Abstract
Background and objectives
Physicians routinely discuss the adverse effects of medications but whether these discussions match older patients’ desire for information is an area that has not been explored. This study compares patient preferences for adverse effect discussions with reported physician practice.
Methods
A cross-sectional survey of a convenience sample of 100 practicing primary care physicians from nine medical groups, and 178 patients recruited from 11 senior centers in the Los Angeles metropolitan area. Physicians listed the adverse effects they typically discuss when prescribing an ACE inhibitor. Patients were given a hypothetical scenario about a new medication prescription and, from a list of adverse effects, they were then asked to circle the three they most wanted to hear about.
Results
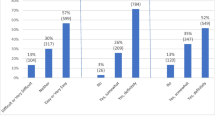
More than 90 % of patients wanted a physician to discuss medication adverse effects; they wanted information about both dangerous (75 % of patients) and common (66 % of patients) adverse effects. However, patients most commonly chose to hear about adverse effects occurring for <1 % of patients, and selected a wide range of adverse effects for discussion. Physicians most frequently reported educating patients about adverse effects which were more common and life-threatening. Patients wishing to discuss additional adverse effects were more worried about adverse effects than those wishing to hear fewer (4.0 vs. 3.4 on a 5-point Likert scale; p = 0.02).
Conclusions
For the studied medication, there was little concordance between the medication adverse effects physicians say they discuss and what patients want to hear. Physicians cannot practically verbally satisfy patients’ information desires about the adverse effects of new medications during time-compressed office visits. Innovative solutions are needed.


Similar content being viewed by others
References
National Center for Health Statistics. Health, United States, 2011: with special feature on socioeconomic status and health [Table 99]. Hyattsville (MD). US Department of Health and Human Services; 2012.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Shrank WH, Polinski JM, Avorn J. Quality indicators for medication use in vulnerable elders. J Am Geriatr Soc. 2007;55(Suppl 2):S373–82.
AHRQ. Questions are the answer. Agency for Healthcare Research and Quality, Rockville (MD). Available from: http://www.ahrq.gov/questionsaretheanswer/level3col_1.asp?nav=3colNav05&content=05_0_prescription. Accessed 27 Dec 2012.
Aspden P, Institute of Medicine (US). Committee on Identifying and Preventing Medication Errors. Preventing medication errors. Quality chasm series. Washington, DC: National Academies Press; 2007. Available from: https://libproxy.usc.edu/login?url=http://ZB5LH7ED7A.search.serialssolutions.com/?V=1.0&L=ZB5LH7ED7A&S=JCs&C=TC0000226812&T=marc.
Huang SW. The Omnibus Reconciliation Act of 1990: redefining pharmacists’ legal responsibilities. Am J Law Med. 1998;24(4):417–42.
Nair K, Dolovich L, Cassels A, et al. What patients want to know about their medications. Focus group study of patient and clinician perspectives. Can Fam Physician. 2002;48:104–10.
Lisper L, Isacson D, Sjoden PO, Bingefors K. Medicated hypertensive patients’ views and experience of information and communication concerning antihypertensive drugs. Patient Educ Couns. 1997;32(3):147–55.
Ziegler DK, Mosier MC, Buenaver M, Okuyemi K. How much information about adverse effects of medication do patients want from physicians? Arch Intern Med. 2001;161(5):706–13.
Bull SA, Hu XH, Hunkeler EM, et al. Discontinuation of use and switching of antidepressants: influence of patient-physician communication. JAMA. 2002;288(11):1403–9.
Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–62.
MacLean CH. Quality indicators for the management of osteoarthritis in vulnerable elders. Ann Intern Med. 2001;135(8 Pt 2):711–21.
Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94.
Powers BJ, Trinh JV, Bosworth HB. Can this patient read and understand written health information? JAMA. 2010;304(1):76–84.
Mechanic D. How should hamsters run? Some observations about sufficient patient time in primary care. BMJ. 2001;323(7307):266–8.
Dickinson D, Raynor DK. What information do patients need about medicines? Ask the patients—they may want to know more than you think. BMJ. 2003;327(7419):861.
Unni E, Farris KB. Determinants of different types of medication non-adherence in cholesterol lowering and asthma maintenance medications: a theoretical approach. Patient Educ Couns. 2011;83(3):382–90.
Duke J, Friedlin J, Ryan P. A quantitative analysis of adverse events and “overwarning” in drug labeling. Arch Intern Med. 2011;171(10):944–6.
Acknowledgments
Derjung M. Tarn, Ariela Wenger, Jeffrey S. Good, Marc Hoffing, Joseph E. Scherger and Neil S. Wenger report no conflicts of interest or financial disclosures.
Dr. Tarn was supported by a University of California–Los Angeles Mentored Clinical Scientist Development Award (5K12AG001004), and by the University of California–Los Angeles Older Americans Independence Center (National Institutes of Health/National Institute on Aging grant P30-AG028748). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Appendix: List of adverse effects provided to patients
Appendix: List of adverse effects provided to patients
The following table lists some of Boncordin’s potential adverse effects. Adverse effects are grouped into non-life-threatening adverse effects that are more or less common, as well as life-threatening adverse effects.
List of potential adverse effects of Boncordin presented to patients
More common adverse effects (% of occurrence) | Less common adverse effects (occur <1 % of the time) | Life-threatening adverse effects (occur <1 % of the time) |
|---|---|---|
Cough (1–10 %) | Hair loss | Significant allergic reaction (anaphylaxis) |
Headache (6 %) | Flushing | Swelling (including of head, neck and intestines) |
Dizziness (4 %) | Joint pains/arthritis | |
Drowsiness (2 %) | Asthma | Shortness of breath |
Worsening kidney function (reversible; doctor will check for this) (2 %) | Dermatitis (itchy skin) | Shock |
Rash | Life-threatening rash in which your skin falls off | |
Numbness or tingling of skin | ||
Muscle aches | ||
Chest pain | ||
Palpitations | ||
Syncope (fainting) | ||
EKG changes | ||
Pancreatitis | ||
Kidney failure | ||
Impotence | ||
Difficulty slee** (insomnia) | ||
Abnormal blood test: sodium | ||
Abnormal white blood count |
Rights and permissions
About this article
Cite this article
Tarn, D.M., Wenger, A., Good, J.S. et al. Do physicians communicate the adverse effects of medications that older patients want to hear?. Drugs Ther Perspect 31, 68–76 (2015). https://doi.org/10.1007/s40267-014-0176-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-014-0176-7