Abstract
Background and Objective
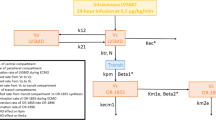
Milrinone is the drug of choice for the treatment and prevention of low cardiac output syndrome (LCOS) in paediatric patients after open heart surgery across Europe. Discrepancies, however, among prescribing guidance, clinical studies and practice pattern require clarification to ensure safe and effective prescribing. However, the clearance prediction equations derived from classical pharmacokinetic modelling provide limited support as they have recently failed a clinical practice evaluation. Therefore, the objective of this study was to evaluate current milrinone dosing using physiology-based pharmacokinetic (PBPK) modelling and simulation to complement the existing pharmacokinetic knowledge and propose optimised dosing regimens as a basis for improving the standard of care for paediatric patients.
Methods
A PBPK drug–disease model using a population approach was developed in three steps from healthy young adults to adult patients and paediatric patients with and without LCOS after open heart surgery. Pre- and postoperative organ function values from adult and paediatric patients were collected from literature and integrated into a disease model as factorial changes from the reference values in healthy adults aged 20–40 years. The disease model was combined with the PBPK drug model and evaluated against existing pharmacokinetic data. Model robustness was assessed by parametric sensitivity analysis. In the next step, virtual patient populations were created, each with 1,000 subjects reflecting the average adult and paediatric patient characteristics with regard to age, sex, bodyweight and height. They were integrated into the PBPK drug–disease model to evaluate the effectiveness of current milrinone dosing in achieving the therapeutic target range of 100–300 ng/mL milrinone in plasma. Optimised dosing regimens were subsequently developed.
Results
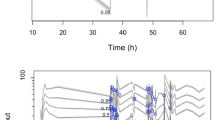
The pharmacokinetics of milrinone in healthy young adults as well as adult and paediatric patients were accurately described with an average fold error of 1.1 ± 0.1 (mean ± standard deviation) and mean relative deviation of 1.5 ± 0.3 as measures of bias and precision, respectively. Normalised maximum sensitivity coefficients for model input parameters ranged from −0.84 to 0.71, which indicated model robustness. The evaluation of milrinone dosing across different paediatric age groups showed a non-linear age dependence of total plasma clearance and exposure differences of a factor 1.4 between patients with and without LCOS for a fixed dosing regimen. None of the currently used dosing regimens for milrinone achieved the therapeutic target range across all paediatric age groups and adult patients, so optimised dosing regimens were developed that considered the age-dependent and pathophysiological differences.
Conclusion
The PBPK drug–disease model for milrinone in paediatric patients with and without LCOS after open heart surgery highlights that age, disease and surgery differently impact the pharmacokinetics of milrinone, and that current milrinone dosing for LCOS is suboptimal to maintain the therapeutic target range across the entire paediatric age range. Thus, optimised dosing strategies are proposed to ensure safe and effective prescribing.






Similar content being viewed by others
References
Ma M, Gauvreau K, Allan CK, et al. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83:1438–45.
Shi S, Zhao Z, Liu X, et al. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest. 2008;134:768–74.
Vogt W, Läer S. Treatment for paediatric low cardiac output syndrome: results from the European EuLoCOS-Paed survey. Arch Dis Child. 2011;96:1180–6.
Vogt W, Läer S. Prevention for pediatric low cardiac output syndrome: results from the European survey EuLoCOS-Paed. Paediatr Anaesth. 2011;12:1176–84.
Vogt W, Läer S. Drug use patterns for the prevention of paediatric low cardiac output syndrome in Europe. Intensive Care Med. 2011;37:1390–1.
Milrinone: Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended (2011). http://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/Paediatric_Regulation/Assessment_Reports/Article_45_work-sharing/Milrinone-_2011_06_Art.45PaedPdAR.pdf. Accessed 9 Jun 2013.
Bailey JM, Hoffman TM, Wessel DL, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. 2004;31:43–59.
Bailey JM, Miller BE, Lu W, et al. The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology. 1999;90:1012–8.
Ramamoorthy C, Anderson GD, Williams GD, et al. Pharmacokinetics and side effects of milrinone in infants and children after open heart surgery. Anesth Analg. 1998;86:283–9.
Garcia Guerra G, Senthilselvan A, Kutsogiannis DJ, et al. Safe administration of milrinone. Pediatr Crit Care Med. 2011;12:A11–A12.
Sanofi aventis. Summary of Product Characteristics for Primacor 1 mg/ml solution for injection (INN: Milrinone).http://www.medicines.org.uk/EMC/medicine/6984/SPC/Primacor+1mg+ml+Solution+for+Injection/. Accessed 9 Jul 2012.
Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002.
Edginton AN, Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47:743–52.
Johnson TN, Boussery K, Rowland-Yeo K, et al. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206.
Yeo KR, Aarabi M, Jamei M, et al. Modelling and predicting drug pharmacokinetics in patients with renal impairment. Expert Opin Clin Pharmacol. 2011;4:261–74.
Zhao P, de Vieira MLT, Grillo JA, et al. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52:91S–108S.
Björkman S, Wada DR, Berling BM, et al. Prediction of the disposition of midazolam in surgical patients by a physiologically based pharmacokinetic model. J Pharm Sci. 2001;90:1226–41.
Johnson TN, Rostami-Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931–56.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34.
Parrott N, Davies B, Hoffmann G, et al. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in neonates and infants. Clin Pharmacokinet. 2011;50:613–23.
Björkman S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: theophylline and midazolam as model drugs. Br J Clin Pharmacol. 2005;59:691–704.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Barrett JS, Della Casa Alberighi O, Läer S, et al. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther. 2012;92:40–9.
Willmann S, Höhn K, Edginton A, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34:401–31.
Zhang Y, Huo M, Zhou J, et al. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–14.
Stroshane RM, Koss RF, Biddlecome CE, et al. Oral and intravenous pharmacokinetics of milrinone in human volunteers. J Pharm Sci. 1984;73:1438–41.
Young RA, Ward A. Milrinone: a preliminary review of its pharmacological properties and therapeutic use. Drugs. 1988;36:158–92.
Alousi AA, Fabian RJ, Baker JF, et al. Milrinone. In: Scriabine A, editor. New drugs annual: cardiovascular drugs, vol. 3. New York: Raven Press; 1985. p. 245–83.
Coleman MD. Human drug metabolism: an introduction. 2nd ed. New York: Wiley; 2010.
Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616.
Woolfrey SG, Hegbrant J, Thysell H, et al. Dose regimen adjustment for milrinone in congestive heart failure patients with moderate and severe renal failure. J Pharm Pharmacol. 1995;47:651–5.
National Kidney Foundation. KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S46–75.
Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest. 1950;29:496–507.
Feneck RO. Effects of variable dose milrinone in patients with low cardiac output after cardiac surgery. European Multicenter Trial Group. Am Heart J. 1991;121:1995–9.
Monnet X, Persichini R, Ktari M, et al. Precision of the transpulmonary thermodilution measurements. Crit Care. 2011;15:R204.
Stetz CW, Miller RG, Kelly GE, et al. Reliability of the thermodilution method in the determination of cardiac output in clinical practice. Am Rev Respir Dis. 1982;126:1001–4.
Arnold JM, Ludmer PL, Wright RF, et al. Role of reflex sympathetic withdrawal in the hemodynamic response to an increased inotropic state in patients with severe heart failure. J Am Coll Cardiol. 1986;8:413–8.
Cinequegrani M, Mesner C, Baggs JG, et al. Effects of continuous milrinone infusion on forearm and hepatic blood flows. Clin Res. 1984;32:155A.
Tobata D, Takao K, Mochizuki M, et al. Effects of dopamine, dobutamine, amrinone and milrinone on regional blood flow in isoflurane anesthetized dogs. J Vet Med Sci. 2004;66:1097–105.
Chen EP, Bittner HB, Davis RD, et al. Milrinone improves pulmonary hemodynamics and right ventricular function in chronic pulmonary hypertension. Ann Thorac Surg. 1997;63:814–21.
Sulek CA, Blas ML, Lobato EB. Milrinone increases middle cerebral artery blood flow velocity after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2002;16:64–9.
Cooper KE, Edholm OG, Mottram RF. The blood flow in skin and muscle of the human forearm. J Physiol. 1955;128:258–67.
Willmann S, Lippert J, Schmitt W. From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol. 2005;1:159–68.
Hillman RS, Ault K, Rinder H. Hematology in clinical practice. 4th ed. New York: McGraw-Hill; 2005.
Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia: W.B. Saunders & Co.; 1995.
Williams LR. Reference values for total blood volume and cardiac output in humans. Washington, DC: Department of Energy; 1994.
Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations (part I). Clin Pharmacokinet. 1991;21:165–77.
Greenblatt DJ, Harmatz JS, Shader RI. Clinical pharmacokinetics of anxiolytics and hypnotics in the elderly: therapeutic considerations (part II). Clin Pharmacokinet. 1991;21:262–73.
Zappitelli M, Bernier P, Saczkowski RS, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–92.
Han WK, Wagener G, Zhu Y, et al. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–82.
Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–9.
Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–9.
Bernstein D, Teitel D, Sidi D, et al. Redistribution of regional blood flow and oxygen delivery in experimental cyanotic heart disease in newborn lambs. Pediatr Res. 1987;22:389–93.
Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765–74.
McNamara PJ, Alcorn J. Protein binding predictions in infants. AAPS PharmSci. 2002;4:19–26.
Chang AC, Atz AM, Wernovsky G, et al. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med. 1995;23:1907–14.
Hayton WL. Maturation and growth of renal function: dosing renally cleared drugs in children. AAPS PharmSci. 2000;2:22–8.
Edginton AN, Schmitt W, Voith B, et al. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45:683–704.
Tsunoo M, Momomura S, Imai Y, et al. Safety, tolerance and pharmacokinetics of milrinone in phase I study in healthy male Japanese subjects (2)—after intravenous infusion. Jpn Pharmacol Ther. 1993;21:201–21.
Bailey JM, Levy JH, Kikura M, et al. Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology. 1994;81:616–22.
Butterworth JF, Hines RL, Royster RL, et al. A pharmacokinetic and pharmacodynamic evaluation of milrinone in adults undergoing cardiac surgery. Anesth Analg. 1995;81:783–92.
Das PA, Skoyles JR, Sherry KM, et al. Disposition of milrinone in patients after cardiac surgery. Br J Anaesth. 1994;72:426–9.
de Hert SG, Moens MM, Jorens PG, et al. Comparison of two different loading doses of milrinone for weaning from cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1995;9:264–71.
Hasei M, Uchiyama A, Nishimura M, et al. Correlation between plasma milrinone concentration and renal function in patients with cardiac disease. Acta Anaesthesiol Scand. 2008;52:991–6.
Benotti JR, Hood WB. Dose-ranging study of intravenous milrinone to determine efficacy and pharmacokinetics. In: Braunwald E, editor. Milrinone: investigation of new inotropic therapy for congestive heart failure. New York: Raven Press; 1984. p. 95–107.
Androne A, Katz SD, Lund L, et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–9.
Fichtl B, Meister W, Schmied R. Serum protein binding of drugs is not altered in patients with severe chronic cardiac failure. Int J Clin Pharmacol Ther Toxicol. 1983;21:241–4.
Ogden CL, Fryar CD, Carroll MD, et al. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data. 2004;347:1–17.
World Health Organization. The WHO child growth standards. http://www.who.int/childgrowth/standards/en/. Accessed 12 Jul 2012.
World Health Organization. Growth reference data for 5-19 years. http://www.who.int/growthref/en/. Accessed 10 Jul 2012.
Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50.
Dorne JL, Walton K, Renwick AG. Human variability in glucuronidation in relation to uncertainty factors for risk assessment. Food Chem Toxicol. 2001;39:1153–73.
Dorne JL, Walton K, Renwick AG. Human variability in the renal elimination of foreign compounds and renal excretion-related uncertainty factors for risk assessment. Food Chem Toxicol. 2004;42:275–98.
Ito K, Houston JB. Prediction of human drug clearance from in vitro and preclinical data using physiologically based and empirical approaches. Pharm Res. 2005;22:103–12.
Jones HM, Parrott N, Jorga K, et al. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45:511–42.
Clewell HJ, Andersen ME. Use of physiologically based pharmacokinetic modeling to investigate individual versus population risk. Toxicology. 1996;111:315–29.
Cameron JW, Rosenthal A, Olson AD. Malnutrition in hospitalized children with congenital heart disease. Arch Pediatr Adolesc Med. 1995;149:1098–102.
Brierley J, Peters M. Hemodynamics of milrinone loading in critically ill children. Crit Care Med. 2006;32:A63.
Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–88.
Benotti JR, Lesko LJ, McCue JE, et al. Pharmacokinetics and pharmacodynamics of milrinone in chronic congestive heart failure. Am J Cardiol. 1985;56:685–9.
Fitzpatrick PG, Cinquegrani MP, Vakiener AR, et al. Hemodynamic and regional blood flow response to milrinone in patients with severe congestive heart failure: a dose-ranging study. Am Heart J. 1987;114:97–105.
Fish DN, Chow AT. The clinical pharmacokinetics of levofloxacin. Clin Pharmacokinet. 1997;32:101–19.
Li F, Nandy P, Chien S, et al. Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob Agents Chemother. 2010;54(1):375–9.
Sun H, Ette EI, Ludden TM. On the recording of sample times and parameter estimation from repeated measures pharmacokinetic data. J Pharmacokinet Biopharm. 1996;24:637–50.
Zuppa AF, Nicolson SC, Barrett JS, et al. Population pharmacokinetics of pentobarbital in neonates, infants, and children after open heart surgery. J Pediatr 2011;159:414–419.e1–3.
Su F, Nicolson SC, Gastonguay MR, et al. Population pharmacokinetics of dexmedetomidine in infants after open heart surgery. Anesth Analg. 2010;110:1383–92.
Tae Y, Kwak JG, Kim B, et al. Population pharmacokinetic analysis and dosing regimen optimization of aprotinin in neonates and young infants undergoing cardiopulmonary bypass. J Clin Pharmacol. 2011;51:1163–76.
van Saet A, de Wildt SN. Prevention of low cardiac output syndrome in children: where is the evidence? Paediatr Anaesth. 2011;21:1173–5.
Massé L, Antonacci M. Low cardiac output syndrome: identification and management. Crit Care Nurs Clin North Am. 2005;17:375–83.
Zuppa AF, Nicolson SC, Adamson PC, et al. Population pharmacokinetics of milrinone in neonates with hypoplastic left heart syndrome undergoing stage I reconstruction. Anesth Analg. 2006;102:1062–9.
Wright DFB, Duffull SB. A Bayesian dose-individualization method for warfarin. Clin Pharmacokinet. 2013;52:59–68.
Krauss M, Burghaus R, Lippert J, et al. Using Bayesian-PBPK modeling for assessment of inter-individual variability and subgroup stratification. In Silico Pharmacol. 2013. doi:10.1186/2193-9616-1-6.
Watson S, Christian KG, Churchwell KB. Side effects of milrinone in the cardiac PICU. Clin Intensive Care. 1999;10:149.
Martinez-Anton A, Sanchez JI, Casanueva L. Impact of an intervention to reduce prescribing errors in a pediatric intensive care unit. Intensive Care Med. 2012;38:1532–8.
Institute for Safe Medication Practices. ISMP’s list of high-alert medications. https://www.ismp.orgtoolshighalertmedications.pdf. Accessed 1 July 2013.
Manolis E, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br J Clin Pharmacol. 2009;68:493–501.
Jönnson S, Henningsson A. Medical Products Agency, Uppsala, Sweden. Regulatory vision of paediatric applications. http://www.emea.europa.eu/docs/en_GB/document_library/Presentation/2009/11/WC500009845.pdf. Accessed 12 Jul 2012.
Zhao P, Zhang L, Grillo JA, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther. 2011;89:259–67.
Anderson BJ, Merry AF. Data sharing for pharmacokinetic studies. Paediatr Anaesth. 2009;19:1005–10.
Knox C, Law V, Jewison T, et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011;39:D1035–41.
Altomare C, Cellamare S, Summo L, et al. Ionization behaviour and tautomerism-dependent lipophilicity of pyridine-2(1H)-one cardiotonic agents. Bioorg Med Chem. 2000;8:909–16.
de Candia M, Fossa P, Cellamare S, et al. Insights into structure-activity relationships from lipophilicity profiles of pyridin-2(1H)-one analogs of the cardiotonic agent milrinone. Eur J Pharm Sci. 2005;26:78–86.
Maganti M, Badiwala M, Sheikh A, et al. Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. 2010;140:790–6.
Maganti MD, Rao V, Borger MA, et al. Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation. 2005;112:I448–52.
Vánky FB, Håkanson E, Tamás E, et al. Risk factors for postoperative heart failure in patients operated on for aortic stenosis. Ann Thorac Surg. 2006;81:1297–304.
Arribas Leal JM, Pascual DA, et al. Epidemiology and new predictors of low cardiac output syndrome after isolated coronary artery bypass grafting. Eur Heart J. 2010;31:68–9.
McKinlay KH, Schinderle DB, Swaminathan M, et al. Predictors of inotrope use during separation from cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2004;18:404–8.
Müller M, Junger A, Bräu M, et al. Incidence and risk calculation of inotropic support in patients undergoing cardiac surgery with cardiopulmonary bypass using an automated anaesthesia record-kee** system. Br J Anaesth. 2002;89:398–404.
Butterworth JF, Legault C, Royster RL, et al. Factors that predict the use of positive inotropic drug support after cardiac valve surgery. Anesth Analg. 1998;86:461–7.
Rao V, Ivanov J, Weisel RD, et al. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112:38–51.
Jungbauer CG, Birner C, Jung B, et al. Kidney injury molecule-1 and N-acetyl-β-D-glucosaminidase in chronic heart failure: possible biomarkers of cardiorenal syndrome. Eur J Heart Fail. 2011;13:1104–10.
Ochs HR, Schuppan U, Greenblatt DJ, et al. Reduced distribution and clearance of acetaminophen in patients with congestive heart failure. J Cardiovasc Pharmacol. 1983;5:697–9.
Miners JO, Mackenzie PI. Drug glucuronidation in humans. Pharmacol Ther. 1991;51:347–69.
Ng CY, Ghabrial H, Morgan DJ, et al. Right heart failure impairs hepatic elimination of p-nitrophenol without inducing changes in content or latency of hepatic UDP-glucuronosyltransferases. J Pharmacol Exp Ther. 2000;295:830–5.
Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure: relationship of cardiac index to kidney function. Drugs. 1990;39:10–21.
Leithe ME, Margorien RD, Hermiller JB, et al. Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation. 1984;69:57–64.
Zelis R, Flaim SF. Alterations in vasomotor tone in congestive heart failure. Prog Cardiovasc Dis. 1982;24:437–59.
Feneck RO, Sherry KM, Withington PS, et al. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2001;15:306–15.
Hiraoka H, Yamamoto K, Okano N, et al. Changes in drug plasma concentrations of an extensively bound and highly extracted drug, propofol, in response to altered plasma binding. Clin Pharmacol Ther. 2004;75:324–30.
Lee E, Chin J, Choi D, et al. Postoperative hypoalbuminemia is associated with outcome in patients undergoing off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:462–8.
Wijeysundera DN, Rao V, Beattie WS, et al. Evaluating surrogate measures of renal dysfunction after cardiac surgery. Anesth Analg. 2003;96:1265–73.
Palomba H, de Castro I, Neto ALC, et al. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int. 2007;72:624–31.
Llopart T, Lombardi R, Forselledo M, et al. Acute renal failure in open heart surgery. Ren Fail. 1997;19:319–23.
Shim CK, Sawada Y, Iga T, et al. Estimation of renal secretory function for organic cations by endogenous N1-methylnicotinamide in rats with experimental renal failure. J Pharmacokinet Biopharm. 1984;12:23–42.
Park JM, Lin YS, Calamia JC, et al. Transiently altered acetaminophen metabolism after liver transplantation. Clin Pharmacol Ther. 2003;73:545–53.
Subramanian RM, Chandel N, Budinger GRS, et al. Hypoxic conformance of metabolism in primary rat hepatocytes: a model of hepatic hibernation. Hepatology. 2007;45:455–64.
Ruokonen E, Takala J, Kari A. Regional blood flow and oxygen transport in patients with the low cardiac output syndrome after cardiac surgery. Crit Care Med. 1993;21:1304–11.
Bulkley GB, Oshima A, Bailey RW. Pathophysiology of hepatic ischemia in cardiogenic shock. Am J Surg. 1986;151:87–97.
Hall C, Mørkrid L, Kjekshus J. Redistribution of peripheral blood flow during acute left ventricular failure in the dog. Scand J Clin Lab Invest. 1988;48:785–94.
Smiseth OA, Riemersma RA, Steinnes K, et al. Regional blood flow during acute heart failure in dogs. Role of adipose tissue perfusion in regulating plasma-free fatty acids. Scand J Clin Lab Invest. 1983;43:285–92.
Sapirstein LA, Sapirstein EH, Bredemeyer A. Effect of hemorrhage on the cardiac output and its distribution in the rat. Circ Res. 1960;8:135–48.
Acknowledgments
I gratefully acknowledge the support of Sanofi Aventis, Germany, for the provision of the original pharmacokinetic datasets for the studies by Stroshane et al. [26] and Bailey et al. [7]. Also, I sincerely thank Dr. Butterworth and Dr. James, Dr. Bailey and F. Szlam, Dr. Uchiyama and Dr. Hasei, and Dr. Woolfrey for providing their original pharmacokinetic datasets for further analysis. I would also like to thank Dr. Coboeken and Dr. Willmann from Bayer Technology Services for technical assistance with Mobi®, and S. Ramusovic for the introduction to Matlab™ and Mobi®. My sincere thanks go to my friend and former colleague Dr. Hsien as well as to Dr. Steinsträßer from Sanofi Aventis for reviewing and commenting on the manuscript. The results presented in this article are part of the PhD work of Ms. Vogt, carried out at Heinrich-Heine-Universität Düsseldorf.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vogt, W. Evaluation and Optimisation of Current Milrinone Prescribing for the Treatment and Prevention of Low Cardiac Output Syndrome in Paediatric Patients After Open Heart Surgery Using a Physiology-Based Pharmacokinetic Drug–Disease Model. Clin Pharmacokinet 53, 51–72 (2014). https://doi.org/10.1007/s40262-013-0096-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0096-z