Abstract
Introduction
Our objective was to provide expert consensus recommendations to improve treatment tolerability through dose adjustments of concomitant antiseizure medications (ASMs) during addition of cenobamate to existing ASM therapy in adult patients with uncontrolled focal seizures.
Methods
A panel of seven epileptologists experienced in the use of ASMs, including cenobamate, used a modified Delphi process to reach consensus. The panelists discussed tolerability issues with concomitant ASMs during cenobamate titration and practical strategies for dose adjustments that may prevent or mitigate adverse effects. The resulting recommendations consider concomitant ASM dose level and specify proactive (prior to report of an adverse effect) and reactive (in response to report of an adverse effect) dose adjustment suggestions based on concomitant ASM pharmacokinetic and pharmacodynamic interactions with cenobamate. Specific dose adjustment recommendations are provided.
Results
We recommend proactively lowering the dose of clobazam, phenytoin, and phenobarbital due to their known drug–drug interactions with cenobamate, and lacosamide due to a pharmacodynamic interaction with cenobamate, to prevent adverse effects during cenobamate titration. Reactive lowering of a concomitant ASM dose is sufficient for other ASMs at standard dosing owing to quick resolution of adverse effects. For carbamazepine and lamotrigine doses exceeding the upper end of standard dosing (e.g., carbamazepine, greater than 1200 mg/day; lamotrigine, greater than 500 mg/day), we encourage consideration of proactive dose reduction at cenobamate 200 mg/day to prevent potential adverse effects. All dose reductions for adverse effects can be repeated every 2 weeks as dictated by the adverse effects. At cenobamate 200 mg/day, we recommend that patients be evaluated for marked improvement of seizures and further dose reductions be considered to reduce potentially unnecessary polypharmacy.
Conclusion
The primary goal of the recommended dose reductions of concomitant ASMs is to prevent or resolve adverse effects, thereby allowing cenobamate to reach the optimal dose to achieve the maximal potential of improving seizure control.
Plain Language Summary
Some people with epilepsy need to take more than one seizure medicine as part of their treatment. Taking more than one seizure medicine, however, can increase the risk of unwanted side effects. One approach to preventing side effects when adding a new seizure medicine is to lower the amount (dose) of existing seizure medicines. Cenobamate is a newer seizure medicine available in the USA for adults with focal seizures (also referred to as partial-onset seizures). Cenobamate, like many seizure medicines, must be titrated over time to a target dose. A group of epilepsy specialists met and developed recommendations for when and how to change the doses of existing seizure medicines when adding cenobamate. The goal of these recommendations is to prevent or reduce side effects like sleepiness or dizziness. The authors recommend that the dose of specific seizure medicines, including clobazam, lacosamide, phenytoin, and phenobarbital, be lowered as cenobamate is started or as cenobamate’s dose is being increased (but before side effects occur). Regular doses of other seizure medicines can be lowered if a side effect occurs because reducing the dose of the other seizure medications can often stop the side effect. These recommendations may help patients successfully reach their optimal dose of cenobamate with fewer side effects, potentially improving their seizure control.
Video Abstract: Dose Adjustment of Concomitant Antiseizure Medications During Cenobamate Treatment: Expert Opinion Consensus Recommendations
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A panel of seven epileptologists developed, via a modified Delphi process, antiseizure medication (ASM) dose-reduction recommendations for initiating cenobamate in adults. |
The primary goal of the recommended dose reductions is to prevent or resolve adverse effects, allowing for the optimal cenobamate dosing. |
Recommendations include proactive dose adjustments prior to report of adverse effects for clobazam, lacosamide, phenytoin, and phenobarbital. |
Reactive dose adjustments in response to adverse effects are recommended for all other ASMs at standard dose levels. |
Infographic:

Digital Features
This article is published with digital features, including a video abstract and infographic, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.21758051.
Introduction
Antiseizure medication (ASM) polypharmacy is standard among patients with medication-resistant epilepsy. During the introduction of a new ASM to an existing treatment regimen that has failed to adequately improve seizure control, pharmacokinetic and pharmacodynamic interactions with concomitant ASMs can produce additive adverse effects [1, 2]. A common approach to care is to adjust the dose of concomitant ASMs rather than to discontinue the newly added ASM [3]. This approach can provide improved tolerability and safety while titrating the new ASM to a potentially effective dose [3]. Reducing the dose of concomitant ASMs allows the new ASM to reach its target dose and thereby allows the determination of its impact on seizure control.
Cenobamate is an ASM approved in the USA (XCOPRI®) and Europe (ONTOZRY®) for the treatment of adults with focal seizures and has shown high responder rates, including high rates of seizure freedom, and good tolerability in adults with uncontrolled focal seizures [4,5,6,7,8,9]. Cenobamate is an orally administered tetrazole carbamate derivative ASM that is different from other carbamate-containing ASMs in that it preferentially attenuates the persistent sodium current instead of the transient sodium current [10, 11]. Although the specific mechanism of action of cenobamate is not fully known, it has novel combined actions as a positive allosteric modulator of the γ-aminobutyric acid (GABAA) receptor through a non-benzodiazepine GABAA receptor site and as a sodium channel blocker by preferentially inhibiting the persistent sodium current [12,13,14]. The dual mechanism of action of cenobamate suggests it has the potential to both prevent seizure initiation and limit seizure spread [15,16,17,18,19].
When cenobamate is added to an ASM regimen, adjustments to concomitant ASMs may be needed to prevent or mitigate potential adverse effects that might arise because of pharmacokinetic or pharmacodynamic interactions among the ASMs [20]. Pharmacokinetic interactions occur early in titration, at lower doses of concomitant ASMs, and the most important involve cytochrome P450 (CYP) isoenzymes [2]. Pharmacokinetic interactions may result in changes in drug exposures and tolerability by increasing the rate of metabolism of an ASM, leading to lower plasma concentrations of the ASM, or by decreasing the rate of metabolism of an ASM, leading to higher plasma concentrations of the ASM [2]. For example, cenobamate inhibits CYP2C19 and clobazam, phenytoin, and phenobarbital are metabolized by the CYP2C19 isoenzyme [4]. When combined with cenobamate, increased plasma concentrations of clobazam’s active metabolite N-desmethylclobazam and of phenytoin and phenobarbital can occur, with the potential for increased adverse effects. Notably, increased plasma concentrations are likely prolonged with N-desmethylclobazam and phenobarbital as a result of their long half-lives, suggesting early dose reduction is needed to prevent prolonged adverse effects.
Pharmacodynamic interactions tend to occur later in titration, at higher doses of concomitant ASMs, and may occur among ASMs with similar mechanisms of action, such as sodium channel blockers. These interactions occur at the site of the ASM action and modify the pharmacologic effect without any change in the plasma concentrations of the ASMs [2]. For example, there are no clinically significant pharmacokinetic effects between lacosamide and cenobamate, which are both sodium channel blockers, but a pharmacodynamic interaction may be occurring if adverse effects increase during coadministration [4]. This pharmacodynamic interaction may occur relatively early in cenobamate titration if the concomitant lacosamide dose is high (i.e., 500 mg/day or higher). It is also possible for pharmacokinetic and pharmacodynamic effects to co-occur as in the cenobamate induction of the CYP3A4 enzyme that metabolizes the common sodium channel blocker carbamazepine [4]. The result is a decrease in the plasma level of carbamazepine and potentially decreased adverse effects, which is in opposition to the potential for pharmacodynamic interactions among these sodium channel blockers that could lead to increased adverse effects.
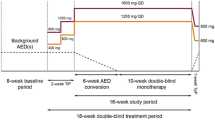
As a result of diverse interactions between cenobamate and other ASMs, there is a possibility of adverse effects both for pharmacokinetic and pharmacodynamic reasons [20]. Detailed recommendations for clinical practice management of concomitant ASMs during cenobamate titration are lacking. The clinical usefulness of such recommendations is indicated by the concomitant ASM dose adjustments performed by investigators in the cenobamate phase 3 safety study [7, 21]. To address this need, a modified Delphi panel-based consensus process, consistent with prior approaches [22,23,24], was initiated to develop recommendations for concomitant ASM dose adjustments. Through our use of cenobamate with patients during the phase 2 and phase 3 clinical program studies and in our clinical practices, we have developed an approach to dose adjustments of concomitant ASMs to maximize tolerability and safety during the addition of cenobamate to existing ASM therapy. Here we provide recommendations for reducing the dose of concomitant ASMs during titration of cenobamate. We distinguish between proactive (i.e., prior to patient-reported adverse effects) and reactive (i.e., following patient-reported adverse effects) dose adjustments, and we suggest amounts of dose reductions. These recommendations are intended for the treatment of an adult population (at least 18 years old).
Methods
Participants and Purpose
A modified Delphi panel-based consensus process that included a survey (round 1) and two virtual face-to-face meetings (round 2 and round 3) was used to develop recommendations for concomitant ASM dose reduction during cenobamate titration. The panelists were seven epileptologists experienced in the use of ASMs, including, specifically, cenobamate. The panelists were identified as experts in cenobamate treatment based on (1) their participation in the cenobamate clinical development program, (2) and/or their participation in cenobamate advisory board meetings (including discussion of concomitant ASM dose-adjustment data), and (3) and/or treatment of more than 50 patients with adjunctive cenobamate. The objective of the panelists was to provide expert opinion consensus recommendations on dose adjustments of concomitant ASMs when cenobamate treatment is added to patients’ existing ASM regimens to prevent or resolve adverse effects and allow cenobamate to reach the optimal dose, to achieve the maximal potential of improving seizure control. All panelists were aware of the objectives of the study, gave consent to participate in the meeting via email, and verbally agreed to participate in the development and publication of the recommendations. The panel was brought together by SK Life Science Inc., the company that manufactures cenobamate. SK Life Science, Inc. did not have any involvement in the direction of the panel’s recommendations or in the approval of the submitted manuscript. This work is based on previously conducted studies and the clinical expertise of the authors in treating patients with epilepsy. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported; therefore, institutional review board (IRB)/ethics approval was not required for the consensus recommendations. Any previously conducted clinical studies were all IRB-approved.
Survey
A survey was developed on the basis of the available evidence of investigator-initiated dose changes in concomitant ASMs during cenobamate treatment in the phase 3 open-label safety study [7, 21], cenobamate pharmacokinetics and drug interactions [25, 26], and cenobamate prescribing information [4]. Prior to the virtual face-to-face roundtable, each panelist responded to the survey questions: “Based on your experience with cenobamate, please note for each concomitant ASM the dose of cenobamate at which you would start decreasing the dose of the concomitant medication and by what increment (either percentage or milligrams) you would dose down.” The survey indicated that dosing downward could be done proactively (i.e., to avoid adverse effects) or reactively (i.e., when adverse effects emerge). Participants were asked to note if their suggestions differed depending on the dose level of the concomitant ASM (e.g., high-dose vs low-dose clobazam). The ASMs included in the survey were carbamazepine, clobazam, eslicarbazepine, lacosamide, lamotrigine, phenobarbital, phenytoin, topiramate, valproate, and cannabidiol.
Procedure
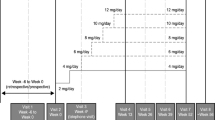
In round 1, panelists independently completed the survey and their responses were collated by a third party (MedVal Scientific Information Services, LLC). The responses were summarized and anonymized by the third party (MedVal) for presentation to the group of panelists in round 2. In round 2, the survey responses and the summarized concomitant ASM dose reduction data from the phase 3 open-label safety study [21] were discussed in a virtual face-to-face roundtable (convened April 15, 2021). Responses in rounds 2 and 3 were not anonymous, as panelists discussed and aligned on their expert opinion recommendations. During the roundtable meeting, panelists discussed the most likely tolerability issues with concomitant ASMs and practical strategies for dose adjustments to prevent or mitigate adverse effects, with a special focus on central nervous system (CNS) dose-related adverse effects, given that these are the most common adverse effects for cenobamate during its titration [7]. Panelists categorized the recommended dose reductions of individual concomitant ASMs as proactive or reactive, discussed when during cenobamate titration each proactive concomitant ASM dose reduction would be needed, and suggested the amount of dose reduction for each concomitant ASM.
Following the roundtable, the recommendations of the panelists were summarized in text as well as in tables and distributed to the panelists for their independent review and comment. In round 3, a second virtual face-to-face meeting (convened October 5, 2021) occurred to resolve areas where consensus had not been achieved. All of the recommendations reported here were accepted by all of the panelists. The resulting recommendations for dose adjustments for concomitant ASMs when cenobamate is added to the ASM regimen take into account concomitant ASM dose (standard dose or high dose) and provide suggestions for proactive and reactive dose adjustments for each concomitant ASM.
Results
Dose Adjustments of Concomitant ASMs
ASMs that have a predictable pharmacokinetic or pharmacodynamic interaction with cenobamate and/or have a long half-life (Table 1) [27] were identified for proactive dose reduction. These ASMs include clobazam, lacosamide, phenytoin, and phenobarbital (Table 2; Fig. 1). As detailed below, we adjust concomitant ASMs with pharmacokinetic interactions early in the titration of cenobamate (see Table 3 for titration schedule) [4, 7], while adjustments of concomitant ASMs with pharmacodynamic interactions often are made later in the titration. In patients receiving polypharmacy with multiple ASMs recommended for proactive reduction, clinicians will need to consider the entirety of the patient’s ASM regimen and use their clinical judgement when deciding which concomitant ASMs and in what order to decrease dosage because changes to one medication may also affect the pharmacokinetics or pharmacodynamics of other concomitant ASMs.
Clobazam
When clinically appropriate, proactive, early dose reduction of clobazam, for example, is recommended at the start of cenobamate 12.5 mg/day if the clobazam dose is 25 mg/day or higher, in order to prevent adverse effects (e.g., the CNS dose-related adverse effect of somnolence, described by patients as sleepiness/drowsiness) when cenobamate is added to the ASM regimen (Table 2). The interaction of cenobamate with clobazam often results in a marked increase in the N-desmethylclobazam active metabolite of clobazam (i.e., possibly increased 2–6 times) [4, 28]. This occurs because N-desmethylclobazam is metabolized by CYP2C19 and cenobamate inhibits CYP2C19 [4, 29]. The consequent increase in the N-desmethylclobazam active metabolite, with a half-life of approximately 72 h, may result in adverse effects, especially the CNS dose-related adverse effects of sleepiness/drowsiness. In our experience, if clobazam dose reductions are made reactively, when the patient reports an adverse effect, such as sleepiness, then the patient may continue to experience the adverse effect for a prolonged period of time (days to weeks) before the sleepiness abates because of the long half-lives of the clobazam active metabolite, N-desmethylclobazam, and of cenobamate [4, 28]. If there is concern that these interactions may be occurring, the patient’s blood levels of the N-desmethylclobazam metabolite, as well as clobazam, can be checked.
For these reasons, we recommend explaining to patients that proactive reduction in clobazam dose is helpful in preventing adverse effects, and when a patient is likely to develop adverse effects from the N-desmethylclobazam level, it is reasonable to recommend a dose reduction. If the patient is receiving a low dose of clobazam (i.e., 10–20 mg/day), the dose may be reduced by 5–10 mg/day at the 100 mg/day cenobamate dose. If the patient is receiving clobazam 25 mg/day, the dose may be reduced by 5 mg/day at the start of the cenobamate 12.5 mg/day starter pack. Clobazam reductions of 5 mg/day may be easier for patients to implement if provided with a supplementary bottle of clobazam. For patients receiving the upper end of standard clobazam doses, that is, 30–40 mg/day, a clobazam dose reduction of 10 mg/day is recommended with initiation of cenobamate treatment, a second clobazam dose reduction of 10 mg/day is recommended at the 50 mg/day dose of cenobamate, and a third clobazam dose reduction of 5–10 mg/day is recommended at the 100 mg/day dose of cenobamate. Additional reactive clobazam dose reductions may be needed for any patient who continues to experience adverse effects. For patients receiving a high dose of clobazam (greater than 40 mg/day), reduction of 10–20 mg/day (25%) starting at cenobamate initiation (12.5 mg/day cenobamate) is recommended, and again at 25 mg/day cenobamate.
Lacosamide
Patients may experience adverse effects (e.g., the CNS dose-related adverse effects of dizziness and ataxia) as cenobamate is added to an ASM regimen that includes lacosamide. This is most likely due to a pharmacodynamic interaction at the sodium channel because both lacosamide and cenobamate act through voltage-gated sodium channels, although with different effects on these channels [12]. Proactive dose reduction of lacosamide is recommended for patients receiving more than 400 mg/day of lacosamide (Table 2). The lacosamide dose may be reduced by 100 mg/day (25%) when the patient reaches the cenobamate dose of 50 mg/day and may again be reduced by 100 mg/day when the cenobamate dose of 100 mg/day is reached. For patients receiving lacosamide at no greater than 400 mg/day, lacosamide dose reduction of 100 mg/day can be reactive, in response to a patient’s report of an adverse effect. Treating physicians should be aware that the reactive reduction in lacosamide dose may be needed early on at low doses of cenobamate. Further dose reduction of lacosamide (e.g., 25% reduction every 2 weeks as needed) may be needed, depending on the patient’s experience with adverse effects.
Phenytoin
The pharmacokinetic interaction between phenytoin (partly metabolized by the CYP2C19 isoenzyme) and cenobamate (inhibits CYP2C19) results in an increase of the plasma concentration of phenytoin by a mean of 84% [4, 30]. At phenytoin blood levels of 15 µg/mL or higher, we recommend a proactive decrease in phenytoin by 100 mg/day (25–33%) when starting cenobamate 25 mg/day (third week of treatment) with an additional reduction of 100 mg/day (25–33%) at cenobamate 50 mg/day (fifth week of treatment) to prevent adverse effects such as CNS dose-related dizziness and ataxia (Table 2). In some patients with phenytoin blood levels of 15 µg/mL or higher, adverse effects may occur earlier at cenobamate 12.5 mg/day and reactive dose decrease of phenytoin should be made for these patients. In patients without a high phenytoin blood level (i.e., less than 15 µg/mL), the approximately 25–33% dose reduction can be reactive following a patient’s report of adverse effects (e.g., dizziness). In our experience, reactive dose adjustments are usually performed at a cenobamate dose of 50–100 mg/day. With occurrence of adverse effects during cenobamate titration, consider checking the level of phenytoin. Further reactive reductions of phenytoin may be necessary, especially with high therapeutic phenytoin levels.
Phenobarbital
The pharmacokinetic interaction of phenobarbital (partly metabolized by the CYP2C19 isoenzyme) and cenobamate (inhibits CYP2C19) results in an increase of the plasma concentration of phenobarbital by a mean of 37% [4]. We recommend a proactive reduction of phenobarbital prior to a patient’s report of an adverse effect (e.g., CNS dose-related sleepiness/drowsiness), with a 25% reduction in phenobarbital dose upon starting cenobamate 50 mg/day (Table 2). If the patient’s phenobarbital level is greater than 25 µg/mL, consider initiating the 25% dose reduction of phenobarbital earlier, at the start of cenobamate 25 mg/day. Further reductions of 25% can be made at 200 mg/day or reactively for the complaint of adverse effects as needed.
Adverse effects that occur during cenobamate titration with other concomitant ASMs can be managed reactively. Lowering the dose of these concomitant ASMs in response to patient reports of adverse effects often results in quick resolution of the adverse effects. We provide guidelines for dose adjustments of concomitant carbamazepine, lamotrigine, and cannabidiol as a result of their pharmacokinetic interactions with cenobamate, as well as for other ASMs in the next sections and Table 4.
Carbamazepine and Lamotrigine
The pharmacokinetic interactions of carbamazepine (metabolized by CYP3A4 and cenobamate moderately induces the CYP3A4 enzyme) and lamotrigine (unspecified interaction) with cenobamate result in reduced plasma concentrations of carbamazepine (by 23%) and lamotrigine (by 21–52%) [4]. The cenobamate package insert suggests an increase in the dose of these concomitant ASMs may be needed; however, in our experience, we do not usually increase the dose of these ASMs [21]. The reduced plasma concentrations of carbamazepine and lamotrigine during cenobamate titration have not usually negatively affected seizure control in our experience; the reductions are likely greatest as cenobamate titration reaches an effective seizure control dose level and these ASMs had previously failed to achieve seizure control in the patient. The reduced plasma concentrations may offset potential adverse effects (e.g., CNS dose-related double vision, dizziness, and sedation with carbamazepine; dizziness with lamotrigine) that can occur as a result of pharmacodynamic effects among sodium channel blockers. If the patient is receiving a high dose of carbamazepine (greater than 1200 mg/day) or lamotrigine (greater than 500 mg/day), we recommend proactive dose reduction of carbamazepine by 100–200 mg/day and lamotrigine by 100 mg/day when the cenobamate dose of 200 mg/day is reached (Table 4). It is possible for patients to experience adverse effects at standard doses of carbamazepine and lamotrigine, due to pharmacodynamic effects of the sodium channel blockers, so dose reduction of carbamazepine and lamotrigine can be done as needed in these patients.
Cannabidiol
Cannabidiol has complicated pharmacokinetics and pharmacodynamics, varied product bioavailability, and is a powerful inhibitor of CYP2C19 and CYP3A4 [28, 31, 32]. There has been relatively little data involving the interaction between cannabidiol and cenobamate because the former was not available during the cenobamate pivotal studies. Our experience has been that the pharmacokinetic interaction of Epidiolex (the prescription formulation of cannabidiol) with cenobamate may result in CNS dose-related adverse effects (e.g., sleepiness). This is especially true with concomitant use of clobazam [33]. We recommend reducing the cannabidiol dose by 25%, or as clinically indicated, in response to a patient’s report of adverse effects (Table 4). Further cannabidiol dose reductions may be needed if the patient continues to experience adverse effects. If the patient is also taking clobazam within the ASM regimen, further reductions in clobazam beyond what was recommended above may be needed as a result of the inhibition of CYP2C19 by cannabidiol and the consequent increase in N-desmethylclobazam [28].
Other Concomitant ASMs
Reactive lowering of the dose of other concomitant ASMs in response to patient reports of adverse effects is largely adequate for clinical management owing to typically rapid resolution of the adverse effects. However, if a patient is on a high dose of the concomitant ASM (e.g., topiramate, 400 mg/day or higher; zonisamide, 600 mg/day or higher; eslicarbazepine, 1600 mg/day or higher; levetiracetam, 4000 mg/day or higher; brivaracetam, 200 mg/day or higher), we recommend consideration of a 25% decrease in the dose of the concomitant ASM when the cenobamate dose reaches 150 mg/day (Table 4). These reductions of high-dose concomitant ASMs may help to avoid adverse effects and/or help to reduce the polypharmacy drug burden for patients.
We recommend reactive dose reduction by approximately 25% anytime adverse effects occur with concomitant ASMs. In our experience, the most commonly occurring CNS dose-related adverse effects by concomitant ASM include the following: valproate (fatigue), topiramate (word-finding difficulties, mathematical/concentration difficulties, fatigue), zonisamide (somnolence, word-finding difficulties), lamotrigine (dizziness), carbamazepine (dizziness), oxcarbazepine (dizziness), eslicarbazepine (dizziness), levetiracetam (somnolence, irritability), brivaracetam (fatigue, somnolence), perampanel (fatigue, dizziness), felbamate (fatigue), cannabidiol (drowsiness), gabapentin (somnolence, fatigue), and pregabalin (somnolence, fatigue). Concomitant ASM doses can continue to be decreased by approximately 25% every 2 weeks as needed for adverse effects, or more frequently as clinical symptoms dictate the need.
Discussion
Our goals of therapy for each patient are seizure freedom (i.e., zero seizures), no adverse effects from ASMs, and the fewest number of ASMs possible. The goal of fewer ASMs is to decrease the adverse effects and potential for long-term consequences of the ASM drug load. Moving toward complete removal of ASMs that are unnecessary and potentially detrimental with chronic exposure should be an important therapy goal. Minimizing adverse effects is key to effective ASM therapy, enhancing treatment adherence, reducing costs related to treatment of adverse effects, and attaining improved quality of life [34,35,36,37]. The addition of a new ASM to improve seizure control because of failure of the existing regimen can lead to increased adverse effects through drug–drug pharmacokinetic and pharmacodynamic interactions [2, 38]. Rather than discontinuing the newly added ASM, a first step to improve tolerability can be to lower the doses of concomitant ASMs that have failed to attain or improve seizure control. This allows for the continued titration of the new ASM with the potential for improved seizure control, an approach that is common within transitional polytherapy [3]. Such optimization of concomitant ASM treatment has been shown to reduce adverse effects without negatively affecting seizure control in patients with drug-refractory epilepsy [39]. Rather than being quickly completed, the optimization process may unfold gradually over 1 month or more to meet the goal of long-term improved patient quality of life.
During the addition of cenobamate to an existing ASM regimen in patients with focal seizures, we recommend proactively lowering the dose of clobazam, lacosamide, phenytoin, and phenobarbital to prevent onset of adverse effects, which most commonly include CNS dose-related adverse effects such as somnolence, dizziness, and ataxia. This recommendation is based on drug–drug interactions—in particular, with those ASMs metabolized by CYP2C19. Cenobamate inhibits CYP2C19, effectively leading to increased blood levels of the affected concomitant ASM, especially affecting the level of clobazam’s active metabolite N-desmethylclobazam, as well as levels of phenytoin and phenobarbital. In the case of concomitant clobazam, the long half-life of clobazam and slow metabolism of the active metabolite N-desmethylclobazam by CYP2C19 necessitate proactive, early dose reduction of clobazam to improve tolerability. Early proactive lowering of concomitant phenytoin (also due to CYP2C19), moderately early proactive lowering of concomitant phenobarbital (also due to CYP2C19), and moderately early proactive reduction of concomitant lacosamide (mostly due to pharmacodynamic reasons although there may be slight CYP2C19 involvement) are also recommended.
For other concomitant ASMs at standard doses during cenobamate addition to the ASM regimen, lowering the dose of the concomitant ASM can be done reactively following a patient’s report of an adverse effect. However, if the concomitant ASM is being used at a high dose, we encourage consideration of dose reduction when the cenobamate dose reaches 150 mg/day. Successfully reducing the dose of concomitant ASMs to prevent or mitigate adverse effects will allow patients to reach their full dose of cenobamate. When the cenobamate dose has reached 200 mg/day, we recommend that patients be evaluated for marked improvement of seizures or seizure freedom. For patients who achieve marked improvement or seizure freedom on cenobamate, further dose reductions and possible removal of concomitant ASMs should be considered to reduce polypharmacy.
Dose reductions and removal of concomitant ASMs were successfully demonstrated in the phase 3 open-label safety study of cenobamate, as shown in the published post hoc efficacy analysis [21]. Adjustments and reduction of concomitant ASMs were allowed as clinically needed but were not mandated in the phase 3 open-label study. Dose reductions were done to improve tolerability and/or to reduce polypharmacy. In the patients who continued cenobamate, the post hoc analysis showed that the clobazam dose was reduced an average of 25.3 mg/day (66% of the baseline dose), lacosamide dose was reduced 176.6 mg/day (35%), phenytoin was reduced an average of 251.6 mg/day (61%), and the phenobarbital average reduction was 40.8 mg/day (40%) [21]. The post hoc analysis also showed average dose reductions of valproate (30%), topiramate (22%), zonisamide (33%), lamotrigine (23%), carbamazepine (46%), oxcarbazepine (35%), eslicarbazepine (51%), levetiracetam (31%), brivaracetam (42%), perampanel (62%), felbamate (33%), gabapentin (75%), and pregabalin (51%), although some of these percentages (e.g., felbamate, gabapentin) are based on a small number of patients. Encouragingly, one quarter of the patients who continued to receive cenobamate treatment over a median treatment duration of 32.9 months had completely discontinued one or more concomitant ASM [21].
Conclusions
This expert opinion consensus panel provides recommendations for dose adjustments of concomitant ASMs when cenobamate is added to an existing ASM regimen that has failed to adequately control focal seizures. The primary goal of the recommended dose reductions of concomitant ASMs is to prevent or resolve adverse effects. This will allow cenobamate to reach the target dose, with potentially improved seizure control for patients. To meet this goal, proactive dose reductions are vital for the concomitant ASMs clobazam, lacosamide, phenytoin, and phenobarbital as a result of their drug–drug interactions with cenobamate and are recommended for other concomitant ASMs being taken at a high dose. Reactive lowering of the dose of concomitant ASMs in response to a patient’s report of adverse effects is sufficient for these other ASMs at a standard dose.
Change history
21 January 2023
A peer-reviewed video abstract and infographic were retrospectively added to this publication.
References
Garnett WR, St Louis EK, Henry TR, Bramley T. Transitional polytherapy: tricks of the trade for monotherapy to monotherapy AED conversions. Curr Neuropharmacol. 2009;7(2):83–95.
Patsalos PN, Perucca E. Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol. 2003;2(8):473–81.
St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009;7(2):96–105.
XCOPRI® (cenobamate tablets), for oral use, CV [prescribing information]. Paramus: SK Life Science, Inc.; 2022.
Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22.
Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48.
Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099–108.
Lattanzi S, Trinka E, Zaccara G, et al. Adjunctive cenobamate for focal-onset seizures in adults: a systematic review and meta-analysis. CNS Drugs. 2020;34(11):1105–20.
Zhang L, Wang J, Wang C. Efficacy and safety of cenobamate in patients with uncontrolled focal seizures: a meta-analysis. Acta Neurol Scand. 2021;144(1):58–66.
Keam SJ. Cenobamate: first approval. Drugs. 2020;80(1):73–8.
Löscher W, Sills GJ, White HS. The ups and downs of alkyl-carbamates in epilepsy therapy: how does cenobamate differ? Epilepsia. 2021;62(3):596–614.
Nakamura M, Cho JH, Shin HS, Jang IS. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. 2019;855:175–82.
Sharma R, Nakamura M, Neupane C, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879: 173117.
Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329–39.
Piredda SG, Woodhead JH, Swinyard EA. Effect of stimulus intensity on the profile of anticonvulsant activity of phenytoin, ethosuximide and valproate. J Pharmacol Exp Ther. 1985;232(3):741–5.
White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38(suppl 1):S9–17.
Anderson LL, Thompson CH, Hawkins NA, et al. Antiepileptic activity of preferential inhibitors of persistent sodium current. Epilepsia. 2014;55(8):1274–83.
Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007;7(1):15–22.
Vreugdenhil M, Hoogland G, van Veelen CW, Wadman WJ. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19(10):2769–78.
Roberti R, De Caro C, Iannone LF, Zaccara G, Lattanzi S, Russo E. Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs. 2021;35(6):609–18.
Rosenfeld WE, Abou-Khalil B, Aboumatar S, et al. Post-hoc analysis of a phase 3, multicenter, open-label study of cenobamate for treatment of uncontrolled focal seizures: effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021;62(12):3016–28.
Asadi-Pooya AA, Beniczky S, Rubboli G, Sperling MR, Rampp S, Perucca E. A pragmatic algorithm to select appropriate antiseizure medications in patients with epilepsy. Epilepsia. 2020;61(8):1668–77.
Nobili L, Beniczky S, Eriksson SH, et al. Expert opinion: managing sleep disturbances in people with epilepsy. Epilepsy Behav. 2021;124: 108341.
Kaufmann E, Bartolomei F, Boon P, et al. European expert opinion on ANT-DBS therapy for patients with drug-resistant epilepsy (a Delphi consensus). Seizure. 2020;81:201–9.
Vernillet L, Greene SA, Kamin M. Pharmacokinetics of cenobamate: results from single and multiple oral ascending-dose studies in healthy subjects. Clin Pharmacol Drug Dev. 2020;9(4):428–43.
Vernillet L, Kamin M. Drug–drug interactions between cenobamate and other antiepileptic drugs: results from phase I studies with carbamazepine, phenobarbital, phenytoin, and divalproex sodium [abstract]. Clin Pharmacol Ther. 2018;103(suppl S1):S91.
Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. 2014;16(4):409–31.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–51.
Giraud C, Treluyer JM, Rey E, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34(4):608–11.
Greene SA, Kwak C, Kamin M, et al. Effect of cenobamate on the single-dose pharmacokinetics of multiple cytochrome P450 probes using a cocktail approach in healthy subjects. Clin Transl Sci. 2021;15(4):899–911.
Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86–95.
Brodie MJ, Ben-Menachem E. Cannabinoids for epilepsy: what do we know and where do we go? Epilepsia. 2018;59(2):291–6.
Klein P, Tolbert D, Gidal BE. Drug–drug interactions and pharmacodynamics of concomitant clobazam and cannabidiol or stiripentol in refractory seizures. Epilepsy Behav. 2019;99: 106459.
Alsfouk BAA, Brodie MJ, Walters M, Kwan P, Chen Z. Tolerability of antiseizure medications in individuals with newly diagnosed epilepsy. JAMA Neurol. 2020;77(5):574–81.
Gilliam FG, Fessler AJ, Baker G, Vahle V, Carter J, Attarian H. Systematic screening allows reduction of adverse antiepileptic drug effects: a randomized trial. Neurology. 2004;62(1):23–7.
Luoni C, Bisulli F, Canevini MP, et al. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52(12):2181–91.
Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet. 2021;397(10282):1363–74.
Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246–55.
Dash D, Aggarwal V, Joshi R, Padma MV, Tripathi M. Effect of reduction of antiepileptic drugs in patients with drug-refractory epilepsy. Seizure. 2015;27:25–9.
Acknowledgements
Funding
The roundtable meeting was funded by SK Life Science, Inc. SK Life Science, Inc. also funded the journal’s Rapid Service Fee.
Medical Writing, Editorial, and Other Assistance
The authors thank Lynanne McGuire, PhD and Don Fallon, ELS, of MedVal Scientific Information Services, LLC (Princeton, NJ, USA), for medical writing and editorial assistance, which were funded by SK Life Science, Inc. MedVal also collated, summarized, and anonymized the initial survey results. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship. All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved.
Author Contributions
All authors contributed to the concept and design, the literature review, and the data analysis and interpretation. All authors critically revised the work and gave approval for its submission.
Disclosures
Michael C. Smith: Consultant/advisor: Azurity, Neurelis, SK Life Science, Inc. Pavel Klein: Consultant/advisor: Abbott, Aquestive, Arvelle, Eisai, Engage, Neurelis, SK Life Science, Inc., UCB Pharma; Speaker: Aquestive, Eisai, Neurelis, Sunovion, UCB Pharma; Research support: Eisai, Lundbeck; Member, Medical Advisory Board for Alliance-Stratus, Scientific Advisory Board for OB Pharma; CEO, PrevEp, LLC. Gregory L. Krauss: Consultant/advisor: Adamas, Eisai, Otsuka, Shire; Research support: Biogen, SK Life Science, Inc., UCB Pharma, Upsher-Smith. Samiya Rashid: Consultant/advisor: Neurelis, Neurocrine, SK Life Science, Inc.; Speaker: Greenwich Biosciences, Neurelis, SK Life Science, Inc. Lawrence G. Seiden: Consultant/advisor: Greenwich Biosciences, SK Life Science, Inc., UCB Pharma; Speaker: Greenwich Biosciences, SK Life Science, Inc., Sunovion, UCB Pharma. John M. Stern: Consultant/advisor: Eisai, Greenwich, LivaNova, SK Life Science, Inc., Neurelis, UCB Pharma. William E. Rosenfeld: Consultant/advisor: Arvelle; SK Life Science, Inc.; Speaker: SK Life Science, Inc., Sunovion, UCB Pharma; Research support: SK Life Science, Inc., UCB Pharma.
Compliance with Ethics Guidelines
This work is based on previously conducted studies and the clinical expertise of the authors in treating patients with epilepsy. No new clinical studies were performed by the authors. No patient-specific efficacy or safety data were reported; therefore, institutional review board (IRB)/ethics approval was not required for the consensus recommendations. Any previously conducted clinical studies were all IRB-approved. All panelists were aware of the objectives of the study, gave consent to participate in the meeting via email, and verbally agreed to participate in the development and publication of the recommendations.
Data Availability
All data generated or analyzed during this study are included in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Smith, M.C., Klein, P., Krauss, G.L. et al. Dose Adjustment of Concomitant Antiseizure Medications During Cenobamate Treatment: Expert Opinion Consensus Recommendations. Neurol Ther 11, 1705–1720 (2022). https://doi.org/10.1007/s40120-022-00400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00400-5