Abstract
Ozanimod (Zeposia®) is the first sphingosine-1-phosphate receptor (S1PR) modulator to be approved for the treatment of adults with moderately to severely active ulcerative colitis in the USA, and in adults with moderately to severely active ulcerative colitis who have had an inadequate or lost response to, or were intolerant of, either conventional therapy or a biologic in the EU. An oral agent, ozanimod is administered once daily as induction and maintenance therapy. In the randomized, double-blind, multinational phase 2 Touchstone and phase 3 True North clinical trials, ozanimod was effective in inducing clinical remission and maintaining remission relative to placebo in adults with moderately to severely active ulcerative colitis. Ozanimod was generally well tolerated in these studies, with manageable or transient adverse events (AEs). Current data from the Touchstone and True North open-label extensions are consistent with the primary studies with respect to therapeutic efficacy and tolerability, with no new safety signals observed. Although further data will be beneficial, ozanimod expands the treatment options for adults with moderately to severely active ulcerative colitis.
Plain Language Summary
Ulcerative colitis is a chronic inflammatory bowel disease involving a dysregulated immune response in the intestinal mucosa. Conventional therapy options for moderate to severe ulcerative colitis are initially effective, but associated with increased risk of adverse events, resistance to treatment, or loss of response over time. Consequently, small molecule drugs have become of interest as alternative treatment options. Ozanimod (Zeposia®) is an oral drug that targets and modulates the activity of sphingosine-1-phosphate receptors to reduce the movement of lymphocytes from the lymph nodes to sites of inflammation. Compared with placebo, ozanimod significantly improved rates of clinical remission and was generally well tolerated in adults with moderately to severely active ulcerative colitis. Findings from open-label extension studies suggest that ozanimod remains efficacious and generally well tolerated with long-term use. Although further data will be beneficial, ozanimod expands the treatment options for adults with moderately to severely active ulcerative colitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.20341755 |
First S1PR modulator approved to treat moderately to severely active ulcerative colitis |
Significantly improves clinical remission rates relative to placebo as induction and maintenance therapy |
Generally well tolerated; infection-related or cardiovascular AEs are manageable and transient |
1 Introduction
Ulcerative colitis is a form of chronic inflammatory bowel disease (IBD) characterized by dysregulation of the mucosal immune response [1]. Affected patients commonly present with rectal bleeding, diarrhoea and weight loss, with some patients requiring hospitalization or, in more severe cases, colectomy [2]. Those younger in age at the time of disease onset (< 40 years) and/or with deep ulcerations, pancolitis, or no signs of endoscopic healing during clinical remission are at a greater risk of more aggressive disease [2]. Ulcerative colitis has a relapsing-remitting pattern of disease and the goal of its treatment is to induce and maintain remission; long-term treatment is therefore usually required [3]. Glucocorticoid therapy and biologic therapy are effective in treating moderate to severe IBD in patients who have had an inadequate response or an intolerance to aminosalicylates; however, their long-term use is associated with increased risk of adverse events (AEs), growing resistance or eventual loss of response [4, 5], as well as drug dependence with glucocorticoid therapy [4]. Patients with moderately to severely active ulcerative colitis experience a reduced quality of life, which may be further impacted by less effective or safe treatments [6].
With a consequent need for durable and convenient treatment options with a greater benefit to risk ratio, a more recent development in ulcerative colitis treatment is small molecule therapy targeting components of active intestinal inflammation [7]. Sphingosine-1-phosphate receptors (S1PR) are involved in cell migration, proliferation and differentiation, with the multiple receptor subtypes (from S1PR1 to 5) involved in different functions [8]. Agonistic activity on S1PR1, which is widely expressed by immune cells and regulates lymphocyte trafficking, prevents lymphocyte movement to inflammation sites by inhibiting their egress from lymph nodes [9].
Oral ozanimod (Zeposia®) is a S1PR modulator originally approved to treat relapsing forms of multiple sclerosis [10, 11]. It is now approved for the treatment of adults with moderately to severely active ulcerative colitis in the USA [11], and in adults with moderately to severely active ulcerative colitis who have had an inadequate or lost response to, or were intolerant of, either conventional therapy or a biologic in the EU [10]. This article provides a narrative review on the pharmacological properties, therapeutic efficacy, and tolerability of ozanimod in this indication; its use in other indications is beyond the scope of this review.
2 Pharmacodynamic Properties of Ozanimod
Ozanimod is a potent S1PR modulator which binds with high affinity to S1PR1 and S1PR5 [12]. In vitro, its major active metabolites (CC112273 and CC1084037) demonstrated a similar level of activity and selectivity for the two receptors [10, 11]. Ozanimod has minimal or no activity on S1PR2, S1PR3 and S1PR4 [12].
While the mechanism of action by which ozanimod produces its therapeutic effects in ulcerative colitis is currently unknown, it may be associated with the ozanimod-induced reduction of lymphocyte migration into the intestine [10, 11]. A greater level of lymphocyte reduction is seen in cells involved in adaptive immune response, while there is minimal impact on cells involved in innate immune response [10]. In a population pharmacokinetic/pharmacodynamic analysis, patients with ulcerative colitis receiving ozanimod 0.92 mg (equivalent to ozanimod HCl 1 mg; n = 885) were estimated to have a mean absolute lymphocyte count (ALC) reduction of 57.2% from baseline and a mean recovery time to normal of 32.8 days following ozanimod discontinuation, with 90% of patients predicted to recover in 3 months [13]. Increasing plasma levels of CC112273 were also associated with ALC reductions of up to 74% from baseline [13].
In randomized controlled clinical trials, ozanimod reduced levels of the intestinal inflammatory markers faecal calprotectin (FCP) and faecal lactoferrin (FLF) during induction therapy in patients with ulcerative colitis; these reductions were maintained through maintenance therapy [14, 15]. In the phase 3 True North clinical trial (Sect. 4.1), ozanimod reduced circulating levels of neutrophil and human neutrophil elastase-mediated degradation of calprotectin (CPa9-HNE), which are markers of increased systemic inflammation, through the induction and maintenance periods [16].
With S1P signalling involved in cardiovascular function, S1PR1 modulators may affect cardiovascular function and heart rate, potentially leading to effects such as bradycardia and atrioventricular block [9, 17]. Ozanimod may cause a transient reduction in heart rate [10, 11], but does not prolong the QTc interval or cause clinically meaningful bradycardia [18].
3 Pharmacokinetic Properties of Ozanimod
Ozanimod and its major active metabolites CC112273 and CC1084037 demonstrate dose-proportional pharmacokinetics over an ozanimod dose range of 0.46–0.92 mg [10, 11]. In vitro data suggest that CC112273 is produced through monoamine oxidase B (MAO-B) and metabolized via cytochrome P450 (CYP) 2C8 and oxido-reductases, and CC1084037 formed from CC112273 through direct reversible metabolism (mediated by carbonyl reductases, aldo-keto reductase 1C1/1C2, and/or 22, and/or 3β- and 11β-hydroxysteroid dehydrogenase), with this interconversion favouring CC112273 [19]. Other metabolic pathways for ozanimod include those of aldehyde dehydrogenase and alcohol dehydrogenase (ALDH/ADH), CYP3A4 and 1A1, and gut microflora to form several other minor active metabolites (e.g. RP101988, RP101075, RP112509), which have similar activity and selectivity for S1PR1 and S1PR5 to the parent drug and undergo further metabolism [10, 11].
With multiple dosing, ≈ 94% of circulating total active substances consist of non-metabolized ozanimod (6%), CC112273 (73%) and CC1084037 (15%) [10, 11] and the areas under the concentration-time curves (AUCs) of CC112273 and CC1084037 are 13-fold and 2.5-fold greater than that of ozanimod [10]. Ozanimod, CC112273 and CC1084037 were bound 98.2%, 99.8%, and 99.3% to human plasma protein, respectively [10, 11]. The times to reach steady state for ozanimod and CC112273 in healthy individuals are 102 h and 45 days, respectively [11], and the time to reach maximum plasma concentration (Tmax) is ≈ 6−8 h for ozanimod and ≈ 10 h for CC112273 [10, 11]. Food intake (regardless of high or low fat and caloric content) in healthy individuals did not affect the exposure of ozanimod or its active metabolites [20].
In healthy individuals, the mean plasma half-life (t½) and apparent oral clearance of ozanimod were ≈ 21 h and ≈ 192 L/h, respectively [19]. The mean t½ of CC112273 was ≈ 195 h. After a single oral 0.92 mg dose of radiolabelled ozanimod, 26% and 37% of radioactivity was found in the urine and faeces, respectively. Most of these contents consisted of inactive metabolites; the concentrations of ozanimod, CC112273 and CC1084037 in urine were negligible [19].
Concomitant use of ozanimod with CYP2C8 inhibitors and inducers may affect the exposure of ozanimod and/or its major active metabolites [10, 11]. Co-administering ozanimod with twice-daily gemfibrozil 600 mg (a potent CYP2C8 inhibitor) increased the AUCs of CC11273 and CC1084037 by ≈ 47% and ≈ 69%, respectively, but did not affect the AUC of ozanimod. Co-administration of once-daily rifampin 600 mg with ozanimod 0.92 mg reduced the AUCs of ozanimod, CC112273 and CC1084037 by ≈ 24%, ≈ 60% and ≈ 55%, respectively [10, 11].
4 Therapeutic Efficacy of Ozanimod
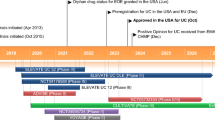
The efficacy of ozanimod in adults with moderately to severely active ulcerative colitis was assessed in two randomized, double-blind, placebo-controlled, multinational studies: the 52-week pivotal phase 3 True North clinical trial (n = 1012) [21] and the 32-week phase 2 Touchstone trial (n = 197) [22]. Both studies involved an induction period and maintenance period, after which patients were eligible to enter the studies’ respective open-label extension (OLE) [21, 22]. This section primarily focuses on the pivotal True North study and is supplemented by findings from Touchstone; the trial design for True North has been illustrated (Fig. 1).
Trial design of the pivotal phase 3 True North clinical trial in adults with moderately to severely active ulcerative colitis [21]. OLE open-label extension, TNFi tumour necrosis factor inhibitor
Supplementary file2 (MP4 10689 kb)
4.1 True North
4.1.1 Trial Design
Patients eligible to enter True North (aged 18−75 years) had a total Mayo score of 6–12 (i.e. moderately to severely active ulcerative colitis) with an endoscopy Mayo subscore of ≥ 2 and rectal bleeding and stool frequency subscores of ≥ 1 each, received stable doses of oral aminosalicylates and/or glucocorticoids for ≥ 2 weeks before screening endoscopy (treatment to be continued at the same dose during the induction period, with the glucocorticoid dose tapered from the start of the maintenance period), and had a documented presence of varicella-zoster virus IgG antibody or complete varicella-zoster vaccination ≥ 30 days before randomization [21]. Those who had no response to induction therapy with ≥ 2 biologic agents to treat ulcerative colitis, a clinically relevant cardiac condition, or a history of uveitis or macular oedema were excluded from the study [21].
The study involved an initial 5-week screening period followed by a 10-week induction period, 42-week maintenance period and a currently ongoing open-label extension (OLE) [Fig. 1] [21]. The induction period included two cohorts: cohort 1, in which patients were randomized 2:1 in a double-blinded manner to receive ozanimod 0.92 mg (equivalent to ozanimod HCl 1 mg; n = 429) or placebo (n = 216) once daily, and cohort 2, an open-label cohort receiving ozanimod only (n = 367). The proportion of patients with prior tumour necrosis factor inhibitor (TNFi) exposure in cohort 1 was capped at 30% after which they were assigned to cohort 2. The proportion of patients with prior TNFi exposure was capped at 50% in cohort 2. Those without previous TNFi exposure were randomized in cohort 1 until the end of enrolment, after which they were assigned to cohort 2. The first week of the induction period involved a 1-week dose escalation of ozanimod [0.23 mg (equivalent to ozanimod HCl 0.25 mg) on days 1–4, 0.46 mg (ozanimod HCl 0.5 mg) on days 5–7 and 0.92 mg thereafter] to reduce the risk of bradycardia, which has been associated with S1P modulator therapy [21].
Ozanimod recipients who had a clinical response at the end of the induction period (i.e. a reduction in total Mayo Clinic score of ≥ 3 points and ≥ 30% from baseline, or a reduction in the three-component Mayo score of ≥ 2 points and ≥ 35% from baseline, in addition to a reduction in the rectal bleeding subscore of ≥ 1 or an absolute rectal bleeding subscore of ≤ 1 point) were eligible to enter the maintenance period, and were randomized 1:1 in a blinded manner to either continue ozanimod therapy or instead receive placebo (Fig. 1) [21]. Placebo recipients with a clinical response at the end of the induction period continued to receive placebo in the maintenance period [21].
The primary efficacy endpoints of True North were the percentages of patients with clinical remission at weeks 10 and 52 (i.e. the end of the induction and maintenance periods), defined as a rectal bleeding subscore of 0, and stool frequency and endoscopy subscores of ≤ 1 and a ≥ 1-point decrease from baseline in stool frequency subscore [21]. The key secondary endpoints were assessed hierarchically at weeks 10 and 52. At week 10, these were (in order) the percentages of patients with clinical response, endoscopic improvement, and mucosal healing (endoscopic improvement and histological remission). At week 52, the hierarchically assessed endpoints were the percentages of patients with clinical response, endoscopic improvement, maintenance of clinical remission (i.e. remission at week 52 in patients achieving remission at week 10), glucocorticoid-free remission (no glucocorticoid use for ≥ 12 weeks), mucosal healing, and durable clinical remission (i.e. remission at weeks 10 and 52, as assessed in all maintenance period participants) [21].
Patients eligible to enter the OLE were those who did not have a clinical response at the end of the induction period, relapsed during the maintenance period (i.e. were no longer responding to treatment) or completed maintenance treatment [23]. Patients who completed ≥ 1 year of the Touchstone OLE were also eligible to enter the True North OLE [23].
4.1.2 Induction and Maintenance Outcomes
Once-daily ozanimod 0.92 mg was effective as induction therapy in patients with moderately to severely active ulcerative colitis [21]. In cohort 1, a significantly greater proportion of ozanimod recipients than placebo recipients achieved clinical remission, with a ≈ 3-fold difference between the two groups (Table 1). Significantly greater proportions of ozanimod recipients achieved the key secondary endpoints; clinical response was achieved by almost two times as many ozanimod recipients than placebo recipients, and endoscopic improvement and mucosal healing achieved by 2.4 and 3.4 times as many ozanimod than placebo recipients (Table 1). Histological remission was achieved by 18.2% of ozanimod recipients, compared with 7.4% of placebo recipients, reflecting an odds ratio of 2.80 (95% CI 1.59–4.93). Efficacy findings from the open-label, ozanimod-only cohort 2 were consistent with the ozanimod group in cohort 1 (Table 1) [21].
Findings from post hoc analyses further support the efficacy of ozanimod in the induction period. Ozanimod appeared to have higher rates of symptomatic response (defined as a ≥ 1 point and ≥ 30% decrease from baseline in partial Mayo score, and a ≥ 1-point decrease from baseline in rectal bleeding subscore or an absolute rectal bleeding subscore of ≤ 1) and remission (defined as rectal bleeding subscore of 0 points, and a stool frequency subscore of ≤ 1 point with a ≥ 1-point decrease from baseline) than placebo [24]. In cohort 1, symptomatic response with ozanimod was seen as early as week 2 (i.e. 1 week post titration) in the overall population [36.1% vs 26.4% with placebo; between-group difference (BGD) 9.6% (95% CI 2.1–17.0)] [24]. Rectal bleeding and stool frequency subscores in ozanimod recipients were decreased by week 2 of the study [21].
Symptomatic response with ozanimod was seen as early as week 2 in TNFi-naïve patients [38.5% vs 29.1% with placebo, BGD 9.4% (95% CI 0.2–18.5)] and week 4 in TNFi-exposed patients [42.2% vs 27.7%, BGD 15.8 (95% CI 1.8–29.8)]. Symptomatic remission with ozanimod was observed as early as week 4 in TNFi-naïve patients [27.2% vs 17.9%, BGD 9.4% (95% CI 1.5–17.4)] and week 8 in TNFi-exposed patients [22.7% vs 12.3%, BGD 11.7% (95% CI 1.3–22.1)] [24]. Ozanimod was similarly effective with or without concomitant corticosteroid therapy in immunomodulator- and biologic-naïve patients who had previously received and failed 5-aminosalicyclate (5-ASA) therapy (typically used for mild to moderate cases of ulcerative colitis) [25].
A significantly greater proportion of ozanimod recipients had achieved clinical remission at the end of the maintenance period (i.e. week 52 of the study) relative to patients who were switched from ozanimod to placebo (Table 2) [21]. Each of the key secondary endpoints were also significantly improved with ozanimod maintenance therapy (Table 2). Over half of the continuous ozanimod recipients who had achieved clinical remission at the end of the induction period had maintained remission at the end of the maintenance period. Histological remission was seen in 33.5% of ozanimod recipients and 16.3% of placebo recipients (odds ratio 2.68; 95% CI 1.70–4.23). Approximately 85% and 60% of ozanimod and placebo recipients did not experience disease relapse (i.e. increase in partial Mayo score of ≥ 2 from week 10, with an absolute partial Mayo score of ≥ 4 and endoscopic subscore of ≥ 2) during the maintenance period [21]. In a post hoc analysis, mucosal healing observed at week 10 was associated with improved clinical outcomes at week 52, with numerically higher proportions of patients with mucosal healing at week 10 achieving clinical remission, corticosteroid-free remission, mucosal healing, endoscopic improvement and histological remission at week 52 than those without [26].
Ozanimod recipients achieved significantly greater work productivity and activity relative to placebo recipients after induction therapy, based on Work Productivity and Activity Impairment-Ulcerative Colitis (WPAI-UC) category scores; score improvements in the assessed categories (i.e. absenteeism, presenteeism, work productivity loss and activity impairment) were reported in those with improved endoscopy score, clinical remission or clinical response than those without [27].
4.1.3 OLE Outcomes
Interim findings from the OLE (n = 823) indicated that benefits with ozanimod therapy were generally maintained after up to 142 weeks of treatment (Table 2) [23]. The proportions of patients achieving clinical remission, clinical response, endoscopic improvement, and glucocorticoid-free remission at week 142 were consistent with those seen at week 46 of the OLE, with some endpoints achieved by numerically higher proportions of patients than after 52 weeks in the True North study. The majority of the patients who were clinical responders at the time of entering the OLE (n = 261) had achieved clinical remission at weeks 46 (70% of patients) and 94 (69% of patients), with most achieving durable clinical response at these time points (95% and 98% of patients, respectively). Week 142 data were not reported in this subgroup due to insufficient data at the time of the interim analysis [23].
In ozanimod recipients in cohort 1 who did not reach clinical response at the end of the induction period and continued receiving ozanimod in the OLE (n = 150), the mean total Mayo score was 8.5 at the end of the induction period (vs 9.2 at baseline), with 23.3% of patients achieving a rectal bleeding subscore of 0 [28]. Symptomatic clinical response was achieved by 44.0% and 48.7% of these patients at weeks 5 and 10 of the OLE [28].
A subgroup of patients who had clinically responded to ozanimod during induction and relapsed after re-randomization to placebo during maintenance received open-label reinduction with ozanimod in the OLE (n = 77) [29]. At weeks 5 and 10 of the OLE, the symptomatic clinical response rates were 55.8% and 58.4%, and the mean partial Mayo scores were 3.5 and 2.1 (vs 6.5 at baseline); reductions were also seen in the partial Mayo subscores of mean rectal bleeding (0.6 and 0.2 vs 1.5 at baseline), stool frequency (1.6 and 1.0 vs 2.6 at baseline) and Physician’s Global Assessment (1.4 and 0.9 vs 2.4% at baseline). Symptomatic clinical response rates were similar between patients previously treated with biologics and those not [29].
4.2 Touchstone
Patients eligible for Touchstone (aged 18–75 years) were those with a Mayo Clinic score of 6–12 and an endoscopic subscore of 2 or 3 (i.e. moderate to severe ulcerative colitis) and a documented presence of varicella-zoster virus IgG antibody or complete varicella-zoster vaccination [22]. Any treatment with oral aminosalicylates or prednisone (≤ 30 mg per day) was to be administered at a stable dose, and any treatment with biological agents or azathioprine, mercaptopurine or methotrexate was to be discontinued 5 half-lives before study treatment and 4 weeks before screening endoscopy [22].
The Touchstone study involved an 8-week induction period and 24-week maintenance period, both double blinded, followed by an optional OLE [22]. In the induction period, patients were randomly assigned in a 1:1:1 ratio to receive once-daily ozanimod 0.46 mg, ozanimod 0.92 mg, or placebo, with ozanimod recipients undergoing dose escalation for one week before receiving their allocated dose for 8 weeks. The primary efficacy endpoint was clinical remission (Mayo score ≤ 2, with no subscore > 1) at the end of the induction period (i.e. after 8 weeks of treatment with ozanimod 0.92 mg); the comparison of ozanimod 0.92 mg versus placebo was assessed prior to that of ozanimod 0.46 mg versus placebo in the hierarchical testing order, followed by the secondary endpoints (also assessed at the end of induction) [22].
Efficacy data from the Touchstone study (n = 197) suggest that once-daily ozanimod 0.92 mg may be effective in these patients relative to placebo [22]. After 8 weeks, 16% of ozanimod 0.92 mg and 6% of placebo recipients achieved clinical remission (p = 0.048). As significance was not reached with respect to clinical remission with ozanimod 0.46 mg, the secondary efficacy endpoints were considered exploratory; clinical response was achieved by 57% of ozanimod 0.92 mg recipients (vs 37% of placebo recipients; nominal p = 0.02), mucosal healing by 34% (vs 12%; nominal p = 0.002) and histological remission by 22% (vs 11%; nominal p = 0.07). Exploratory analyses indicated that the efficacy of ozanimod 0.92 mg was maintained after 32 weeks of treatment (i.e. at the end of the maintenance period) with respect to the proportions of patients achieving clinical remission (21% vs 6% with placebo; nominal p = 0.01), clinical response (51% vs 20%; nominal p < 0.001), mucosal healing (33% vs 12%; nominal p = 0.005) and histological remission (31% vs 8%; nominal p < 0.001) [22].
Data from the Touchstone OLE (n = 170) also generally support the long-term efficacy of ozanimod, with most patients achieving clinical response (93%) and remission (83%) after up to 200 weeks of treatment with ozanimod 0.92 mg [30].
5 Tolerability of Ozanimod
Ozanimod was generally well tolerated in patients with moderately to severely active ulcerative colitis in phase 2 and 3 clinical trials [10, 11]. After 52 weeks in the True North study, AEs had occurred in ≈ 40% and 49% of ozanimod recipients in the induction and maintenance periods (vs 38% and 37% of placebo recipients) [21]. Pooled data from the induction periods of the True North and Touchstone studies showed that the most common AEs with ozanimod 0.92 mg (incidence ≥ 2%) with an incidence ≥ 1% greater than placebo were upper respiratory infection (5% vs 4% of patients in the respective groups), liver test increased (5% vs 0%), headache (4% vs 3%), pyrexia and nausea (3% vs 2% each), and arthralgia (3% vs 1%) [11]. In the True North maintenance period, AEs in ozanimod recipients with an incidence of ≥ 4% and ≥ 1% greater than placebo were increased liver test (11% vs 2%) and headache (5% vs < 1%) [11].
Serious AEs (SAEs) occurred in ≈ 5% of ozanimod recipients in both the induction and maintenance periods of True North (vs 3% and 8% of placebo recipients in the respective periods), with those related to treatment occurring in four patients (i.e. 0.5%) during the induction period and none during maintenance [vs two (0.9%) and one (0.4%) placebo recipients; SAEs not specified] [21]. During the maintenance period, hypertensive crisis as a SAE was reported in one patient in each of the ozanimod and placebo groups, neither of whom discontinued treatment as a result. In the induction and maintenance periods, 3.5% and 1.3% of ozanimod recipients discontinued treatment due to AEs (vs 3.2% and 2.6% of placebo recipients). The most common cause of treatment discontinuation in the maintenance period was disease relapse (13.5% vs 33.9% of ozanimod and placebo recipients) [21]. No cases of progressive multifocal leukoencephalopathy (PML) were reported in ulcerative colitis trials [31].
Findings from the shorter Touchstone study were consistent with those seen in True North [22]. After 32 weeks of treatment, SAEs occurred in three ozanimod 0.92 mg recipients (worsening ulcerative colitis in two patients, adenoma of the colon in one patient) and six placebo recipients (worsening ulcerative colitis in three patients, iron-deficiency anaemia in one patient, herpes zoster infection and autoimmune haemolytic anaemia in one patient, jaundice in one patient). AEs leading to treatment discontinuation occurred in 1% of ozanimod 0.92 mg and 6% of placebo recipients [22].
Pooled data from all controlled and uncontrolled ulcerative colitis studies (including phase 2 and 3 studies and the Touchstone and True North OLEs) as well as data that also included a multiple sclerosis study [32, 33], suggested no new patterns of AEs or cumulative toxicity over a mean treatment duration of 22 months of treatment in ulcerative colitis patients [32,33,34].
In a pooled analysis based on data across multiple ulcerative colitis studies, including the Touchstone and True North studies and their OLEs, tolerability findings in patients who received ozanimod concomitantly with serotonin-norepinephrine reuptake inhibitors (SNRIs) or selective serotonin reuptake inhibitors (SSRIs) [n = 62] were comparable to those who did not, and no cases of serotonin syndrome were reported [35]. The most commonly reported (incidence > 2% in either group) serotonin syndrome-related treatment-related AEs (TEAEs) were nausea (4.8% vs 3.8% in patients who received concomitant SNRI/SSRI and those who did not) and pyrexia (3.2% vs 2.9%), and the most commonly reported hypertension-related TEAEs (incidence ≥ 1%) were hypertension (6.5% vs 4.4%) and blood pressure increased (1.6% vs 0.4%) [35].
5.1 Adverse Events of Special Interest
Cardiovascular AEs, which may be related to the S1PR modulator class (Sect. 2) were generally transient and low in incidence in phase 3 trials [21, 22]. Following the first dose of ozanimod in True North and Touchstone participants, the mean heart rate decreased to a nadir (decrease of 0.7 bpm from baseline) 5 h post-administration and returned to baseline at 6 h [36]. One of the 34 ozanimod recipients in the two studies who had a previous history of cardiac disorders experienced asymptomatic bradycardia on the first day of treatment [36]. In True North, 1.8% and 1.3% of ozanimod recipients in the induction and maintenance periods (vs 0.9% and 2.9% of continuous placebo recipients) experienced a cardiac-related AE; none were reported in the ozanimod to placebo group in the maintenance period [37]. Cardiac AEs occurring in more than one ozanimod recipient were reported only in the induction period and included bradycardia [five patients (0.6%)], and palpitations and tachycardia [three patients (0.4%) each]; none of these occurred in placebo recipients. Pooled data across phase 3 trials in patients with ulcerative colitis and multiple sclerosis suggest that the incidence of bradycardia with ozanimod was low and primarily occurred in the induction period; longer-term use of ozanimod was not associated with an increased risk of major cardiovascular AEs [37].
The mean ALC in patients who received ozanimod throughout the True North induction and maintenance periods was reduced to 43–45% of baseline levels (0.79 × 109/L) at the end of the induction period and maintained until the end of the maintenance period [38]. In patients who received ozanimod induction therapy and were switched to placebo for the maintenance period, the mean ALC recovered within 8 weeks and reached baseline levels within 18 weeks following the switch to placebo. No patient had ALC levels < 0.5 × 109/L at the end of the maintenance period. The mean ALC in placebo recipients remained between 1.8–2.1 × 109/L over the 52-week treatment period. In all groups, no patient with an ALC of < 0.2 × 109/L experienced concurrent serious or opportunistic infections [38], and ALCs were noted to be consistent between those who developed a herpes zoster infection and those who did not [39].
In a pooled analysis across the True North and Touchstone studies and their OLEs (n = 1158 and 508 ozanimod and placebo recipients), the incidence rate (IR) of infections per 1000 patient-years of exposure (PYE) was 228.1 in ozanimod recipients and 314.3 in placebo recipients [40]. The IR for serious infections was 13.2 and 28.4 in ozanimod and placebo recipients, and that for opportunistic infections (all non-serious) was 14.8 and 8.1 in the respective patient groups; most opportunistic infections were driven by herpes zoster infections (IR 13.2 and 8.1) [40]. Of the ozanimod recipients in True North (n = 796), 1.0% reported herpes zoster during induction or maintenance treatment (IR 1.8 per 100 PYE); all cases were treated successfully with oral antivirals while they remained on ozanimod therapy, and no patient discontinued ozanimod on account of herpes zoster infection [39].
In True North, three ozanimod recipients in the induction period and one in the maintenance period discontinued ozanimod therapy due to abnormal liver function tests [21]. Across True North and Touchstone, alanine aminotransferase (ALT) level elevations of ≥ 3-fold the upper limit of normal (ULN) were reported in 2.6% of ozanimod and 0.5% of placebo recipients in the induction period, and in 2.3% and 0% in the maintenance period [10]. Most ozanimod recipients (96%) experiencing ALT elevations of ≥ 3-fold the ULN had ALT levels returning to < 3-fold the ULN within ≈ 2–4 weeks of continued ozanimod therapy. ALT elevations of ≥ 5-fold the ULN occurred in 0.9% of ozanimod and 0.5% of placebo recipients in the induction period and 0.9% and 0% of patients in the maintenance period [10].
6 Dosage and Administration of Ozanimod
Oral ozanimod is indicated for the treatment of adults with moderately to severely active ulcerative colitis in the USA [11], and in adults with moderately to severely active ulcerative colitis who have had an inadequate or lost response to, or were intolerant of, either conventional therapy or a biologic in the EU [10]. Ozanimod should be initiated with a 7-day titration (starting from 0.23 mg once daily), after which the recommended dosage is 0.92 mg once a day; the capsules may be taken with or without food [10, 11]. Patients should be assessed for complete blood count, cardiac and liver function, vaccination status, and current or prior medications before and during ozanimod therapy [10, 11].
Ozanimod is contraindicated in those with immunodeficiency (due to its immunosuppressive effect), severe liver impairment, and a history or presence of second-degree atrioventricular block Type II, third-degree atrioventricular block or sick sinus syndrome, unless the patient has a functioning pacemaker [10, 11]. Ozanimod causes a temporary reduction in peripheral blood lymphocyte count (Sect. 5.1), which may increase susceptibility to infections. Concomitant use with monoamine oxidase inhibitors or CYP2C8 inducers is not recommended. PML has been reported with S1PR modulator use; ozanimod therapy should be suspended if PML is suspected, and discontinued if PML is confirmed [10, 11].
Local prescribing information should be consulted for detailed information regarding further contraindications, dose titration schedules, use in special patient populations, and warnings and precautions.
7 Current Status of Ozanimod in Ulcerative Colitis
The goal of ulcerative colitis management in adults is to produce a durable response and sustained periods remission that do not require steroid therapy [3]. Guidelines recommend inducing remission of moderately to severely active ulcerative colitis with oral corticosteroids (e.g. prednisolone), particularly for those who were refractory to sulfasalazine or aminosalicylate therapy [41], but not for maintaining remission as long-term exposure to systemic corticosteroids has considerable safety risks [42]. Anti-TNF agents (e.g. infliximab, adalimumab and golimumab), vedolizumab, tofacitinib or ustekinumab are also recommended to induce remission and may be continued as maintenance therapy if the patient responds to induction therapy [42, 43], although there may be a loss of response to these biologics over time [5].
The development of oral small molecule drugs has become an area of interest in the search for effective, convenient and well-tolerated therapies for long-term ulcerative colitis management [5]. Those that are currently available for treating ulcerative colitis include the Janus kinase inhibitors tofacitinib, upadacitinib (both approved in the USA and the EU) and filgotinib (EU only), and ozanimod, the first S1PR modulator and (following tofacitinib) second small molecule drug to be approved for the treatment of ulcerative colitis [44]. The selective and potent binding of ozanimod to S1PR1 and S1PR5 reduces lymphocyte migration to the intestine, which may then reduce inflammation in the colon (Sect. 2). The pharmacokinetics of ozanimod allow for once-daily dosing and are not affected by food intake, although they may be affected by concomitant CYP2C8 inhibitors or inducers (Sect. 3).
In clinical trials, ozanimod was effective as both induction and maintenance therapy in adults with moderately to severely active ulcerative colitis (Sect. 4). In True North, significantly greater proportions of patients had achieved clinical remission with ozanimod relative to placebo at the end of the induction and maintenance periods (Sect. 4.1.2); similar findings were seen in the Touchstone study (Sect. 4.2). OLE data from True North and Touchstone suggest that ozanimod was effective for up to 142 weeks and 200 weeks, respectively, with respect to clinical remission and response (Sect. 4). Of note, symptomatic response and remission were generally seen earlier in TNFi-naïve patients compared with TNFi-exposed patients in the True North induction period (Sect. 4.1.2). Studies designed to specifically investigate the use of ozanimod in patients who do not respond to or tolerate TNFi therapy, as well as those who have lost response and relapsed, will be beneficial in further determining the scope of its use in this indication.
Ozanimod was generally well tolerated in these patients, with a tolerability profile consistent between the induction and maintenance periods (Sect. 5). AEs of special interest (AESIs), including abnormal liver function tests decreased, ALC, infections, and cardiac-related events, were generally low in incidence and/or manageable and transient, with minimal numbers of patients discontinuing treatment on account of these (Sect. 5.1); it should be noted that patients with recent, clinically significant cardiovascular disease (e.g. myocardial infarction, unstable angina) were not included in the True North or Touchstone trials. Current tolerability data from the True North and Touchstone OLEs are consistent with those from the primary studies, suggesting that ozanimod is well tolerated with long-term use.
Further studies support the use of ozanimod in moderately to severely active ulcerative colitis. Preliminary real-world data in a small group of patients treated for up to 8 weeks (n = 16) are promising and support clinical trial findings in the efficacy and tolerability of ozanimod [45]. Multiple indirect analyses suggest that ozanimod is comparable to other recommended drugs for this indication. In a systematic review and network meta-analysis, ozanimod appeared to have comparable efficacy to other existing small molecule drugs and biologics for ulcerative colitis (or greater, e.g. adalimumab for endoscopic improvement). No differences in AEs and SAEs were observed between active interventions, but ozanimod was associated with the highest rate of SAEs [44]. In matching-adjusted indirect comparison analyses, ozanimod appeared comparably efficacious relative to adalimumab, vedolizumab [46] and ustekinumab [47], but more efficacious than adalimumab as induction therapy (with respect to clinical response and endoscopic improvement) [46]. In TNFi-naïve patients, vedolizumab was considered to be the most efficacious as maintenance therapy with respect to differences with placebo in clinical response rates [46]. Ozanimod was associated with a significantly lower rate of infectious AEs than with adalimumab [46] and ustekinumab [47]. As these are indirect analyses, this data should be interpreted with caution; more robust, direct head-to-head analyses would be valuable to compare the use of ozanimod with other treatments.
Guidelines for the treatment of moderate to severe ulcerative colitis have yet to include ozanimod as they precede its approval. All treatment options for ulcerative colitis are associated with their own benefits and risks; for instance, while tofacitinib and upadacitinib have been shown to be effective in this indication, they are (unlike ozanimod) associated with a boxed warning in the US prescribing information concerning the risk of serious infections or malignancies [48, 49], which may be a barrier to some patients. Further studies on ozanimod, including head-to-head analyses and studies assessing the cost-effectiveness of ozanimod relative to other treatments, will be valuable in more firmly establishing the place of ozanimod in the management of ulcerative colitis and in determining which patient population may benefit the most from ozanimod therapy.
In conclusion, current data indicate that ozanimod is an effective and generally well tolerated treatment to both induce and maintain disease remission in ulcerative colitis. Although further data will be beneficial, ozanimod expands the treatment options for adults with moderately to severely active ulcerative colitis.
Data Selection Ozanimod: 285 records identified
Duplicates removed | 53 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 86 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 97 |
Cited efficacy/tolerability articles | 22 |
Cited articles not efficacy/tolerability | 27 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were ozanimod, RPC-1063, ZEPOSIA, ulcerative colitis. Records were limited to those in English language. Searches last updated 3 August 2022. | |
Change history
01 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40265-022-01772-6
References
Wyatt NJ, Speight RA, Stewart CJ, et al. Targeting leukocyte trafficking in inflammatory bowel disease. BioDrugs. 2021;35(5):473–503.
Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–70.
Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384–413.
Dubois-Camacho K, Ottum PA, Franco-Munoz D, et al. Glucocorticosteroid therapy in inflammatory bowel diseases: from clinical practice to molecular biology. World J Gastroenterol. 2017;23(36):6628–38.
Ma C, Battat R, Dulai PS, et al. Innovations in oral therapies for inflammatory bowel disease. Drugs. 2019;79(12):1321–35.
Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021;53(7):803–8.
Shivaji UN, Nardone OM, Cannatelli R, et al. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol Hepatol. 2020;5(9):850–61.
Verstockt B, Vetrano S, Salas A, et al. Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2022. https://doi.org/10.1038/s41575-021-00574-7.
Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol. 2019;10:212.
Bristol Myers Squibb Pharma EEIG. Zeposia® (ozanimod hydrochloride): EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 3 Aug 2022.
Bristol Myers Squibb Pharma EEIG. Zeposia® (ozanimod hydrochloride) capsules, for oral use: US prescribing information. 2021. https://dailymed.nlm.nih.gov. Accessed 3 Aug 2022.
Scott FL, Clemons B, Brooks J, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173(11):1778–92.
Tatosian D, Shen J, Chen L, et al. Population pharmacokinetics and pharmacodynamics of ozanimod in ulcerative colitis [abstract]. Gastroenterology. 2022;162(Suppl. 3):S16.
Sandborn W, Feagan B, Wolf D, et al. Effect of ozanimod on fecal calprotectin and fecal lactoferrin, biomarkers of intestinal inflammation, in the phase 2 TOUCHSTONE study of patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2021;27(Suppl. 1):S6.
Ghosh S, D’Haens G, Jairath V, et al. Ozanimod reduced fecal calprotectin levels in patients with ulcerative colitis in the phase 3 True North study [abstract no. P012]. Am J Gastroenterol. 2020;115(Suppl. 1):S3.
Harris S, Wu C, Li Y, et al. The effect of ozanimod on circulating neutrophils: results from the True North study of patients with moderately to severely active ulcerative colitis [abstract plus poster no. Tu1466]. In: DDW 2022. 2022.
Jozefczuk E, Guzik TJ, Siedlinski M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol Res. 2020;156: 104793.
Tran JQ, Hartung JP, Olson AD, et al. Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc study. Clin Pharmacol Drug Dev. 2018;7(3):263–76.
Surapaneni S, Yerramilli U, Bai A, et al. Absorption, metabolism, and excretion, in vitro pharmacology, and clinical pharmacokinetics of ozanimod, a novel sphingosine 1-phosphate receptor modulator. Drug Metab Dispos. 2021;49(5):405–19.
Tran JQ, Hartung JP, Tompkins CA, et al. Effects of high- and low-fat meals on the pharmacokinetics of ozanimod, a novel sphingosine-1-phosphate receptor modulator. Clin Pharmacol Drug Dev. 2018;7(6):634–40.
Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280–91.
Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374(18):1754–62.
Danese S, Colombel J, Ponich T, et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis [abstract no. DOP44]. J Crohns Colitis. 2022;16(Suppl. 1):i093–4.
Siegmund B, Axelrad J, Pondel M, et al. Rapidity of ozanimod-induced symptomatic response and remission in patients with moderately to severely active ulcerative colitis: results from the induction period of True North [abstract no. DOP43 plus presentation]. In: ECCO Annual Congress. 2022.
Sands BE, Dignass A, Irving P, et al. Ozanimod is an efficacious oral therapy after, 5-ASA failure in immunomodulator- and biologic-naive patients with ulcerative colitis: post hoc analysis from True North [abstract plus poster no. P316]. In: ECCO Annual Congress. 2022.
Reinisch W, Axelrad J, Ahmad H, et al. Early mucosal healing at week 10 with ozanimod predicts clinical outcomes at week 52: post hoc analysis of the phase 3 True North clinical trial [poster no. P431]. In: ECCO Annual Congress. 2022.
Floden L, Pham TP, Kumar J, et al. Evaluation of work productivity and activity impairment in moderate-to-severe ulcerative colitis participants treated with ozanimod in the phase 3 True North study [abstract plus poster no. EP1029]. In: DDW 2022. 2022.
Panaccione R, Afzali A, Hudesman D, et al. Extended therapy with ozanimod for delayed responders to ozanimod in moderately to severely active ulcerative colitis: data from the True North open-label extension study [abstract plus poster no. Tu1450]. In: DDW 2022. 2022.
Afzali A, Chiorean MV, Lawlor G, et al. Recapture of response with ozanimod in patients with moderately to severely active ulcerative colitis who withdrew therapy: data from the True North open-label extension study [abstract plus presentation no. 969]. In: DDW 2022. 2022.
Sandborn WJ, Feagan BG, Hanauer S, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J Crohns Colitis. 2021;15(7):1120–9.
US Center for Drug Evaluation and Research. Zeposia® (ozanimod hydrochloride): US summary review. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/209899Orig1s000MedR.pdf. Accessed 3 Aug 2022.
Danese S, Wolf DC, Alekseeva O, et al. Long-term safety of ozanimod in patients with moderately to severely active ulcerative colitis (UC) and relapsing multiple sclerosis (RMS) studies [abstract plus poster no. P0442]. United Eur Gastroenterol J. 2021;9(Suppl. 8):527–8.
Rubin DT, Wolf DC, Alekseeva O, et al. Long-term safety of ozanimod in patients with moderately to severely active ulcerative colitis (UC) and relapsing multiple sclerosis (RMS) studies [abstract no. S853]. Am J Gastroenterol. 2021;116(Suppl.):S397.
D’Haens GR, Colombel JF, Lichtenstein GR, et al. Safety of ozanimod in patients with moderately to severely active ulcerative colitis over time: pooled analysis from phase 2, phase 3, and open-label extension trials [abstract no. 128]. Gastroenterology. 2021;160(Suppl. 6):S-35.
Colombel JF, Charles L, Petersen A, et al. Safety of concurrent administration of ozanimod and serotonergic antidepressants in patients with ulcerative colitis [abstract no. P0441 and poster]. United Eur Gastroenterol J. 2021;9(Suppl. 8):526–7.
Long M, Cross R, Calkwood J, et al. Ozanimod first-dose cardiac effects in patients with moderately to severely active ulcerative colitis and relapsing multiple sclerosis [abstract no. P038]. Am J Gastroenterol. 2021;116(Suppl. 1):S9–10.
Armuzzi A, Cross R, Lichtenstein G, et al. Long-term cardiac safety of ozanimod in phase 3 clinical program of ulcerative colitis and relapsing multiple sclerosis [abstract no. DOP45]. J Crohns Colitis. 2022;16(Suppl. 1):i094–5.
D’Haens G, Irving P, Colombel JF, et al. Effect of ozanimod treatment and discontinuation on absolute lymphocyte count in patients with moderately to severely active ulcerative colitis: results from a phase 3 randomized trial [abstract plus poster no. P0386]. United Eur Gastroenterol J. 2021;9(Suppl. 8):480–1.
Siegmund B, Melmed GY, Irving P, et al. Incidence and outcomes of herpes zoster in the ozanimod phase 3 ulcerative colitis and relapsing multiple sclerosis clinical program [abstract plus poster no. P402]. J Crohns Colitis. 2022;16(Suppl. 1):i395–6.
Rieder F, Wolf DC, Charles L, et al. Incidence of infections in patients with moderately to severely active ulcerative colitis treated with ozanimod and relationship to significant lymphopenia: results from a pooled safety analysis [abstract no. Fr513]. In: Gastroenterology. 2021.
Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl. 3):s1–106.
Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17.
Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA Clinical Practice Guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–61.
Lasa JS, Olivera PA, Danese S, et al. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–70.
Cohen N, Choden T, Choi D, et al. Real world effectiveness and safety of ozanimod: initial results from a large tertiary center [abstract]. Gastroenterology. 2022;162(Suppl. 3):S109.
Dubinsky MC, Betts KA, LaPensee K, et al. Comparative efficacy and safety of ozanimod vs adalimumab and vedolizumab in patients with moderately to severely active ulcerative colitis [abstract no. S694]. Am J Gastroenterol. 2021;116(Suppl.):S314.
Dubinsky MC, Betts KA, Eren D, et al. Comparative efficacy and safety of ozanimod and ustekinumab in patients with moderately to severely active ulcerative colitis [abstract plus poster no. Su1500]. In: DDW 2022. 2022.
Pfizer Inc. Xeljanz® (tofacitinib) tablets, for oral use: US prescribing information. 2018. https://dailymed.nlm.nih.gov. Accessed 3 Aug 2022.
AbbVie Inc. RINVOQ™ (upadacitinib) extended-dose tablets, for oral use: US prescribing information. 2019. https://dailymed.nlm.nih.gov. Accessed 3 Aug 2022.
Acknowledgments
During the peer review process the manufacturer of ozanimod was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Julia Paik is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: J. Gubatan, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA, USA; J. M. Venner, Department of Internal Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Paik, J. Ozanimod: A Review in Ulcerative Colitis. Drugs 82, 1303–1313 (2022). https://doi.org/10.1007/s40265-022-01762-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01762-8