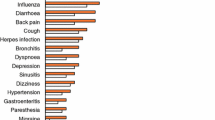
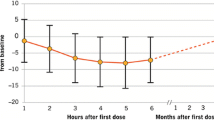
Abstract
Drug development for multiple sclerosis (MS), as with any other neurological disease, faces numerous challenges, with many drugs failing at various stages of development. The disease-modifying therapies (DMTs) first introduced for MS are only moderately effective, but given the lack of competition, they have been widely accepted in clinical practice. Although safety and efficacy continue to be the two main metrics by which drugs will be judged, the newer agents in the market also face challenges of a more comparative nature—are they more efficacious than the currently available drugs on the market? Are they safer or better tolerated? Do they offer any practical advantages over current treatments? Fingolimod represented a milestone following its approval as an oral drug for MS in 2010, offering patients a far more convenient administration route. However, association with cardiovascular complications has led to a more cautious approach in its initial prescribing, now requiring cardiac monitoring for the first 6 h as well as subsequent monitoring of blood pressure and for macular oedema. Natalizumab, amongst licensed drugs, represents the current benchmark for efficacy. The risk of progressive multifocal leukoencephalopathy during natalizumab treatment is now more quantifiable. Other monoclonal antibodies are in various phases of development. Marketing authorisation for alemtuzumab has been filed, and whilst trial data suggest that its efficacy outperforms both licensed drugs and others in development, there is a significant risk of secondary autoimmunity. Its once-yearly administration, however, seems particularly advantageous. Rituximab is unlikely to be developed further as its license will expire, but ocrelizumab, another monoclonal antibody directly targeting B cells, is currently in phase 2 development and looks promising. Daclizumab is also moderately efficacious but may struggle to establish itself given its monthly subcutaneous dosing. There are new oral drugs in development, and it is likely that BG-12 will be licensed this year. This has been licensed for psoriasis so there are good safety data in humans that may also hold true in MS; however, its three times daily dosage will probably impact on patient compliance. Laquinimod has lower efficacy than BG-12 but appears safe and could find a place as a first-line agent. Teriflunomide has just been licensed by the US FDA and may challenge the current injectable first-line therapies as it has a similar efficacy but the advantage of being taken orally. However, risk of teratogenicity may caution against its use in some women of child-bearing potential. This review will examine drugs that have been recently approved as well as those that are in late phase 2 or 3 development as treatment for relapsing MS, highlighting their mechanism of action as well as the clinical trial and safety data before discussing their potential for success in an increasingly florid and complex DMT armamentarium.


Similar content being viewed by others
References
Schwartz RS. Paul Ehrlich’s magic bullets. N Engl J Med. 2004;350(11):1079–80.
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343(13):898–904.
Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67(7):1242–9.
Jiang H, Milo R, Swoveland P, et al. Interferon beta-1b reduces interferon gamma-induced antigen-presenting capacity of human glial and B cells. J Neuroimmunol. 1995;61(1):17–25.
Genc K, Dona DL, Reder AT. Increased CD80(+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest. 1997;99(11):2664–71.
Teleshova N, Bao W, Kivisakk P, et al. Elevated CD40 ligand expressing blood T-cell levels in multiple sclerosis are reversed by interferon-beta treatment. Scand J Immunol. 2000;51(3):312–20.
Hallal-Longo DEM, Mirandola SR, Oliveira EC, et al. Diminished myelin-specific T cell activation associated with increase in CTLA4 and Fas molecules in multiple sclerosis patients treated with IFN-beta. J Interferon Cytokine Res. 2007;27(10):865–73.
Muraro PA, Leist T, Bielekova B, et al. VLA-4/CD49d downregulated on primed T lymphocytes during interferon-beta therapy in multiple sclerosis. J Neuroimmunol. 2000;111(1–2):186–94.
Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74(1):S17–24.
Duquette P, Girard M, Despault L, et al. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–61.
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–94.
Ebers GC, PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352(9139):1498–504.
Murdoch D, Lyseng-Williamson KA. Subcutaneous recombinant-interferon-beta-1a (Rebif®): a review of its use in relapsing-remitting multiple sclerosis. Drugs. 2005;65(9):1295–312.
Bloomgren G, Sperling B, Cushing K, et al. Assessment of malignancy risk in patients with multiple sclerosis treated with intramuscular interferon beta-1a: retrospective evaluation using a health insurance claims database and postmarketing surveillance data. Ther Clin Risk Manag. 2012;8:313–21.
Neuhaus O, Farina C, Wekerle H, et al. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56(6):702–8.
Dhib-Jalbut S. Glatiramer acetate (Copaxone) therapy for multiple sclerosis. Pharmacol Ther. 2003;98(2):245–55.
Kala M, Miravalle A, Vollmer T. Recent insights into the mechanism of action of glatiramer acetate. J Neuroimmunol. 2011;235(1–2):9–17.
Chen C, Liu X, Wan B, et al. Regulatory properties of copolymer I in Th17 differentiation by altering STAT3 phosphorylation. J Immunol. 2009;183(1):246–53.
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–76.
Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49(3):290–7.
Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Neurology. 1998;50(3):701–8.
Johnson KP, Brooks BB, Ford CC, et al. Results of the long-term (eight-year) prospective, open-label trial of glatiramer acetate for relapsing multiple sclerosis [poster]. Neurology. 2002;58(7):A458.
Ford C, Goodman AD, Johnson K, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16(3):342–50.
Martinelli V, Radaelli M, Straffi L, et al. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci. 2009;30(Suppl 2):S167–70.
Vollmer T, Stewart T, Baxter N. Mitoxantrone and cytotoxic drugs’ mechanisms of action. Neurology. 2010;74(1):S41–6.
Li J-M, Yang Y, Zhu P, et al. Mitoxantrone exerts both cytotoxic and immunoregulatory effects on activated microglial cells. Immunopharmacol Immunotoxicol. 2012;34(1):36–41.
Millefiorini E, Gasperini C, Pozzilli C, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing–remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244(3):153–9.
Edan G, Miller D, Clanet M, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: a randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62(2):112–8.
Hartung HP, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–25.
Pratt RG, Boehm GA, Kortepeter CM, et al. Mitoxantrone treatment of multiple sclerosis: safety considerations. Neurology. 2005;65(12):1997.
Marriott JJ, Miyasaki JM, Gronseth G, et al. Evidence report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74(18):1463–70.
Martinelli V, Cocco E, Capra R, et al. Acute myeloid leukemia in Italian patients with multiple sclerosis treated with mitoxantrone. Neurology. 2011;77(21):1887–95.
Rivera V, Weinstock-Guttman B, Beagan J, et al. Final results from the Registry to Evaluate Novantrone Effects in Worsening Multiple Sclerosis study. Mult Scler. 2009;15(9):S254–5.
Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390–401.
Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–23.
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354(9):924–33.
Iaffaldano P, D’Onghia M, Trojano M. Safety profile of Tysabri: international risk management plan. Neurol Sci. 2009;30:159–62.
Clifford DB, DeLuca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438–46.
Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol. 2011;10(8):745–58.
Multiple Sclerosis Resource Centre. MSRC. 2012. http://www.msrc.co.uk/index.cfm/fuseaction/show/pageid/3563. Accessed 1 Aug 2012.
Aktas O, Kuery P, Kieseier B, et al. Fingolimod is a potential novel therapy for multiple sclerosis. Nat Rev Neurol. 2010;6(7):373–82.
Chun J, Hartung H-P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33(2):91–101.
Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355(11):1124–40.
Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
Radue EW, O’ Connor P, Polman CH, et al. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol. 2012;69(10):1259–69.
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–15.
Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing–remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10(6):520–9.
FDA. FDA drug safety communication: revised recommendations for cardiovascular monitoring and use of multiple sclerosis drug Gilenya (fingolimod). FDA. 2012. http://www.fda.gov/Drugs/DrugSafety/ucm303192.htm. Accessed 1 Aug 2012.
EMA. European Medicines Agency questions and answers on the review of Gilenya document reference number EMA/254587/2012 EMEA/H/C/002202/A20/0008. EMA. 2012. http://www.emea.europa.eu/docs/en_GB/document_library/Medicine_QA/2012/04/WC500125689.pdf. Accessed 1 Aug 2012.
Sorensen PS. Balancing the benefits and risks of disease-modifying therapy in patients with multiple sclerosis. J Neurol Sci. 2011;311:S29–34.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Riechmann L, Clark M, Waldmann H, et al. Resha** human antibodies for therapy. Nature. 1988;332(6162):323–7.
Magdelaine-Beuzelin C, Kaas Q, Wehbi V, et al. Structure–function relationships of the variable domains of monoclonal antibodies approved for cancer treatment. Crit Rev Oncol Hematol. 2007;64(3):210–25.
Lutterotti A, Martin R. Getting specific: monoclonal antibodies in multiple sclerosis. Lancet Neurol. 2008;7(6):538–47.
Bielekova B, Becker BL. Monoclonal antibodies in MS mechanisms of action. Neurology. 2010;74(1):S31–40.
Hohlfeld R, Wekerle H. Drug insight: using monoclonal antibodies to treat multiple sclerosis. Nat Clin Pract Neurol. 2005;1(1):34–44.
**a MQ, Hale G, Lifely MR, et al. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993;293(Pt 3):633–40.
Hale G, Rebello P, Brettman LR, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–55.
Rebello P, Cwynarski K, Varughese M, et al. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3(4):261–7.
Rommer PS, Stuve O, Goertsches R, et al. Monoclonal antibodies in the therapy of multiple sclerosis: an overview. J Neurol. 2008;255(Suppl 6):28–35.
Jones JL, Coles AJ. Spotlight on alemtuzumab. Int MS J. 2009;16(3):77–81.
Gribben JG, Hallek M. Rediscovering alemtuzumab: current and emerging therapeutic roles. Br J Haematol. 2009;144(6):818–31.
Minagar A, Alexander JS, Sahraian MA, et al. Alemtuzumab and multiple sclerosis: therapeutic application. Expert Opin Biol Ther. 2010;10(3):421–9.
Rowan WC, Hale G, Tite JP, et al. Cross-linking of the CAMPATH-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol. 1995;7(1):69–77.
Masuyama J, Yoshio T, Suzuki K, et al. Characterization of the 4C8 antigen involved in transendothelial migration of CD26(hi) T cells after tight adhesion to human umbilical vein endothelial cell monolayers. J Exp Med. 1999;189(6):979–90.
Watanabe T, Masuyama J, Sohma Y, et al. CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120(3):247–59.
Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology. 2009;128(2):260–70.
Nuckel H, Frey UH, Roth A, et al. Alemtuzumab induces enhanced apoptosis in vitro in B-cells from patients with chronic lymphocytic leukemia by antibody-dependent cellular cytotoxicity. Eur J Pharmacol. 2005;514(2–3):217–24.
Stanglmaier M, Reis S, Hallek M. Rituximab and alemtuzumab induce a nonclassic, caspase-independent apoptotic pathway in B-lymphoid cell lines and in chronic lymphocytic leukemia cells. Ann Hematol. 2004;83(10):634–45.
Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253(1):98–108.
Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82(3):807–12.
Hill-Cawthorne GA, Button T, Tuohy O, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):298–304.
Thompson SAJ, Jones JL, Cox AL, et al. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30(1):99–105.
Cox AL, Thompson SA, Jones JL, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. 2005;35(11):3332–42.
Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999;46(3):296–304.
Paolillo A, Coles AJ, Molyneux PD, et al. Quantitative MRI in patients with secondary progressive MS treated with monoclonal antibody Campath 1H. Neurology. 1999;53(4):751–7.
Coles AJ, Compston DAS, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–801.
Hirst CL, Pace A, Pickersgill TP, et al. Campath 1-H treatment in patients with aggressive relapsing remitting multiple sclerosis. J Neurol. 2008;255(2):231–8.
Fox EJ, Sullivan HC, Gazda SK, et al. A single-arm, open-label study of alemtuzumab in treatment-refractory patients with multiple sclerosis. Eur J Neurol. 2012;19(2):307–11.
Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing–remitting multiple sclerosis: post hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol. 2011;10(4):338–48.
Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon beta-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology. 2012;78(14):1069–78.
Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing–remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–28.
Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–39.
Pace AA, Zajicek JP. Melanoma following treatment with alemtuzumab for multiple sclerosis. Eur J Neurol. 2009;16(4):E70–1.
Cossburn M, Pace AA, Jones J, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology. 2011;77(6):573–9.
Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain J Neurol. 2007;130:1089–104.
Kitsos DK, Tsiodras S, Stamboulis E, et al. Rituximab and multiple sclerosis. Clin Neuropharmacol. 2012;35(2):90–6.
Hartung HP. Atacicept: a new B lymphocyte-targeted therapy for multiple sclerosis. Nervenarzt. 2009;80(12):1462–72.
Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing–remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400.
Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88.
Voso MT, Pantel G, Rutella S, et al. Rituximab reduces the number of peripheral blood B-cells in vitro mainly by effector cell-mediated mechanisms. Haematologica. 2002;87(9):918–25.
Gurcan HM, Keskin DB, Stern JN, et al. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9(1):10–25.
Kessel A, Rosner I, Toubi E. Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol. 2008;34(1):74–9.
Stuve O, Leussink VI, Frohlich R, et al. Long-term B-lymphocyte depletion with rituximab in patients with relapsing–remitting multiple sclerosis. Arch Neurol. 2009;66(2):259–61.
Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease—the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007;3(2):86–95.
Piccio L, Naismith RT, Trinkaus K, et al. Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol. 2010;67(6):707–14.
Naismith RT, Piccio L, Lyons JA, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74(23):1860–7.
Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40.
Morschhauser F, Marlton P, Vitolo U, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol. 2010;21(9):1870–6.
Genovese MC, Kaine JL, Lowenstein MB, et al. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(9):2652–61.
Kausar F, Mustafa K, Sweis G, et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert Opin Biol Ther. 2009;9(7):889–95.
Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing–remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–87.
Rigby W, Tony HP, Oelke K, et al. Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum. 2012;64(2):350–9.
Stohl W, Gomez-Reino J, Olech E, et al. Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis. 2012;71(8):1289–96.
Martin R. Anti-CD25 (daclizumab) monoclonal antibody therapy in relapsing–remitting multiple sclerosis. Clin Immunol. 2012;142(1):9–14.
Mottershead M, Neuberger J. Daclizumab. Expert Opin Biol Ther. 2007;7(10):1583–96.
Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103(15):5941–6.
Wuest SC, Edwan JH, Martin JF, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17(5):604–9.
Snyder JT, Shen J, Azmi H, et al. Direct inhibition of CD40L expression can contribute to the clinical efficacy of daclizumab independently of its effects on cell division and Th1/Th2 cytokine production. Blood. 2007;109(12):5399–406.
Perry JS, Han S, Xu Q, et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci Transl Med. 2012;4(145):145ra06.
Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A. 2004;101(23):8705–8.
Rose JW, Watt HE, White AT, et al. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol. 2004;56(6):864–7.
Rose JW, Burns JB, Bjorklund J, et al. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69(8):785–9.
Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol. 2009;66(4):483–9.
Bielekova B, Richert N, Herman ML, et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology. 2011;77(21):1877–86.
Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurol. 2010;9(4):381–90.
Gold R, Giovannoni G, Selmaj K, et al. A randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of daclizumab HYP monotherapy in relapsing–remitting multiple sclerosis: primary results of the SELECT trial. Neurology. 2012;78.
Giovannoni G, Gold R, Selmaj K. Primary results of the SELECTION trial of daclizumab HYP in relapsing multiple sclerosis. In: Proceedings of the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2012 Oct 10–13; Lyon, France; 2012.
Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol. 2009;66(4):471–9.
Ohayon J, Oh U, Richert N, et al. CNS vasculitis in a patient with MS on daclizumab monotherapy. Neurology. 2013;80(5):453–7.
Schattenkirchner M. The use of leflunomide in the treatment of rheumatoid arthritis: an experimental and clinical review. Immunopharmacology. 2000;47(2–3):291–8.
Mladenovic V, Domljan Z, Rozman B, et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995;38(11):1595–603.
Strand V, Cohen S, Schiff M, et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Leflunomide Rheumatoid Arthritis Investigators Group. Arch Intern Med. 1999;159(21):2542–50.
Smolen JS, Kalden JR, Scott DL, et al. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. European Leflunomide Study Group. Lancet. 1999;353(9149):259–66.
Fox RI, Herrmann ML, Frangou CG, et al. Mechanism of action for leflunomide in rheumatoid arthritis. Clin Immunol. 1999;93(3):198–208.
Limsakun T, Menguy-Vacheron F. Pharmacokinetics of oral teriflunomide, a novel oral disease-modifying agent under investigation for the treatment of multiple sclerosis [poster]. Neurology. 2010;74(9):A415.
Limsakun T, Menguy-Vacheron F. Effects of cholestyramine on the elimination of teriflunomide in healthy male volunteers [poster]. Mult Scler J. 2010;16(8):1004.
Limsakun T, Menguy-Vacheron F. Effect of repeated oral doses of teriflunomide on a single oral dose of midazolam in healthy subjects. Mult Scler J. 2010;16(8):1004.
Claussen MC, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clin Immunol. 2012;142(1):49–56.
Greene S, Watanabe K, Braatz-Trulson J, et al. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol. 1995;50(6):861–7.
Cherwinski HM, Cohn RG, Cheung P, et al. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther. 1995;275(2):1043–9.
Ruckemann K, Fairbanks LD, Carrey EA, et al. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem. 1998;273(34):21682–91.
Siemasko KF, Chong AS, Williams JW, et al. Regulation of B cell function by the immunosuppressive agent leflunomide. Transplantation. 1996;61(4):635–42.
Korn T, Magnus T, Toyka K, et al. Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide—mechanisms independent of pyrimidine depletion. J Leukoc Biol. 2004;76(5):950–60.
Siemasko K, Chong AS, Jack HM, et al. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol. 1998;160(4):1581–8.
Xu X, Williams JW, Bremer EG, et al. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J Biol Chem. 1995;270(21):12398–403.
Miljkovic D, Samardzic T, Mostarica Stojkovic M, et al. Leflunomide inhibits activation of inducible nitric oxide synthase in rat astrocytes. Brain Res. 2001;889(1–2):331–8.
Merrill JE, Hanak S, Pu SF, et al. Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J Neurol. 2009;256(1):89–103.
O’Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66(6):894–900.
Freedman MS, Wolinsky JS, Wamil B, et al. Teriflunomide added to interferon-beta in relapsing multiple sclerosis: a randomized phase II trial. Neurology. 2012;78(23):1877–85.
Confavreux C, Li DK, Freedman MS, et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler. 2012;18(9):1278–89.
O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–303.
Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1435–9.
Warnatz K, Peter HH, Schumacher M, et al. Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Ann Rheum Dis. 2003;62(1):50–7.
Rahmlow M, Shuster EA, Dominik J, et al. Leflunomide-associated progressive multifocal leukoencephalopathy. Arch Neurol. 2008;65(11):1538–9.
Brent RL. Teratogen update: reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology. 2001;63(2):106–12.
Mrowietz U, Christophers E, Altmeyer P. Treatment of psoriasis with fumaric acid esters: results of a prospective multicentre study. Br J Dermatol. 1998;138(3):456–60.
Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br J Dermatol. 1999;141(3):424–9.
Litjens NH, Burggraaf J, van Strijen E, et al. Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol. 2004;58(4):429–32.
Lee DH, Linker RA, Gold R. Spotlight on fumarates. Int MS J. 2008;15(1):12–8.
Schilling S, Goelz S, Linker R, et al. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145(1):101–7.
de Jong R, Bezemer AC, Zomerdijk TP, et al. Selective stimulation of T helper 2 cytokine responses by the anti-psoriasis agent monomethylfumarate. Eur J Immunol. 1996;26(9):2067–74.
Asadullah K, Schmid H, Friedrich M, et al. Influence of monomethylfumarate on monocytic cytokine formation—explanation for adverse and therapeutic effects in psoriasis? Arch Dermatol Res. 1997;289(11):623–30.
Litjens NH, Rademaker M, Ravensbergen B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004;34(2):565–75.
Loewe R, Holnthoner W, Groger M, et al. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. J Immunol. 2002;168(9):4781–7.
Wierinckx A, Breve J, Mercier D, et al. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol. 2005;166(1–2):132–43.
Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain J Neurol. 2011;134(Pt 3):678–92.
Treumer F, Zhu K, Glaser R, et al. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121(6):1383–8.
Vandermeeren M, Janssens S, Borgers M, et al. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun. 1997;234(1):19–23.
Schimrigk S, Brune N, Hellwig K, et al. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. 2006;13(6):604–10.
Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372(9648):1463–72.
Kappos L, Gold R, Miller DH, et al. Effect of BG-12 on contrast-enhanced lesions in patients with relapsing–remitting multiple sclerosis: subgroup analyses from the phase 2b study. Mult Scler. 2012;18(3):314–21.
Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098–107.
Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–97.
Ogilvie S, Lewis Jones S, Dawe R, et al. Proteinuria with fumaric acid ester treatment for psoriasis. Clin Exp Dermatol. 2011;36(6):632–4.
Fernandez O. Oral laquinimod treatment in multiple sclerosis. Neurologia. 2011;26(2):111–7.
Noseworthy JH, Wolinsky JS, Lublin FD, et al. Linomide in relapsing and secondary progressive MS: part I: trial design and clinical results. North American Linomide Investigators. Neurology. 2000;54(9):1726–33.
Bruck W, Wegner C. Insight into the mechanism of laquinimod action. J Neurol Sci. 2011;306(1–2):173–9.
Tuvesson H, Hallin I, Persson R, et al. Cytochrome P450 3A4 is the major enzyme responsible for the metabolism of laquinimod, a novel immunomodulator. Drug Metab Dispos. 2005;33(6):866–72.
Yang JS, Xu LY, **ao BG, et al. Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-beta in Lewis rats. J Neuroimmunol. 2004;156(1–2):3–9.
Brunmark C, Runstrom A, Ohlsson L, et al. The new orally active immunoregulator laquinimod (ABR-215062) effectively inhibits development and relapses of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;130(1–2):163–72.
Runstrom A, Leanderson T, Ohlsson L, et al. Inhibition of the development of chronic experimental autoimmune encephalomyelitis by laquinimod (ABR-215062) in IFN-beta k.o. and wild type mice. J Neuroimmunol. 2006;173(1–2):69–78.
Wegner C, Stadelmann C, Pfortner R, et al. Laquinimod interferes with migratory capacity of T cells and reduces IL-17 levels, inflammatory demyelination and acute axonal damage in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;227(1–2):133–43.
Zou LP, Abbas N, Volkmann I, et al. Suppression of experimental autoimmune neuritis by ABR-215062 is associated with altered Th1/Th2 balance and inhibited migration of inflammatory cells into the peripheral nerve tissue. Neuropharmacology. 2002;42(5):731–9.
Gurevich M, Gritzman T, Orbach R, et al. Laquinimod suppress antigen presentation in relapsing–remitting multiple sclerosis: in vitro high-throughput gene expression study. J Neuroimmunol. 2010;221(1–2):87–94.
Thone J, Ellrichmann G, Seubert S, et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. Am J Pathol. 2012;180(1):267–74.
Polman C, Barkhof F, Sandberg-Wollheim M, et al. Treatment with laquinimod reduces development of active MRI lesions in relapsing MS. Neurology. 2005;64(6):987–91.
Comi G, Pulizzi A, Rovaris M, et al. Effect of laquinimod on MRI-monitored disease activity in patients with relapsing–remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;371(9630):2085–92.
Comi G, Jeffery D, Kappos L, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med. 2012;366(11):1000–9.
Vollmer T. A placebo-controlled and active comparator phase III trial (BRAVO) for relapsing remitting multiple sclerosis. Abstract 148. ECTRIMS/ACTRIMS; 2011.
Fox E, Wynn D, Cohan S, et al. A randomized clinical trial of autologous T-cell therapy in multiple sclerosis: subset analysis and implications for trial design. Mult Scler. 2012;18(6):843–52.
Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing–remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74(13):1033–40.
Gold SM, Voskuhl RR. Estrogen treatment in multiple sclerosis. J Neurol Sci. 2009;286(1–2):99–103.
Sorensen P, Drulovic J, Havrdova E, et al. Magnetic resonance imaging (MRI) efficacy of ofatumumab in relapsing-remitting multiple sclerosis—results of a phase II study. Neurology. 2011;76(9):A85.
Miller DH, Weber T, Grove R, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11(2):131–9.
Vollmer T, Selmaj K, Bar-Or A, et al. A double-blind, placebo-controlled, phase 2, 26-week DreaMS trial of a selective S1P receptor agonist ONO-4641 in patients with relapsing–remitting multiple sclerosis. Neurology. 2012;79(11):E90.
Komiya T, Sato K, Shioya H, et al. Efficacy and immunomodulatory actions of ONO-4641, a novel selective agonist for sphingosine 1-phosphate receptors 1 and 5, in preclinical models of multiple sclerosis. Clin Exp Immunol. 2013;171(1):54–62.
Li D. Siponimod (BAF312) treatment leads to early MRI benefits in relapsing-remitting multiple sclerosis patients: results from a phase 2 study. Abstract P494. In: Proceedings of the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2012 Oct 10–13; Lyon, France; 2012.
Havrdova E. Abstract 168. In: Proceedings of the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2012 Oct 10–13; Lyon, France; 2012.
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Conflicts of interest
Dr Ali has received a travel grant from Merck Serono and from Novartis; these are not relevant to this manuscript. Dr Nicholas has received a clinical grant from Biogen, consultancy fees for participation in advisory boards and lecture fees from Biogen, Novartis and Merck Serono. He has received travel grants from Biogen, Novartis and Teva Pharmaceuticals. Dr Nicholas is grateful for support from the NIHR Biomedical Research Centre. These disclosures are not considered relevant to this manuscript. Dr Muraro has received travel grants and speaker honoraria from Bayer Healthcare, Bayer Pharma and Merck Serono. He is supported by the Medical Research Council (ref. no. G0800679), the UK MS Society (ref. nos. 938/10 and 944/10) and the Italian MS Society (ref. no. 2010/R/24). These disclosures are not considered relevant to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, R., Nicholas, R.S.J. & Muraro, P.A. Drugs in Development for Relapsing Multiple Sclerosis. Drugs 73, 625–650 (2013). https://doi.org/10.1007/s40265-013-0030-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0030-6