. C., Jain, N., Singh, P. K., Singh, G. P., & Prabhu, K. V. (2020). Map** genomic regions of moisture deficit stress tolerance using backcross inbred lines in wheat (Triticum aestivum L.). Scientific Reports, 10(1), 1–17.
Ramalingam, P., Kamoshita, A., Deshmukh, V., Yaginuma, S., & Uga, Y. (2017). Association between root growth angle and root length density of a near-isogenic line of IR64 rice with DEEPER ROOTING 1 under different levels of soil compaction. Plant Production Science, 20(2), 162–175.
Rich, S. M., Christopher, J., Richards, R., & Watt, M. (2020). Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. Journal of Experimental Botany, 71(16), 4751–4762.
Richard, C. A., Hickey, L. T., Fletcher, S., Jennings, R., Chenu, K., & Christopher, J. T. (2015). High-throughput phenoty** of seminal root traits in wheat. Plant Methods, 11(1), 1–11.
Rogers, E. D., & Benfey, P. N. (2015). Regulation of plant root system architecture: Implications for crop advancement. Current Opinion in Biotechnology, 32, 93–98.
Saradadevi, R., Bramley, H., Palta, J. A., & Siddique, K. H. (2016). Stomatal behaviour under terminal drought affects post-anthesis water use in wheat. Functional Plant Biology, 44(3), 279–289.
Schenk, H. J., & Jackson, R. B. (2002). The global biogeography of roots. Ecological Monographs, 72(3), 311–328.
Severini, A. D., Wasson, A. P., Evans, J. R., Richards, R. A., & Watt, M. (2020). Root phenotypes at maturity in diverse wheat and triticale genotypes grown in three field experiments: Relationships to shoot selection, biomass, grain yield, flowering time, and environment. Field Crops Research, 255, 107870.
Siddique, K. H. M., Belford, R. K., & Tennant, D. (1990). Root: Shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant and Soil, 121(1), 89–98.
Tardieu, F., Draye, X., & Javaux, M. (2017). Root water uptake and ideotypes of the root system: Whole-plant controls matter. Vadose Zone Journal. https://doi.org/10.2136/vzj2017.05.0107
Thorup-Kristensen, K., Halberg, N., Nicolaisen, M., Olesen, J. E., Crews, T. E., Hinsinger, P., Kirkegaard, J., Pierret, A., & Dresbøll, D. B. (2020). Digging deeper for agricultural resources, the value of deep rooting. Trends in Plant Science, 25(4), 406–417.
Topp, C. N., Bray, A. L., Ellis, N. A., & Liu, Z. (2016). How can we harness quantitative genetic variation in crop root systems for agricultural improvement? Journal of Integrative Plant Biology, 58(3), 213–225.
Uga, Y. (2021). Challenges to design-oriented breeding of root system architecture adapted to climate change. Breeding Science, 71, 3–12.
Uga, Y., Kitomi, Y., Ishikawa, S., & Yano, M. (2015). Genetic improvement for root growth angle to enhance crop production. Breeding Science, 65(2), 111–119.
Uga, Y., Sugimoto, K., Ogawa, S., Rane, J., Ishitani, M., Hara, N., Kitomi, Y., Inukai, Y., Ono, K., Kanno, N., & Inoue, H. (2013). Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics, 45(9), 1097–1102.
Watt, M., Moosavi, S., Cunningham, S. C., Kirkegaard, J. A., Rebetzke, G. J., & Richards, R. A. (2013). A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Annals of Botany, 112(2), 447–455.
York, L. M., Nord, E., & Lynch, J. (2013). Integration of root phenes for soil resource acquisition. Frontiers in Plant Science, 4, 355.
Acknowledgements
1. ICAR fellowship to Ph.D. student GR Rathod (ICAR-IARI) is gratefully acknowledged. 2. Authors wish to convey sincere thanks to ICAR-Indian Agricultural Research Institute (IARI), New Delhi, India for financial support to the in-house project entitled ‘‘Deciphering physiological, biochemical and molecular mechanisms of abiotic stress tolerance and nutrient use efficiency of crop plants’’ (2014-2021).
Author information
Authors and Affiliations
Contributions
GRR, RP, CV designed the experiment, GRR, RP collected the samples GRR, RP, VP recorded data. All the authors have contributed equally in analyzing the data, writing of the manuscript, gone through it and approved it for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rathod, G.R., Pandey, R., Chinnusamy, V. et al. Deeper root system architecture confers better stability to photosynthesis and yield compared to shallow system under terminal drought stress in wheat (Triticum aestivum L.). Plant Physiol. Rep. 27, 250–259 (2022). https://doi.org/10.1007/s40502-022-00652-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-022-00652-1