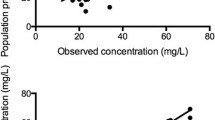
Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a form of cardiopulmonary life support frequently utilized in catastrophic lung and or cardiac failure. Patients on ECMO often receive vancomycin therapy for treatment or prophylaxis against Gram-positive organisms. It is unclear if ECMO affects vancomycin pharmacokinetics, thus we modeled the pharmacokinetic behavior of vancomycin according to ECMO-specific variables.
Methods
Adult patients receiving vancomycin and Veno-Arterial-ECMO between 12/1/2016 and 10/1/2017 were prospectively enrolled. Extracorporeal membrane oxygenation settings and four sets of pre- and post-oxygenator vancomycin concentrations were collected for each patient. Compartmental models were built and assessed ECMO flow rates on vancomycin clearance and potential circuit sequestration. Bayesian posterior concentrations of the pre- and post-oxygenator concentrations were obtained for each patient, and summary pharmacokinetic parameters were calculated. Simulations were performed from the final model for efficacy and toxicity predictions.
Results
Eight patients contributed 64 serum concentrations. Patients were a median (interquartile range) age of 58.5 years (50.8–62.3) with a calculated creatinine clearance of 39 mL/min (29.5–62.5) and ECMO flow rates of 3980 mL/min (interquartile range = 3493.75–4132.5). A three-compartment model best fit the data (Bayesian: plasma pre-oxygenation R2 = 0.99, post-oxygenation R2 = 0.99). Vancomycin clearance was not impacted by ECMO flow rate (p = 0.7). Simulations demonstrated that vancomycin 1 g twice daily was rarely sufficient for minimum inhibitory concentrations > 0.5 mg/L. Doses ≥ 1.5 g twice daily often exceeded toxicity thresholds for exposure.
Conclusions
Extracorporeal membrane oxygenation flow rates did not influence vancomycin clearance between flow rates of 3500 and 5000 mL/min and vancomycin was not sequestered in ECMO. Common vancomycin regimens resulted in suboptimal efficacy and/or excessive toxicity. Individual therapeutic drug monitoring is recommended for patients on ECMO.



Similar content being viewed by others
References
Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015. https://doi.org/10.3978/j.issn.2072-1439.2015.07.17.
Ratnani I, Tuazon Di, Zainab A, Uddin F. The role and impact of extracorporeal membrane oxygenation in critical care. Methodist Debakey Cardiovasc J. 2018;14(2):110–19.
Vogel AM, Lew DF, Kao LS, Lally KP. Defining risk for infectious complications on extracorporeal life support. J Pediatr Surg. 2011. https://doi.org/10.1016/j.jpedsurg.2011.09.013.
Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017. https://doi.org/10.1016/j.ijantimicag.2017.02.025.
Begg EJ, Barclay ML, Kirkpatrick CJM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 1999. https://doi.org/10.1046/j.1365-2125.1999.00850.x.
Hsu MS, Chiu KM, Huang YT, Kao KL, Chu SH, Liao CH. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect. 2009. https://doi.org/10.1016/j.jhin.2009.07.016.
Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FLL. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2016. https://doi.org/10.1016/j.jfma.2015.05.017.
Shekar K, Roberts JA, Mcdonald CI, Fisquet S, Barnett AG, Mullany DV, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012. https://doi.org/10.1186/cc11679.
Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010. https://doi.org/10.1007/s00134-010-2041-z.
Amaker RD, Dipiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996.
Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005. https://doi.org/10.1111/j.1365-2125.2005.02432.x.
Hoie E, Swigart S, Leuschen M, Willett L, Bolam D, Goodrich P, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–5.
Moore JN, Healy JR, Thoma BN, Peahota MM, Ahamadi M, Schmidt L, et al. A population pharmacokinetic model for vancomycin in adult patients receiving extracorporeal membrane oxygenation therapy. CPT Pharmacometrics Syst Pharmacol. 2016. https://doi.org/10.1002/psp4.12112.
Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, et al. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care. 2014. https://doi.org/10.1186/s13054-014-0632-8.
Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho YH, et al. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0141016.
Beckman Coulter. AU5800 series clinical chemistry analyzers 2019. https://www.beckmancoulter.com/en/products/chemistry/au5800. Accessed 12 May 2020.
Leary R, Jelliffe R, Schumitzky A, Van Guilder M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models. Proc IEEE Symp Comput Med Syst. 2001. https://doi.org/10.1109/CBMS.2001.941750.
Neely MN, Van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012. https://doi.org/10.1097/FTD.0b013e31825c4ba6.
Team RC. R: a language and environment for statistical computing. J Comput Graph Stat. 2015.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976. https://doi.org/10.1159/000180580.
Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. 2013. https://doi.org/10.1002/9781118548387.
Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, et al. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother. 2015. https://doi.org/10.1093/jac/dkv067.
Goutelle S, Bourguignon L, Maire PH, Van Guilder M, Conte JE, Jelliffe RW. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother. 2009. https://doi.org/10.1128/AAC.01520-08.
Stove V, Coene L, Carlier M, De Waele JJ, Fiers T, Verstraete AG. Measuring unbound versus total vancomycin concentrations in serum and plasma: methodological issues and relevance. Ther Drug Monit. 2015. https://doi.org/10.1097/FTD.0000000000000122.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2020;77(11):835–864. https://doi.org/10.1093/ajhp/zxaa036.
Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz051.
Lodise TP, Rosenkranz SL, Finnemeyer M, Evans S, Sims M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with MRSA bloodstream infections (PROVIDE). Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz460.
Avedissian SN, Pais GM, O’Donnell JN, Lodise TP, Liu J, Prozialeck WC, et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother. 2019. https://doi.org/10.1093/jac/dkz167.
Álvarez R, Cortés LEL, Molina J, Cisneros JM, Pachón J. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. 2016. https://doi.org/10.1128/AAC.03147-14.
Roberts JA, Taccone FS, Udy AA, Vincent J-L, Jacobs F, Lipman J. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 2011. https://doi.org/10.1128/aac.01708-10.
Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017. https://doi.org/10.1097/CCM.0000000000002652.
Kim DW, Yeo HJ, Yoon SH, Lee SE, Lee SJ, Cho WH, et al. Impact of bloodstream infections on catheter colonization during extracorporeal membrane oxygenation. J Artif Organs. 2016. https://doi.org/10.1007/s10047-015-0882-5.
O’Donnell JN, Ghossein C, Rhodes NJ, Peng J, Lertharakul T, Pham CK, et al. Eight unexpected cases of vancomycin associated acute kidney injury with contemporary dosing. J Infect Chemother. 2017. https://doi.org/10.1016/j.jiac.2016.12.011.
O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, et al. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/AAC.00416-17.
Downes KJ, Hayes M, Fitzgerald JC, Pais GM, Liu J, Zane NR, et al. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother. 2019. https://doi.org/10.1093/jac/dkz325.
Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014. https://doi.org/10.1128/AAC.01653-13.
Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O’Donnell JN, Pais G, et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother. 2016. https://doi.org/10.1128/AAC.00591-16.
Funding
Marc H. Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award number R21AI149026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding for assays in this study were paid for utilizing discretionary research funds from the Scheetz Laboratory. All other efforts by the study authors were donated or part of normal work activities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ahmed Mahmoud, Sean N. Avedissian, Abbas Al-Qamari, Tiffany Bohling, and Michelle Pham have no conflicts of interest that are directly relevant to the content of this article. Marc H. Scheetz received a research grant with Nevakar and has a patent (US 2019/0099500 A1) pending.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
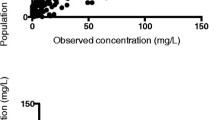
See Fig. 4 and Tables 4, 5, 6 and 7.
Concentrations did not significantly differ when comparing the time-matched pre- and post-oxygenator concentrations (mean difference − 0.22 mg/L, 0.90 mg/L SD, p = 0.18) indicating little sequestration (Fig. 4 of the Appendix). Results of the non-compartmental analysis from the Bayesian posterior-predicted concentrations (i.e., pre-oxygenator concentrations) for the eight patients are summarized in Table 3. Briefly, within the eight study patients, the median (IQR) clearance and volume of distribution at steady-state values were 3.4 (1–3.87) L/h and 43.91 (40.65–51.4) L, respectively.
Rights and permissions
About this article
Cite this article
Mahmoud, A.A., Avedissian, S.N., Al-Qamari, A. et al. Pharmacokinetic Assessment of Pre- and Post-Oxygenator Vancomycin Concentrations in Extracorporeal Membrane Oxygenation: A Prospective Observational Study. Clin Pharmacokinet 59, 1575–1587 (2020). https://doi.org/10.1007/s40262-020-00902-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00902-1