Abstract
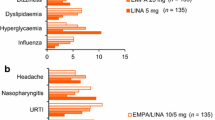
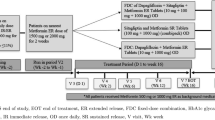
Saxagliptin/dapagliflozin fixed-dose combination tablets (Qtern®) are indicated in the EU for the improvement of glycaemic control in adults with type 2 diabetes mellitus (T2DM), either when treatment with metformin and/or a sulfonylurea plus a monocomponent of saxagliptin/dapagliflozin provides inadequate glycaemic control, or when the patient is already being treated with the free combination of saxagliptin + dapagliflozin. This narrative review summarizes pharmacological, efficacy and tolerability data relevant to the use of saxagliptin/dapagliflozin in this indication. The agents have complementary mechanisms of action, and saxagliptin/dapagliflozin fixed-dose combination tablets are bioequivalent to free combination of saxagliptin + dapagliflozin. In three phase III trials, saxagliptin + dapagliflozin + metformin was more effective at providing glycaemic control than saxagliptin + metformin or dapagliflozin + metformin in previously treated patients with T2DM and inadequate glycaemic control on metformin monotherapy or metformin plus one of the monocomponents. The combination is associated with decreased bodyweight and a low risk of hypoglycaemia. As the first dipeptidyl peptidase-4 (DPP-4) inhibitor/sodium-glucose co-transporter (SGLT2) inhibitor fixed-dose combination available in the EU for glycaemic control in patients with T2DM, saxagliptin/dapagliflozin is a useful new option in this setting.

Similar content being viewed by others
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–42.
National Institute for Health and Excellence. Type 2 diabetes in adults: management. NICE guideline ng28. 2016. http://www.nice.org.uk. Accessed 16 Jan 2017.
International Diabetes Federation. Global guidelines for type 2 diabetes. 2012. http://www.idf.org. Accessed 16 Jan 2017.
Scheen AJ. DPP-4 inhibitor plus SGLT-2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016;12(12):1407–17.
Bell DS. Combine and conquer: advantages and disadvantages of fixed-dose combination therapy. Diabetes Obes Metab. 2013;15(4):291–300.
European Medicines Agency. QTern® (saxagliptin hydrochloride/dapagliflozin propanediol monohydrate tablets): EU summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 16 Jan 2017.
Plosker GL. Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs. 2014;74(18):2191–209.
Dhillon S. Saxagliptin: a review in type 2 diabetes. Drugs. 2015;75(15):1783–96.
Yang LP. Saxagliptin: a review of its use as combination therapy in the management of type 2 diabetes mellitus in the EU. Drugs. 2012;72(2):229–48.
Hansen L, Iqbal N, Ekholm E, et al. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr Pract. 2014;20(11):1187–97.
Ekholm E, Hansen L, Iqbal N, et al. Combined treatment with saxagliptin + dapagliflozin improves beta cell function and reduces insulin levels by increased insulin clearance [abstract no. 731]. Diabetologia. 2015;58(Suppl 1):S349.
Mathieu C, Ranetti AE, Li D, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38(11):2009–17.
Matthaei S, Catrinoiu D, Celinski A, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38(11):2018–24.
Rohwedder K, Elkholm E, Cook W, et al. Dual treatment with saxagliptin + dapagliflozin provides a similar increase in urinary glucose excretion with fewer genitourinary infections compared with dapagliflozin [abstract no. 1208-P]. Diabetes. 2015;64(Suppl 1):A312.
Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376–83.
Vakkalagadda B, Vetter ML, Rana J, et al. Bioequivalence of saxagliptin/dapagliflozin fixed-dose combination tablets compared with coadministration of the individual tablets to healthy subjects. Pharmacol Res Perspect. 2015;3(6):1–12.
Vakkalagadda B, Lubin S, Reynolds L, et al. Lack of a pharmacokinetic interaction between saxagliptin and dapagliflozin in healthy subjects: a randomized crossover study. Clin Ther. 2016;38(8):1890–9.
Boulton DW, Kasichayanula S, Keung CFA, et al. Simultaneous oral therapeutic and intravenous 14C-microdoses to determine the absolute oral bioavailability of saxagliptin and dapagliflozin. Br J Clin Pharmacol. 2013;75(3):763–8.
European Medicines Agency. Qtern® assessment report, procedure no. EMEA/H/C/004057/0000. 2016. http://www.ema.europa.eu. Accessed 16 Jan 2017.
Mathieu C, Herrera Mamolejo M, Gonzalez Gonzalez JG, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(11):1134–7.
Matthaei S, Aggarwal N, Garcia-Hernandez P, et al. One-year efficacy and safety of saxagliptin add-on in patients receiving dapagliflozin and metformin. Diabetes Obes Metab. 2016;18(11):1128–33.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
Ptaszynska A, Cohen SM, Messing EM, et al. Assessing bladder cancer risk in type 2 diabetes clinical trials: the dapagliflozin drug development program as a ‘case study’. Diabetes Ther. 2015;6(3):357–75.
Warren H, Raison N, Dasgupta P. Pioglitazone and bladder cancer. BJU Int. 2016;118(1):16–7.
European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. CPMP/EWP/1080/00 Rev. 1. 2012. http://www.ema.europa.eu. Accessed 16 Jan 2017.
Tkac I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2016. doi:10.2337/dc15-1707.
Abdul-Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes? Diabetes Care. 2015;38(3):373–5.
Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J. 2016;37(19):1526–34.
Acknowledgements
During the peer review process, the manufacturer of saxagliptin/dapagliflozin was also offered an opportunity to review this article. Any changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Karly Garnock-Jones is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D. Bhatnagar, Division of Diabetes, Endocrinology and Gastroenterology, School of Medical Sciences, University of Manchester, Manchester, England; G. Dimitriadis, 2nd Department of Internal Medicine, Research Institute & Diabetes Center, Athens University Medical School, Attikon University Hospital, Haidari, Greece; J. G. Eriksson, Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland and Folkhalsan Research Center, Helsinki, Finland.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P. Saxagliptin/Dapagliflozin: A Review in Type 2 Diabetes Mellitus. Drugs 77, 319–330 (2017). https://doi.org/10.1007/s40265-017-0697-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0697-1