Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare opportunistic infection of the central nervous system caused by the John Cunningham virus (JCV) that has been associated with therapeutic immunosuppression in patients with multiple sclerosis (MS). So far, more than 600 cases of PML have been reported in association with natalizumab administration. There have also been confirmed cases of PML in individuals who received fingolimod and dimethyl fumarate without previous natalizumab treatment. The new licensed disease-modifying therapies for MS carry the risk of immunosuppressant and so of JCV reactivation. Various factors have been identified with increased risk of develo** PML, including a positive JCV serology, natalizumab administration for >2 years, and prior use of immunosuppressive agents. Clinicians can employ such tools for patients’ risk stratification, but the incidence of PML among patients receiving natalizumab therapy has not changed. In this review we outline the current state of understanding of PML pathogenesis and patients’ risk stratification. The landscape of MS is dramatically changing and knowledge of the side effects of the licensed therapies is imperative to enable optimal decision making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Progressive multifocal leukoencephalopathy (PML) is a rare entity, but treatment of multiple sclerosis with immunosuppressive and immunomodulant agents exposes patients to the risk of John Cunningham virus (JCV) reactivation. |
We need new and stronger prediction tools for stratifying patients into categories of PML risk. |
We need more accurate epidemiological studies on PML in multiple sclerosis. |
1 Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS). It often affects young people, with a considerable economic burden and impact on quality of life [1]. The course of the disease varies greatly between individuals: some patients accumulate minimal disability over their lives in the case of the relapsing-remitting form (RRMS), whilst others experience much more disability, either after a variable period of RR phase (i.e., secondary progressive MS [SPMS]) or immediately from the beginning of the disease (i.e., primary progressive MS [PPMS]) [2]. Current treatment options for MS mainly target inflammatory processes, and over recent years the treatment armamentarium of MS has increased tremendously [3]. However, we still face difficult decisions about when to initiate, escalate, or withdraw therapies. Such decisions require proper assessment of relative risks, costs, and benefits of recently discovered and emerging therapies [4, 5]. Moreover, treating patients with MS who suffer from primary or secondary disability accrual continues to be frustrating and ineffective [6].
Progressive multifocal leukoencephalopathy (PML) is a rare CNS infection that is a potentially fatal complication associated with the pharmacologic management of MS [7]. It is generally related to the immunosuppressant state resulting from the use of different disease-modifying therapies such as natalizumab, an immunomodulant drug [7, 8].
2 Methodology
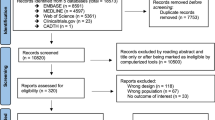
Experimental and clinical studies were chosen for review by searching electronic databases (e.g., PubMed, Science Citation Index Expanded, Conference Proceedings Citation Index–Science, and clinicaltrials.gov) and bibliographies/citations of previously published reviews. We updated this search on 30 July 2016. The searching was restricted to studies in English. Records were screened and the methodological quality of the included studies were assessed independently by two reviewers (ED, AZ) under the supervision of another reviewer (HT) using the principles recommended in the levels of evidence espoused by the American Academy of Neurology.
3 Progressive Multifocal Leukoencephalopathy (PML) Phenomenon
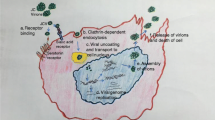
PML is a severe, potentially fatal, CNS infection. It is caused by reactivation of the John Cunningham virus (JCV). While JCV is present in 50–70 % of the general population, PML occurs in about 0.2/100,000 of the general population [9]. The risk of PML is increased in patients with chronic inflammatory diseases or autoimmune diseases, even in the absence of treatments that induce an immunosuppressive status [9] (see Fig. 1).
Progressive multifocal leukoencephalopathy (PML) is a rare and usually fatal viral disease. The prevalence is extremely wide among various systemic diseases. AIDS acquired immunodeficiency syndrome, HAART highly active antiretroviral therapy, HIV human immunodeficiency virus, ISA immunosuppressive agents, JCV John Cunningham virus, MS multiple sclerosis
The prevalence of PML is not increased in patients with MS; on the contrary, it is increased in other systemic diseases, and the main systemic diseases associated with PML are the vasculitides, most notably Wegener’s granulomatosis and poly- or dermatomyositis (with an overall prevalence of 2/100,000) [10]. A few cases have been reported in patients with scleroderma or Sjogren’s syndrome with CD4+ counts <300/mm3 [10], whilst the prevalence is increased in systemic lupus erythematosus (SLE), up to 4/100,000 [10]. A majority (60 %) of patients with SLE who experienced PML had received immunosuppressants, chiefly cyclophosphamide and azathioprine [10]. One SLE-PML case has been reported involving previous treatment with leflunomide and two cases with rituximab [10]. In 40 % of SLE patients with PML, a modest lymphopenia was seen, suggesting that SLE independently increases the risk of PML. The mechanism underlying this effect is still unknown [10, 11]. PML is also associated with human immunodeficiency virus 1 and 2 (HIV-1 and HIV-2) [12, 13]. It is currently one of the AIDS-defining illnesses in HIV-infected patients. HIV-associated PML typically follows the immune recovery after the initiation of highly active antiretroviral therapy (HAART) and it usually develops when the CD4 cell count is very low (<200/µL). Rarely, the PML in HIV-infected patients has been reported in the setting of better immunological function (CD4 counts >500/µL) [14–20].
PML is also associated with lymphoproliferative disorders and patients often show first neurological symptoms after complete remission. Then, the exordium of PML during the remission phase of lymphoproliferative diseases could be related to the high dose of therapy or to the hematopoietic stem cell treatment [21]. Additional application of rituximab also seems to delay the onset of PML, probably due to a delay in the recovery of B lymphocytes, but possibly also by prolongated T cells reconstitution, demonstrated by the decreased levels of both T and B cells [22]. In detail, levels of both T and B cells were decreased indicating a failure in recovery of these cells. In conclusion, opportunistic JCV infection causing PML should be suspected, even when the onset of neurological symptoms occur several months or years after remission of the lymphoproliferative disease [23].
Any hypothesis of PML pathogenesis must account for a number of factors and their description is beyond the scope of this review. Briefly, it is considered that immune system cells participate in the pathogenesis of infection leading to PML [24]. The role of B cells in JCV infection and PML is likely more complex than initially thought. Indeed, on the one hand, B cells represent a potential reservoir for JCV and may disseminate the virus to the CNS while, on the other hand, they likely play a regulatory role in the immune response that controls JCV infection [24]. The association between rituximab and PML suggests that B cells may help to control JCV infection through functions other than antibody production. B cells secreting T helper 1 (Th1)-type cytokines such as interferon (IFN)-γ probably enhance the Th1 response and thereby help to establish effective CD8 T cell activity against JCV [24]. In addition, regulatory T cell (Treg) responses are enhanced in B cell-depleted human and mouse models. These Treg responses could be induced by post-rituximab repopulating B cells, which could be predominantly interleukin-10 (IL-10)-producing cells. A better understanding of the complex relationship between JCV and B cells may have significant implications for the prevention and treatment of PML [24].
Furthermore, B lymphocytes infected with JCV were found in bone marrow specimens and after investigation of cerebral biopsy samples it was shown that these cells could be implicated as carriers of the virus from the periphery to the brain [25].
Investigation of peripheral blood mononuclear cells and bone marrow in HIV-1-infected and non-infected patients showed the presence of JCV DNA in bone marrow samples in both PML and non-PML patients, highlighting the importance of bone marrow as a viral reservoir [26].
Studies conducted on natalizumab-treated patients with MS showed higher prevalence of viral DNA in CD34+ cells than CD19+ cells [27]. The direct observation of intracellular viremia in CD34+ and CD19+ cells suggests that these cells can harbor JCV [27]. One of the unique effects of natalizumab is its forced migration of CD34+ cells from the bone marrow to the peripheral circulation [27]. Since JCV may remain persistent in these cells, it is not surprising that latently infected CD34+ cells find their way into the blood and might participate in PML pathogenesis [27].
In another pathogenetic model, JCV should replicate in the setting of decreased immune surveillance related to medications, although there is strong evidence for an increased number of JCV prototypes in immunosuppressed individuals without PML [28]. More often investigated is the correlation between the duration of immunosuppression and PML incidence [28–30].
The hallmark of PML is demyelination of axons caused by the lysis of infected oligodentrocytes by JCV. Clinical presentation depends on the extent of demyelination and brain structures involved.
As stated, there are other therapies and underlying diseases that are associated with the occurrence of PML, but in these patients, the incidence of PML is several orders of magnitude less than with either AIDS patients or MS patients treated with natalizumab. We need to gain insight into the mechanism that ties natalizumab with a high risk of develo** PML [31].
The presence of anti-JCV antibodies in serum or plasma is considered a risk factor for PML development, and the detection of anti-JCV antibodies using a 2-step enzyme-linked immunosorbent assay (ELISA) has been proven to reliably stratify the risk of PML [32]. The reported prevalence of anti-JCV antibodies using the 2-step ELISA ranges from approximately 50 to 70 % in natalizumab-treated MS patients (see later). However, risk stratification has been demonstrated for natalizumab only [32]. It is unknown whether a JCV antibody index would be predictive of PML in other populations or whether duration of treatment or prior immunosuppression has any effect on the risk of PML with fingolimod, dimethyl fumarate, or other agents. The mortality rate is dependent on many factors, but perhaps most important is the underlying nature of the abnormality that has predisposed a patient to PML and whether it is reversible or not.
The incidence of PML in MS patients is 1/250 overall and 1/70 in the highest risk group, and in HIV-infected patients it has an incidence of 1/100 [14]. Therefore, the definition of PML as a rare event has to be revised; because it is difficult to know the incidence of a disease until it is not reportable, it is often under-diagnosed. PML has to be recognized as part of a differential diagnosis and is a substantial neurological complication in many underlying diseases.
No effective anti-viral treatment for established PML is currently available. Although virostatic treatment with mefloquine or by mirtazapine has been proposed, they are not of any value in PML [7]. Immune reconstitution is the only option to halt the disease when the virus has invaded the brain. Unfortunately, in some cases this immune reconstitution leads to an excessively overreacting immune response referred to as immune reconstitution inflammatory syndrome (IRIS) [33, 34].
Whereas demyelination of axons in PML is caused by the lysis of infected oligodentrocytes by JCV, in PML-IRIS this demyelination can be initiated or further enhanced by excessive destruction of brain tissue by the host’s immune system. For that reason, PML-IRIS is usually treated with high doses of corticosteroids [17] and recently with maraviroc (a CCR5 antagonist) [34], treatments that would be counter-intuitive in PML and may result in clinical conditions of high morbidity and mortality [35].
In general, PML has a mortality rate of 30–50 % in the first few months following diagnosis, depending on the severity of the underlying disease and received treatment [35]. With respect to MS, the data of PML mortality are obtained from patient information by the treating physicians as well as from Biogen Idec’s registry (the company with full ownership of natalizumab marketing for MS); the data showed about 80 % survival, but with a great difference between the US (62 %) and Europe (93 %) [36, 37]. Of the survivors, it is estimated that 30 % are left with severe neurologic impairment, approximately 50 % with moderate impairment, and 15 % with mild impairment [35]. Recent comparisons of survivors with fatal cases revealed the following differences: median age (43 in survivors, 53 in fatalities), median time from symptom onset to PML diagnosis (27 days in survivors, 41 days in fatalities), and median JCV loads in cerebrospinal fluid (CSF) at diagnosis (132,000 copies/mL in survivors, 287,000 copies/mL in fatalities) [35].
It is important to note that the mortality rate of PML has varied greatly in recent years due to the detailed monitoring programs. However, in the US the mortality rate is higher than in Europe and it could be due to the different approach to the ‘MS reality’ [36, 37]. The key to obtaining a clear picture of PML phenomenon is to guarantee high surveillance levels. Other prognostic factors include number of gadolinium (gd +) enhancement lesions on magnetic resonance imaging (MRI), and the exposure to previous immunosuppressant therapy [37]. Seroprevalence studies have demonstrated conflicting results regarding the distribution of JCV antibodies. Data indicate that there are differences in both the geographic and age distributions of the antibody, suggesting that seroprevalence increases with age. Therefore, longitudinal data on JCV seroconversion rates over time are needed to assess the probability for anti-JCV antibody-negative patients to change antibody status from negative to positive over time [38, 39]. Furthermore, important prognostic factors are the characteristics of MRI lesions, in particular their localization and if they are unilobar or multilobar [40]. Unilobar lesions are usually found in asymptomatic patients, whereas widespread lesions were typical of symptomatic patients. In both type of patients, lesions more frequently involve the frontal lobe [40]. Furthermore, the results reported from Cambridge University showed that PML patients who were asymptomatic at diagnosis had better survival and less functional disability than those who were symptomatic at diagnosis [40]. More data are needed to improve these elements.
4 Multiple Sclerosis (MS) Medications Associated with PML
Several medications have been prescribed for PML in MS patients, including natalizumab, fingolimod, and dimethyl fumarate. Alemtuzumab and rituximab have been associated with cases of PML in other autoimmune diseases (see Table 1). Recently, the case of an MS patient with an inherent immune disorder who developed PML during monotherapy with IFNβ1a was reported.
4.1 Natalizumab
Natalizumab treatment appears to increase the risk of develo** PML. Natalizumab was withdrawn from market in early 2005 after two patients with MS developed PML, causing death in one [41]. PML was also reported in a patient treated with natalizumab for Crohn’s disease [42].
A relationship between natalizumab therapy and the onset of PML was supported by the detection of a new and rising JC viral load in serum that preceded the onset of neurologic symptoms [43]. Natalizumab was reintroduced to the US and European markets in mid-2006 for use only as monotherapy to treat RRMS. Thereafter, additional cases of PML associated with natalizumab began to emerge. Although cases of PML are seen in patients with MS after receiving as few as seven monthly natalizumab doses, the highest incidence of PML, 1/75 or higher, occurs after 24 monthly doses [44].
The pathogenesis of PML during natalizumab treatment is still unclear [45]. As we have said, the current prevailing hypothesis is that the modulation of immune cell trafficking into the CNS by natalizumab increases the likelihood of PML development [45].
4.1.1 Monitoring PML Risk in MS Patients Treated with Natalizumab
So far, the described risk factors for natalizumab-associated PML are previous infection with JCV as suggested by baseline seropositivity for anti-JCV antibodies, level of JCV-antibodies titer, prior immunosuppressant treatment, and duration of natalizumab exposure [46].
Plavina et al. [31] examined the association between anti-JCV antibody index (anti-JCV antibody level as measured using the STRATIFY JCV assay) and PML risk in anti-JCV–positive patients enrolled in natalizumab clinical trials and post marketing studies. Anti-JCV antibody index data were available from 71 natalizumab-treated PML patients at least 6 months prior to PML diagnosis and from 2522 non-PML anti-JCV antibody-positive patients. In cross-sectional analyses, anti-JCV antibody index was not associated with duration of natalizumab treatment (≤24 versus >24 infusions) or prior immunosuppressant use but was significantly associated with PML risk. A different relationship was observed between anti-JCV antibody index and PML by prior immunosuppressant use. Then, the association between anti-JCV antibody index and PML risk was assessed using all available longitudinal data in anti-JCV–positive patients without prior immunosuppressant use, and estimated odds ratios across a range of thresholds of interest varied from 7 to 23 for the occurrence of PML at higher versus lower index [31].
The risk of PML in natalizumab-treated anti-JCV antibody-negative patients with MS is ≤0.1/1000 [46]. Taken together, JCV serology is a sensitive biomarker for PML risk, but it is very dynamic.
Furthermore, it is important to distinguish between seroconversion (a JCV− patient converting to JCV+) and an increase in seroprevalence (the percentage of JCV+ patients within a cohort).
It was demonstrated that not every patient with MS is susceptible to JCV seroconversion by treatment, but natalizumab might facilitate it in patients who are susceptible. There has recently been an extensive study of 7724 patients and their JCV serostatus in a group of control patients [47]. The authors clearly show that when adjusted for age, sex, and country of origin, the duration of MS treatment has no influence on JCV seroprevalence, leaving treatment with natalizumab as the only factor in our study, as sex and country of origin do not change in longitudinal cohorts [47]. Because as yet there are no studies on the influence of other treatments on JCV index values, and despite a very recent study also supporting this hypothesis, we cannot be certain that it was the treatment with natalizumab that led to the rising index values in our study [47]. However, because there were no correlations with age in JCV+ patients and these patients have certainly been treated for longer with disease-modifying drugs, it can be speculated that it is specifically the treatment with natalizumab that induces rising JCV index values (and, therefore, anti-JCV titers) [47]. As long as these biological backgrounds are not fully elucidated, it seems prudent to include the theory of (re)infection with JCV as a source for seroconversion. However, since there are patients who shed the virus in their urine without being antibody seropositive, it seems unlikely that the process leading to seropositivity is solely linked to (re)exposure to JCV [45].
JCV patients should reassess their status regularly and should check their JCV index values until they have reached the highest risk category, after which JCV serology loses some of its usefulness [48]. The fact that treatment with natalizumab is associated with a very high rate of seroconversion and rising index values does not diminish its clinical efficacy, but calls for more elaborate strategies for PML risk stratification according to current scientific developments, also regarding patients with prior use of immunosuppressants, where the JCV index is not helpful [48].
Recently, new biomarkers—lymphocyte cell adhesion molecule, L-selectin, and the presence of lipid-specific immunoglobulin M oligoclonal bands in cerebrospinal fluid—for stratifying the PML risk in natalizumab-treated MS patients were proposed [49, 50]. The percentage of L-selectin-expressing CD4+ T cells was significantly lower in patients treated long-term with natalizumab (40.2 %) when compared with patients not receiving natalizumab treatment (47.2 %; p = 0.016) or with healthy controls (61.0 %; p < 0.0001) [49, 50]. Moreover, an unusually low percentage (ninefold lower; 4.6 %) was highly correlated with the risk of develo** PML in the patient group with available pre-PML samples when compared with non-PML natalizumab-treated patients (p ≤ 0.0001) [49].
IgM bands in the cerebrospinal fluid are a recognized marker of highly inflammatory MS. Twenty-four MS patients who developed PML during natalizumab treatment and another 343 who did not have this opportunistic infection during natalizumab treatment were recruited in a multicenter study [50]. IgM bands were independently associated with decreased PML risk (OR 45.9, 95 % CI 5.9–339.3). Higher risk was observed in patients positive for anti-JCV antibodies and negative for IgM bands (OR 59.71, 95 % CI 13.6–262.2). These data suggested that higher inflammation activity (evidenced by the presence of IgM bands) may be associated with lower PML risk in MS patients treated with natalizumab [50]. However, the value of L-selectin as a risk marker remains controversial and recent data by Biogen investigators suggests that it is of no value. Both L-selectin and CSF IgM bands need to be swathed in caution.
Although there are no pathognomonic findings that differentiate PML from MS, a brain MRI scan that includes fluid-attenuated inversion recovery (FLAIR) and T1- and T2-weighted sequences, with and without gd + lesions, should be performed to assess patients with neurological changes suggestive of PML [51]. Comparison with a baseline scan may assist with interpretation of the findings on the new MRI.
Different programs have been studied for the evaluation of PML risk. One such program is TOUCH™, designed to assess the risk of PML associated with natalizumab, which aims to minimize the risk of PML, the mortality rate, and disability and to promote informed risk–benefit decisions regarding natalizumab use [52]. The risks of the treatment are addressed through the distribution program, along with education of prescribers, pharmacists, infusion center staff, and patients about potential PML infection [52].
4.2 Fingolimod
Fingolimod, a sphingosine 1-phosphate receptor modulator, is the first oral treatment approved for the treatment of RRMS [53]. The rate of PML under fingolimod therapy not attributed to previous natalizumab treatment is estimated to be approximately 1/26,000 patients overall (based on data cut-off as of 31 August 2016) [54]. Total exposure to fingolimod was 154,000 MS patients with a cumulative exposure of approximately 343,000 patient-years [54, 55]. Estimated number of patients treated for ≥2 years in post-marketing was approximately 62,000 [54, 55].
Regulatory approval for once-daily dosing of fingolimod 0.5 mg as disease-modifying therapy for RRMS was first granted in September 2010. It was approved as first-line therapy without restriction in the US and Switzerland [56, 57]. Due to safety concerns described above, other agencies (Health Canada, the European Medicines Agency) approved fingolimod as second-line therapy for patients who failed IFNβ therapy or who have a rapidly progressive course [58]. Fingolimod reduces the activity of the immune system, in particular of T cells. For this reason, patients treated with this medication may be at higher risk for infections and diseases, including PML and certain cancers [59]. Important elements in all cases were the absence of prior history of sustained grade 4 lymphopenia, all patients had exposure to fingolimod >2 years (30–54 months), the age ranged from 49 to 63 years, and none of the patients died.
The first case was a 49-year-old who developed probable PML after taking fingolimod for approximately 4 years. The patient had a 5-year history of MS and had previously been treated with IFNβ1a for 10 months in addition to short-term corticosteroids before and during fingolimod treatment [60]. The second case was a 54-year-old who developed PML after taking fingolimod for approximately 2.5 years. The patient had a 13- to 14-year history of MS and had previously been treated with IFNβ1b for approximately 11 years, as well as with mesalazine for ulcerative colitis for the last 4 years [60]. Information describing these two cases was added to the Warnings and Precautions and Patient Counseling Information sections of the drug label, as well as to the Patient Medication Guide [60].
An August 17, 2015 notice on the company’s Gilenya Information Center webpage announced a third case of PML. Again, the patient did not have prior exposure to natalizumab treatment. The patient had a history of colorectal cancer treated with chemotherapy and radiation treatment [61].
In August 2013, the US Food and Drug Administration reported that a patient developed PML after taking fingolimod, but PML could not be conclusively linked to fingolimod in this case because the patient had previously been treated with natalizumab, and during fingolimod treatment had received multiple courses of intravenous corticosteroids [61]. In April 2012, Novartis reported a case of PML in a patient receiving fingolimod, but this patient had also previously been treated for >3 years with natalizumab before switching to fingolimod [54].
4.3 Dimethyl Fumarate
Dimethyl fumarate is the methyl ester of fumaric acid [62]. The drug activates nuclear factor pathways which are involved in the physiologic response to oxidative stress [62]. The precise mechanism of action is not clearly characterized but it is thought that the drug exerts neuroprotective effects in patients with MS with the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcriptional pathway [63]. Other studies have proposed additional immunomodulatory actions for dimethyl fumarate mediated through nitric oxide, interleukins, tumor necrosis factor-alpha (TNFα), or other cytokines [64–68]. The medication has been used for many years in the treatment of dermatologic diseases, including psoriasis. The first cases of PML in patients using dimethylfumarate occurred in individuals with this diagnosis [69].
There are some details about these cases which deserve attention. One patient was not noted to have had prolonged lymphopenia; and two of the patients in question had additional risk factors that may have made them susceptible to PML. In detail, one patient had sarcoidosis that had been treated with methotrexate and corticosteroids, and in the second patient, a previous exposure to the monoclonal antibody efalizumab for a not well recognized diagnosis of cancer had been recorded [70, 71].
In patients receiving dimethyl fumarate specifically for MS, four cases of PML have been identified so far. The first patient was a 54-year-old female who died in October 2014 with diagnoses of PML and aspiration pneumonia [68]. She received dimethyl fumarate 240 mg three times daily for approximately 4.5 years, and for more than 3 years she had persistent lymphopenia (290–580 cells/mm3). In August of 2014 she began to exhibit severe gait disorder and speech disturbance. A subsequent evaluation using an MRI and CSF resulted in the diagnosis of PML [72]. The patient had not undergone treatment with immunosuppressants at any time before or since her diagnosis [72]. Another two cases showed similar clinical and laboratory history. In particular, they had very low lymphocyte counts—below 500 cells/mm3 (grade 3 lymphopenia). On October 2015, the fourth case was described, and it was of particular interest. Here, PML occurred with a grade 2 lymphopenia, which is much more common, occurring in about 10–20 % of MS patients taking this drug [42]. It is also important to note that in some cases there was a hiatus in the follow-up laboratory studies, although during the treatment it was not observed [73]. Therefore, the patients might still develop a JCV infection even if they have a normal lymphocyte count and previously tested negative for anti-JCV antibodies.
A new recommendation to reduce the risk of PML in dimethyl fumarate patients has been elaborated in Europe: if PML is suspected in any patient, stop dimethyl fumarate immediately and investigate appropriately; for example, an MRI scan, an ultrasensitive polymerase chain reaction (PCR) assay for JCV DNA, and monitor full blood count every 3 months. Consider interrupting dimethyl fumarate if lymphocyte counts fall below 0.5 × 109/L for more than 6 months. If treatment is stopped, monitor lymphocyte counts until they return to normal [74].
The new advice when treating patients with severe prolonged lymphopenia in which dimethyl fumarate treatment is continued is to consider further MRI imaging as part of increased vigilance for PML, in accordance with national and local recommendations, and to counsel patients again on the risk of PML [74].
4.4 Alemtuzumab
Alemtuzumab is a monoclonal antibody that targets a cell surface glycoprotein (CD52) that is expressed on T and B cells during differentiation. The drug’s activity targets these cells for lysis [75]. To date, no cases of PML have been identified in conjunction with alemtuzumab therapy for MS except for a single carry-over case treated before with natalizumab. However, patients using the drug for other indications have been diagnosed with PML. A review of the World Health Organization Collaborating Center (UMC’s VigiBase) revealed 14 reported cases of PML in alemtuzumab users and the details of about five of these cases have been published [75]. Four cases of PML occurred in patients diagnosed with chronic lymphocytic leukemia [76]. The other case occurred in a lung transplant patient who was receiving prednisone, azathioprine, and tacrolimus. Alemtuzumab was administered as part of a therapeutic regimen for allograft rejection [76]. The onset of PML with alemtuzumab exposure seems to occur quite rapidly, as the mean latency to diagnosis in the 14 reported patients was approximately 1 month from initial treatment [76].
4.5 Rituximab
Rituximab is a chimeric monoclonal antibody that binds to the transmembrane phosphoprotein CD20, a receptor that is highly B-cell specific and expressed on pre- and inactive mature B cells resulting in their being targeted for destruction. Although rituximab is not approved for the treatment of MS, it is used off label for this purpose in some patients [77]. There are no reported cases of PML occurring in an MS patient as a result of rituximab therapy. Current available data indicates that 52 patients with lymphoproliferative disorders, two with SLE, one with rheumatoid arthritis, and one each with autoimmune pancytopenia and autoimmune thrombocytopenia were identified with rituximab-related PML [78]. Data from Genentech indicate that approximately 2 million doses have been given to 1 million patients, among whom 157 cases of PML have been observed (137 with lymphoproliferative disorders, 6 with RA, 8 with SLE, and 6 with AIDS). There have been no cases reported with its use in MS or other neurological disorders [79].
One patient with PML was previously treated with natalizumab [78]. The patient was a 42-year-old female diagnosed with MS in 2009, who was given natalizumab as fìrst-line therapy due to aggressive disease presentation. In late 2011, she became JCV-antibody positive, and continued on natalizumab. Approximately 1 year later, she developed double vision. MRI changes were interpreted as an MS exacerbation, and she was switched to rituximab therapy due to a presumed failure with natalizumab. After one dose of rituximab 1000 mg, her B-cell levels dropped and remained undetectable for 15 months. Roughly 4 months after administration, the patient’s condition began to rapidly deteriorate, and the lesions on MRI progressed. At this time, a diagnosis of PML was considered; however, CSF was negative for JCV DNA on two separate occasions. The patient worsened and was hospitalized. A subsequent CSF evaluation was positive for JCV, and the diagnosis was confirmed. Over time, the patient recovered [78].
It has been hypothesized that the effect of rituximab on B cells (their depletion) causing an environment of decreased immune surveillance in the CNS, or by stimulating a profound mobilization of cells out of the bone marrow that could theoretically traffic the virus across the blood–brain barrier, may be the reason PML risk exists in patients using the medication [79].
4.6 Interferon
A case of PML on IFNβ1a monotherapy was recently described [80]. The case was about a 46-year-old woman with RRMS on immunomodulatory treatment with IFNβ1a intramuscularly. She developed progressive left hemiparesis and the MRI scan showed progressive subcortical lesions affecting U-fibers with spotty enhancement in the right hemisphere and left frontal lobe suggestive of PML. CSF was twice tested positive for JCV by PCR [80]. An immunological workup revealed a serum hypogammaglobulinemia and a mild lymphopenia (1190/mm3) with reduced CD4 T-cell count (140/mm3); a picture compatible with the diagnosis of a common variable immunodeficiency syndrome; so the PML was more likely related to the primary immunodeficiency than to the treatment. HIV infection and other causes of secondary immunodeficiency were ruled out and, finally, a diagnosis of PML was established [80]. The treatment with IFNβ1a was stopped immediately and treatment with mirtazapine (30 mg per day), mefloquine (250 mg per day), filgrastim (30 MU [0.6 mg/ml] for 5 consecutive days) and intravenous immunoglobulins (1 g/kg body weight, because of the hypogammaglobulinemia) was started. A rapid clinical stabilization and partial recovery of the hemiparesis was obtained [80].
5 Conclusion and Future Perspectives
The term ‘drug-related PML’ was introduced as a result of an increasing number of incidences of PML in MS patients treated with drugs which induced an immunosuppressive status. Such terminology caused serious concerns among all MS stakeholders [81].
Proposed studies that aim at predicting PML risk appear unworkable because they would require at least 1000 controls along with four patients having PML, and would need to exceed 2 years [14].
Therefore, at this time, it appears inevitable that most of the data leading to these and future guidelines will need to rely on retrospective and partly anecdotal evidence [81].
This not only relates to risk prediction of PML, but is also true (1) for assessing the prognostic relevance of PML detection at an asymptomatic stage using MRI, (2) for therapeutic approaches to handle PML when diagnosed, and (3) for new approaches to improve PML workup. To date, the ability to more accurately predict PML in MS patients is based on testing for the presence of JCV, since JCV is an absolute prerequisite for PML development. However, we need better risk predictors to offer patients with MS when giving them the option to accept the risk of PML, in return for improved quality of life while on drug treatment [79]. Is it possible to mitigate the risk? We believe the predictive value of serological testing for JCV is affected by the rarity of PML.
The greater understanding of individual patient risk provided by better tests may help balance the concern of PML with the benefits that natalizumab offers to patients, particularly those who are anti-JCV antibody-positive with a low index value (≤1.5) in whom, before the application of stratification by index, the decision whether or not to continue natalizumab treatment would be difficult [82]. The stability of the anti-JCV antibody test over time and the prognostic significance of a rising index are not known. Therefore, the consensus group suggests reviewing this recommendation as new data emerges. Supporting clinicians in counseling patients on the risk benefit of starting natalizumab and, once on treatment, on the early detection of PML before it can cause significant disability or death will be the future challenge. Such early or presymptomatic diagnosis requires improved identification of at-risk patients, sustained clinical vigilance, and evidence-led MRI monitoring protocols [82]. Despite these limitations, it is important to perform such studies to move the develo** field forward. However, clinicians need to exercise caution when giving risk numbers based on retrospective incidence to patients, in particular in conjunction with, for example, genetically regulated levels of antibodies towards JCV in serum, which may allow a more individualized risk prediction. The latter published data are intriguing, but nonetheless, are based on a large number of cases and controls, and rely on the same incidences that previously failed to hold up over time. The future challenge and our duty as academic physicians will be to build networks of high-level expertise in order to collect larger and more harmonized data sets on patients with MS treated with pharmaceuticals that are associated with known and unknown hazards. This will hopefully further increase the power of, and the confidence in, new guidelines on stratification and monitoring of patients at risk.
Considering all immunosuppressive drugs as a potential trigger of PML is absolutely misleading. The epidemiological data showed differences among the investigated drugs in terms of PML risk, but any comparison is not possible so far. A framework for assessing relative risk for immunosuppressive therapies based on the best epidemiological data available is urgently needed.
The JCV reactivation in the CNS is not clear. Recently, one answer was proposed: the involvement of TNFα/NF-κB/NFAT4 signaling in JCV regulation in cultured human glial cells from HIV patients [83]. Unfortunately, no such studies have been performed in the field of MS.
For all of these reasons, we advocate clinical pharmacovigilance for all MS patients in therapies with immunosuppressant drugs. While it is possible that an underlying genetic susceptibility for PML in general will be uncovered in the future, the current evidence suggests instead a collection of multiple, individually underlying susceptibilities. In this regard, the development of common surveillance protocol is mandatory.
Given the recent cases of PML with fingolimod and dimethyl fumarate, determining a patient’s JCV antibody status is probably warranted, taking in account the cited limitations of this testing. Routine MRI should include diffusion weighted imaging (DWI) because it is of particular value in the evaluation of patients suspected of PML, as peripheral hyperintensity and central hypointensity on DWI images are classic. DWI may have utility in differentiating early PML from MS relapse and may be used to monitor patients treated for PML [84]. Although not yet in widespread clinical use, Diffusion tensor imaging (DTI) may be able to detect PML earlier than conventional imaging, before PML is clinically manifest [85]. No lymphopenia threshold has been established to drive MS therapy discontinuation or to trigger an alarm for PML risk [5]. Some evidence points to an elevated risk of PML with prolonged levels of approximately 500 cells/mm3 or less [82], but no definitive data are available so far.
The reports of PML in association with a primary immunodeficiency disorder are scarce. However, attention is now focusing on any suitable candidate genetic asset that could help in explaining PML as a primary immunodeficiency (not clinically recognizable except in the context of JCV).
Personalized treatment is highly desirable in MS because it is an immensely heterogeneous disease. Currently, a combination of clinical features and imaging parameters in MRI is used to classify active and non-active patients and treatment responders and non-responders [84]. Research on the pharmacogenomics of MS is increasing but no useful biomarker for clinical practice has emerged.
An emerging field is the role of age as a prognostic PML element. Aging of the immune system (immunosenescence) is characterized by changes in native and adaptive immune response (both humoral and cellular), resulting in high susceptibility to infection or virus reactivation. However, it is possible to suppose that long-term exposure to the virus, in a latent form in the CNS, increases the probability of JCV reactivation. Therefore, the relationship between PML and age has to be defined; in particular, we have to clarify if it is more linked to changes in host immune system or to the virulence of JCV.
The most important challenge regarding MS therapeutic interventions will be to tailor the therapy to the needs of the patient and to the aggressiveness of the disease, balancing the risks/effects of any proposed treatment.
Treatment for MS is dramatically changing and we are going in the direction where avoiding immunosuppressive therapies is neither practical nor desirable. In personalized therapy, treating to specific bio-targets will become a popular concept [82]. We strong believe that neurologists specializing in MS must help patients set meaningful goals and give the patients the means to achieve them.
In this scenario, the patient and the physician would collaborate in the decision making process regarding the administration of natalizumab. The discussion could include the relative efficacy, safety issues, and side-effect profiles, route of administration, convenience, adherence, long-term implications, and medical monitoring issues [86]. The future of therapy is changing rapidly and the new therapeutic agents should show good efficacy and safety profiles and have tests that allow for the stratification of risks. We have to bear in mind that MS is a long-life disease and the long-term outcomes for patients with MS need to be vastly improved. The overall goal will be balancing the risks posed by the medications with the risks posed by the disease.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–31.
Confavreux C, Vukusic S. The clinical course of multiple sclerosis. Hand b Clin Neurol. 2014;122:343–69.
Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics. 2016;13(1):47–57.
Torkildsen Ø, Myhr KM, Bø L. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol. 2016;23(1):18–27.
Lugaresi A, di Ioia M, Travaglini D, Pietrolongo E, Pucci E, Onofrj M. Risk-benefit considerations in the treatment of relapsing-remitting multiple sclerosis. Neuropsychiatr Dis Treat. 2013;9:893–914.
Ontaneda D, Fox RJ. Progressive multiple sclerosis. Curr Opin Neurol. 2015;28(3):237–43.
Zaheer F, Berger JR. Treatment related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther Adv Drug Saf. 2012;3(5):227–39.
Lallana EC, Fadul CE. Toxicities of immunosuppressive treatment of autoimmune neurologic diseases. Curr Neuropharmacol. 2011;9(3):468–77.
Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 2012;25(3):471–506.
Amend KL, Turnbull B, Foskett N, Napalkov P, Kurth T, Seeger J. Incidence of progressive multifocal leukoencephalopathy in patients without HIV. Neurology. 2012;75(15):1326–32.
Tyler KL. Emerging viral infections of the central nervous system: part 2. Arch Neurol. 2009;66(9):1065–74.
Bienaime A, Colson P, Moreau J. Progressive multifocal leukoencephalopathy in HIV-2-infected patient. AIDS. 2006;20(9):1342–3.
Verma A. Neurological manifestations of human immunodeficiency virus infection in adults. Neurol Clin Pract. 2004;2:1581–602.
Vendrely A, Bienvenu B, Gasnault J, et al. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol (Berl). 2005;109(4):449–55.
Delobel P, Brassat D, Delisle MB, Scaravilli F, Clanet M. Progressive multifocal leukoencephalopathy in an HIV patient with normal CD4 T-cell count and magnetic resonance imaging. AIDS. 2004;18(4):702–4.
Mascarello M, Lanzafame M, Lattuada E, Concia E, Ferrari S. Progressive multifocal leukoencephalopathy in an HIV patient receiving successful long-term HAART. J Neurovirol. 2011;17(2):196–9.
Weissert R. Progressive multifocal leukoencephalopathy. J Neuroimmunol. 2011;231(1–2):73–7.
Sainz-de-la-Maza S, Casado JL, Pérez-Elías MJ, Moreno A, Quereda C, Moreno S, et al. Incidence and prognosis of immune reconstitution inflammatory syndrome in HIV-associated progressive multifocal leucoencephalopathy. Eur J Neurol. 2016;23(5):919–25.
Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72(17):1458–64.
Di Giambenedetto S, Vago G, Pompucci A, Scoppettuolo G, Cingolani A, Marzocchetti A, et al. Fatal inflammatory AIDS-associated PML with high CD4 counts on HAART: a new clinical entity? Neurology. 2004;63(12):2452–3.
Garcia-Suarez J, de Miguel D, Krsnik I, Banas H, Arribas I, Burgaleta C. Changes in the natural history of progressive multifocal leukoencephalopathy in HIV-negative lymphoproliferative disorders: impact of novel therapies. Am J Hematol. 2005;80:271–81.
Worch J, Makarova O, Burkhardt B. Immunreconstitution and infectious complications after rituximab treatment in children and adolescents: what do we know and what can we learn from adults? Lenz G, Dreyling M, eds. Cancers. 2015;7(1):305–328. doi:10.3390/cancers7010305.
Freim Wahl SG, Folvik MR, Torp SH. Progressive multifocal leukoencephalopathy in a lymphoma patient with complete remission after treatment with cytostatics and rituximab: case report and review of the literature. Clin Neuropathol. 2007;26(2):68–73.
Durali D, de Goër de Herve M-G, Gasnault J, Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. doi:10.3389/fimmu.2015.00241.
Tan CS, Dezube BJ, Bhargava P, Autissier P, Wuthrich C, Miller J, et al. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. J Infect Dis. 2009;199:881–8. doi:10.1086/597117.
Planas R, Jelcic I, Schippling S, Martin R, Sospedra M. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur J Immunol. 2012;42:790–8.
Tan CS, Koralnik IJ. Beyond progressive multifocal leukoencephalopathy: expanded pathogenesis of JC virus infection in the central nervous system. Lancet Neurol. 2010;9(4):425–37. doi:10.1016/S1474-4422(10)70040-5.
De Gascun CF, Carr MJ. Human polyomavirus reactivation: disease pathogenesis and treatment approaches. Clin Dev Immunol. 2013;2013:373579. doi:10.1155/2013/373579.
Houff SA, Berger JR. The bone marrow, B cells, and JC virus. J NeuroVirol. 2008;14(5):341–3.
Monaco MC, Major EO. Immune system involvement in the pathogenesis of JC virus induced PML: what is learned from studies of patients with underlying diseases and therapies as risk factors. Front Immunol. 2015;6:159.
Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, Lee S, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76(6):802–12.
Calabrese LH, Molloy E, Berger J. Sorting out the risks in progressive multifocal leukoencephalopathy. Nat Rev Rheumatol. 2015;11(2):119–23.
Bauer J, Gold R, Adams O, Lassmann H. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome (IRIS). Acta Neuropathol. 2015;130(6):751–64.
Giacomini PS, Rozenberg A, Metz I, Araujo D, Arbour N, Bar-Or A, Maraviroc in Multiple Sclerosis–Associated PML–IRIS (MIMSAPI) Group. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med. 2014;370(5):486–8. doi:10.1056/NEJMc1304828.
Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M, Progressive Multifocal Leukeoncephalopathy Consortium. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255–73. doi:10.1177/1756285615602832.
Biogen Idec communication: PML incidence in patients receiving TYSABRI (natalizumab); 2016. http://www.biogenidec.com/therapies.aspx?ID=5489. Accessed July 2016.
Dong-Si T, Gheuens S, Gangadharan A, Wenten M, Philip J, McIninch J, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy.
Etxeberria A, Outteryck O, Ongagna JC, et al. Annual rate of JCV seroconversion in a French cohort of MS patients under natalizumab. Lyon: European Committee for Treatment and Research in Multiple Sclerosis; 2012. p. P996
Lanzillo R, Liuzzi R, Vallefuoco L, Moccia M, Amato L, Vacca G, et al. JC virus antibody index in natalizumab-treated patients: correlations with John Cunningham virus DNA and C-reactive protein level. Ther Clin Risk Manag. 2014;10:807–14. doi:10.2147/TCRM.S63295.
Dong-Si T, Richman S, Wattjes MP. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Annals of Clinical and Translational Neurology. 2014;1(10):755–64.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108413.htm. Accessed July 2016.
Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8.
http://multiple-sclerosis-research.blogspot.com/2015/12/clinicspeak-natalizumab-pml-risk-update.html. Accessed July 2016.
Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol (Epub 2016 Feb 3).
Mc Guigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87(2):117–25.
Bozic C, Subramanyam M, Richman S, Plavina T, Zhang A, Ticho B. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol. 2014;21:299–304.
Berger JR, Houff SA, Gurwell J, Vega N, Miller CS, Danaher RJ. JC virus antibody status underestimates infection rates. Ann Neurol. 2013;74:84–90.
Schwab N, Schneider-Hohendorf T, Posevitz V, Breuer J, Göbel K, Windhagen S, et al. L-selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81(10):865–71.
Villar LM, Costa-Frossard L, Masterman T, Fernandez O, Montalban X, Casanova B. Lipid-specific immunoglobulin M bands in cerebrospinal fluid are associated with a reduced risk of develo** progressive multifocal leukoencephalopathy during treatment with natalizumab. Ann Neurol. 2015;77(3):447–57.
http://www.fda.gov/downloads/Drugs/Drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm288126.pdf. Accessed July 2016.
https://www.touchprogram.com/TTP/images/Guidance_For_Evaluation_of_New_Neurologic_Symptoms_in_Patients_Receiving_TYSABRI.pdf. Accessed July 2016.
Singer B, Ross AP, Tobias K. Oral fingolimod for the treatment of patients with relapsing forms of multiple sclerosis. Int J Clin Pract. 2011;65(8):887–95.
Novartis data on file. https://www.novartis.it. Accessed July 2016.
https://www.novartis.it. Data as of 31st May 2016, Novartis Pharmaceuticals Q2 2016 Financial Report dated July 2016. Cumulative exposure in clinical trials and from marketing experience. Accessed July 2016.
Gajofatto A, Turatti M, Monaco S, Benedetti MD. Clinical efficacy, safety, and tolerability of fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf. 2015;7:157–67.
US Food Drug Administration FDA Drug Safety Communication: FDA warns about cases of rare brain infection with MS drug Gilenya (fingolimod) in two patients with no prior exposure to immunosuppressant drugs. from:http://www.fda.gov/Drugs/DrugSafety/ucm456919.htm. Accessed 25 Sept 2015.
Calic Z, Cappelen-Smith C, Hodgkinson SJ, McDougall A, Cuganesan R, Brew BJ. Treatment of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome with intravenous immunoglobulin in a patient with multiple sclerosis treated with fingolimod after discontinuation of natalizumab. J Clin Neurosci. 2015;22(3):598–600.
Killestein J, Vennegoor A, van Golde AE, Bourez RL, Wijlens ML, Wattjes MP. PML-IRIS during fingolimod diagnosed after natalizumab discontinuation. Case Rep Neurol Med. 2014;2014:307872.
https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm. Accessed July 2016.
http://www.fda.gov/Drugs/DrugSafety/ucm456919.htm. Accessed July 2016.
Xu Z, Zhang F, Sun F, Gu K, Dong S, He D. Dimethyl fumarate for multiple sclerosis. Cochrane Database Syst Rev. 2015;4:CD011076.
NFE2L2 nuclear factor, erythroid 2-like 2 [Homo sapiens (human)] Gene ID: 4780, updated on 29-Sep-2013. Genes & Expression Database [Internet]. National Center for Biotechnology Information. Available from:http://www.ncbi.nlm.nih.gov/gene/4780. Accessed July 2016.
Wilms H, Sievers J, Rickert U, et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1ß, TNF-α and IL-6 in an in vitro model of brain inflammation. J Neuroinflamm. 2010;19(7):30.
Vandermeeren M, Janssens S, Wouters H, et al. Dimethylfumarate is an inhibitor of cytokine-induced nuclear translocation of NF-kappa B1, but not RelA in normal human dermal fibroblast cells. J Invest Dermatol. 2001;116(1):124–30.
Treumer F, Zhu K, Gläser R, et al. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol. 2003;121(6):1383–8.
Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(Pt 3):678–92.
Lin SX, Lisi L, Dello Russo C, et al. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro. 2011;3(2):75–84.
Van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med. 2013;368(17):1658–9.
Nieuwkamp DJ, Murk JL, van Oosten BW, Cremers CH, Killestein J, Viveen MC, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med. 2015;372(15):1474–6.
Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476–8.
Dammeier N, Schubert V, Hauser TK, Bornemann A, Bischof F. Case report of a patient with progressive multifocal leukoencephalopathy under treatment with dimethyl fumarate. BMC Neurol. 2015;15:108.
Hughes S, Fourth PML Case With Tecfidera in MS Calls for Vigilance. Medscape. 2015. http://www.medscape.com/viewarticle/856148.
http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/10/news_detail_002423.jsp&mid=WC0b01ac058004d5c1. Accessed July 2016.
Coles AJ. Alemtuzumab therapy for multiple sclerosis. Neurotherapeutics. 2013;10(1):29–33.
Mentzer D, Prestel J, Adams O, Gold R, Hartung HP, Hengel H, et al. Case definition for progressive multifocal leukoencephalopathy following treatment with monoclonal antibodies. J Neurol Neurosurg Psychiatry. 2012;83(9):927–33.
Castillo-Trivino T, Braithwaite D, Bacchetti P, Waubant E. Rituximab in relapsing and progressive forms of multiple sclerosis: a systematic review. PLoS One. 2003;8(7):e66308.
Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40.
Genentech (2012) PML with Rituximab. Berger JR (ed). San Francisco.
Lehmann HC, Krüger K, Fink GR, Schroeter M. Progressive multifocal leukoencephalopathy after interferon beta-1a monotherapy. J Neurol. 2015;262(3):771–3.
Warnke C, Menge T, Hartung HP, Racke MK, Cravens PD, Bennett JL, et al. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors and can it be avoided? Arch Neurol. 2010;67(8):923–30.
Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurol. 2012;78:507–8.
Dong-Si T, Richman S, Wattjes MP, Wenten M, Gheuens S, Philip J, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1(10):755–64.
Derfuss T. Personalized medicine in multiple sclerosis: hope or reality? BMC Med. 2012;10:116.
Brück W, Ralf G, Lund BT, et al. Therapeutic decisions in multiple sclerosis: moving beyond efficacy. JAMA Neurol. 2013;70(10):1315–24. doi:10.1001/jamaneurol.2013.3510.
Honce JM, Nagae L, Nyberg E. Neuroimaging of natalizumab complications in multiple sclerosis: PML and other associated entities. Mult Scler Int. 2015;2015:809252. doi:10.1155/2015/809252.
Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K. Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care. 2013;15(1):36–45. doi:10.7224/1537-2073.2011-038.
Acknowledgments
The authors are grateful to Gregory Scott, PhD, for his editing contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript complies with ethical standards; it has been approved by the ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Funding
No sources of funding were used to assist in the preparation of this study.
Conflicts of Interest
Francesco Patti has served on the scientific advisory board for Teva, Biogen-Idec, Bayer-Schering, Novartis, and has received honoraria as a speaker for Teva, Biogen, Merck-Serono, Bayer-Schering, Genzyme/Sanofi, and Novartis. Hayrettin Tumani serves on a scientific advisory board, as a consultant for, and/or received funding for research projects and travel from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, Siemens, and Teva; serves on editorial board for MSI (Multiple Sclerosis International) and NPBR (Neurology, Psychiatry and Brain Research); and receives research support from BMBF, University of Ulm, Landesstiftung BW. Emanuele D’Amico, Carmela Leone, and Aurora Zanghì have no conficts of interest that are directly relevant to the content of this study.
Author contributions
Emanuele D’Amico contributed to conception and design. He participated in drafting and revising the article. Hayrettin Tumani and Aurora Zanghì contributed to the draft and revising the article; Francesco Patti contributed in revising the article. He gave final approval of the version to be submitted and any revised version.
Rights and permissions
About this article
Cite this article
D’Amico, E., Zanghì, A., Leone, C. et al. Treatment-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: A Comprehensive Review of Current Evidence and Future Needs. Drug Saf 39, 1163–1174 (2016). https://doi.org/10.1007/s40264-016-0461-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0461-6