Abstract
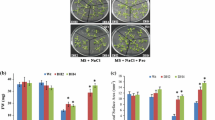
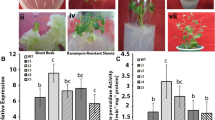
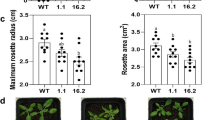
With No Lysine kinase (WNK) belongs to the ser/thr protein kinases category in which the conserved catalytic lysine (K) residue is present in subdomain I instead of being in subdomain II. WNKs alleviate various abiotic stresses through a variety of signal transduction pathways governing various stress tolerance mechanisms in plants. These mechanisms include accumulation of osmo-regulator entities such as proline, activation of various antioxidant enzymes such as catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (POD) as well as certain non-enzyme molecules like secondary metabolites. All of these mechanisms flush out harmful reactive species and maintain cellular integrity in plants. The present study evaluated biochemical properties of wild-type (WT) and OsWNK9 transgenic Arabidopsis lines against the salt and drought stress conditions. Transgenic lines showed high levels of proline accumulation, reduced membrane damage and hydrogen peroxide content compared to WT plants. Moreover, the transgenic lines exhibited the improved activity of antioxidant enzymes such as catalase and ascorbate peroxidase along with the dynamism in peroxidase activity. The total antioxidant capacity in terms of DPPH free radical scavenging percentage was highest in the transgenic lines in comparison to the WT. The Na+, K+ and Na+/K+ measurements among the WT and transgenic lines suggested that the transgenic lines efficiently maintained the intracellular ionic balance and thereby reduces the extent of the cell death. Altogether, transgenic Arabidopsis lines evinced more tolerance to salinity and drought stress than WT plants signifying the involvement of OsWNK9 gene in the regulation of abiotic stress tolerance.








Similar content being viewed by others
References
Aebi, H. E. (1984). Catalase in vitro. Methods of Enzymatic Analysis, 105, 121–126.
Anderson, M. D., Prasad, T. K., & Stewart, C. R. (1995). Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiology, 109(4), 1247–1257.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207.
Cheng, Y. J., Kim, M. D., Deng, X. P., Kwak, S. S., & Chen, W. (2013). Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1, a sweet potato transcription factor. Journal of Microbiology and Biotechnology, 23(12), 1737–1746.
de Azevedo Neto, A. D., Prisco, J. T., Enéas-Filho, J., de Abreu, C. E., & Gomes-Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environmental and Experimental Botany, 56(1), 87–94.
Du, C., Zhao, P., Zhang, H., Li, N., Zheng, L., & Wang, Y. (2017). The Reaumuria trigyna transcription factor RtWRKY1 confers tolerance to salt stress in transgenic Arabidopsis. Journal of Plant Physiology, 215, 48–58.
Gagnon, K. B., England, R., & Delpire, E. (2006). Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. American Journal of Physiology-Cell Physiology, 290(1), C134–C142.
Hanks, S. K., & Hunter, T. (1995). Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. The FASEB Journal, 9(8), 576–596.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Hong-Hermesdorf, A., Brüx, A., Grüber, A., Grüber, G., & Schumacher, K. (2006). A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Letters, 580(3), 932–939.
Huang, C. L., Cha, S. K., Wang, H. R., **e, J., & Cobb, M. H. (2007). WNKs: Protein kinases with a unique kinase domain. Experimental & Molecular Medicine, 39(5), 565.
James, D., Borphukan, B., Fartyal, D., Ram, B., Singh, J., Manna, M., et al. (2018). Concurrent overexpression of OsGS1; 1 and OsGS2 genes in transgenic rice (Oryza sativa L.): Impact on tolerance to abiotic stresses. Frontiers in Plant Science, 9, 1–19.
Kahle, K. T., Rinehart, J., Ring, A., Gimenez, I., Gamba, G., Hebert, S. C., et al. (2006). WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na–K–Cl and K–Cl cotransporters. Physiology, 21(5), 326–335.
Kahle, K. T., Wilson, F. H., & Lifton, R. P. (2005). Regulation of diverse ion transport pathways by WNK4 kinase: A novel molecular switch. Trends in Endocrinology and Metabolism, 16(3), 98–103.
Kang, H. M., & Saltveit, M. E. (2002). Antioxidant enzymes and DPPH-radical scavenging activity in chilled and heat-shocked rice (Oryza sativa L.) seedlings radicles. Journal of Agricultural and Food Chemistry, 50(3), 513–518.
Kumar, K., Rao, K. P., Biswas, D. K., & Sinha, A. K. (2011). Rice WNK1 is regulated by abiotic stress and involved in internal circadian rhythm. Plant Signaling & Behavior, 6(3), 316–320.
Liu, A. L., Zou, J., Liu, C. F., Zhou, X. Y., Zhang, X. W., Luo, G. Y., et al. (2013). Over-expression of OsHsfA7 enhanced salt and drought tolerance in transgenic rice. BMB Reports, 46(1), 31.
Ma, T. L., Wu, W. H., & Wang, Y. (2012). Transcriptome analysis of rice root responses to potassium deficiency. BMC Plant Biology, 12(1), 161.
Manuka, R., Saddhe, A. A., & Kumar, K. (2015). Genome-wide identification and expression analysis of WNK kinase gene family in rice. Computational Biology and Chemistry, 59, 56–66.
Manuka, R., Saddhe, A. A., & Kumar, K. (2018). Expression of OsWNK9 in Arabidopsis conferred tolerance to salt and drought stress. Plant Science, 270, 58–71.
McCormick, J. A., & Ellison, D. H. (2011). The WNKs: A typical protein kinases with pleiotropic actions. Physiological Reviews, 91(1), 177–219.
Munns, R., Wallace, P. A., Teakle, N. L., & Colmer, T. D. (2010). Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In R. Sunkar (Ed.), Plant stress tolerance (1st ed., pp. 371–382). New York: Humana Press.
Mzid, R., Zorrig, W., Ayed, R. B., Hamed, K. B., Ayadi, M., Damak, Y., et al. (2018). The grapevine VvWRKY2 gene enhances salt and osmotic stress tolerance in transgenic Nicotiana tabacum. 3 Biotech, 8(6), 277.
Naing, A. H., Park, K. I., Ai, T. N., Chung, M. Y., Han, J. S., Kang, Y. W., et al. (2017). Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biology, 17(1), 65.
Nakamichi, N., Murakami-Kojima, M., Sato, E., Kishi, Y., Yamashino, T., & Mizuno, T. (2002). Compilation and characterization of a novel WNK family of protein kinases in Arabiodpsis thaliana with reference to circadian rhythms. Bioscience, Biotechnology, and Biochemistry, 66(11), 2429–2436.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867–880.
O’Reilly, M., Marshall, E., Speirs, H. J., & Brown, R. W. (2003). WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. Journal of the American Society of Nephrology, 14(10), 2447–2456.
Pushpalatha, G., Subrahmanyam, D., Sreenu, K., Ram, T., Subbarao, L. V., Parmar, B., et al. (2013). Effect of salt stress on seedling growth and antioxidant enzymes in two contrasting rice introgression lines. Indian Journal of Plant Physiology, 18(4), 360–366.
Pütter, J. (1974). Peroxidases. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (2nd ed., pp. 685–690). Weinhan: Verlag Chemie.
Racchi, M. (2013). Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants, 2(4), 340–369.
Rahmani, F., & Padervand, A. H. (2016). Differential response to physiological drought stress in tolerant and susceptible cultivars of canola. Indian Journal of Plant Physiology, 21(3), 333–340.
Sarmast, M. K., Salehi, H., & Niazi, A. (2015). Biochemical differences underlie varying drought tolerance in four Festuca arundinacea Schreb. genotypes subjected to short water scarcity. Acta Physiologiae Plantarum, 37(9), 192.
Sofo, A., Scopa, A., Nuzzaci, M., & Vitti, A. (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Sciences, 16(6), 13561–13578.
Tsuchiya, T., & Eulgem, T. (2010). The Arabidopsis defense component EDM2 affects the floral transition in an FLC-dependent manner. The Plant Journal, 62(3), 518–528.
Uchida, S., Sohara, E., Rai, T., & Sasaki, S. (2014). Regulation of with-no-lysine kinase signaling by Kelch-like proteins. Biology of the Cell, 106(2), 45–56.
Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Science, 151(1), 59–66.
Veríssimo, F., & Jordan, P. (2001). WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene, 20(39), 5562.
Wang, Y., Liu, K., Liao, H., Zhuang, C., Ma, H., & Yan, X. (2008). The plant WNK gene family and regulation of flowering time in Arabidopsis. Plant Biology, 10(5), 548–562.
Wang, Y., Suo, H., Zheng, Y., Liu, K., Zhuang, C., Kahle, K.T., et al. (2010). The soybean root‐specific protein kinase GmWNK1 regulates stress‐responsive ABA signaling on the root system architecture. The Plant Journal, 64(2), 230–242.
Wang, Y., Suo, H., Zhuang, C., Ma, H., & Yan, X. (2011). Overexpression of the soybean GmWNK1 altered the sensitivity to salt and osmotic stress in Arabidopsis. Journal of Plant Physiology, 168(18), 2260–2267.
Wang, F., Tong, W., Zhu, H., Kong, W., Peng, R., Liu, Q., et al. (2016). A novel Cys 2/His 2 zinc finger protein gene from sweetpotato, IbZFP1, is involved in salt and drought tolerance in transgenic Arabidopsis. Planta, 243(3), 783–797.
Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Wiliams, C., & Desitter, I. (2001). Human hypertension caused by mutations in WNK kinases. Science, 293, 1107–1112.
**e, M., Wu, D., Duan, G., Wang, L., He, R., Li, X., et al. (2014). AtWNK9 is regulated by ABA and dehydration and is involved in drought tolerance in Arabidopsis. Plant Physiology and Biochemistry, 77, 73–83.
Xu, B. E., English, J. M., Wilsbacher, J. L., Stippec, S., Goldsmith, E. J., & Cobb, M. H. (2000). WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. Journal of Biological Chemistry, 275(22), 16795–16801.
Yao, X., Horie, T., Xue, S., Leung, H. Y., Katsuhara, M., Brodsky, D. E., et al. (2010). Differential sodium and potassium transport selectivities of the rice OsHKT2; 1 and OsHKT2; 2 transporters in plant cells. Plant Physiology, 152(1), 341–355.
Zhang, B., Liu, K., Zheng, Y., Wang, Y., Wang, J., & Liao, H. (2013). Disruption of AtWNK8 enhances tolerance of Arabidopsis to salt and osmotic stresses via modulating proline content and activities of catalase and peroxidase. International Journal of Molecular Sciences, 14(4), 7032–7047.
Zhao, J., Guo, S., Chen, S., Zhang, H., & Zhao, Y. (2009). Expression of yeast YAP1 in transgenic Arabidopsis results in increased salt tolerance. Journal of Plant Biology, 52(1), 56.
Acknowledgements
This work is supported by financial assistance from the Science and Engineering Research Board, Department of Science and Technology (India) (SB/FT/LS-312/2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manuka, R., Karle, S.B. & Kumar, K. OsWNK9 mitigates salt and drought stress effects through induced antioxidant systems in Arabidopsis. Plant Physiol. Rep. 24, 168–181 (2019). https://doi.org/10.1007/s40502-019-00448-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-00448-w