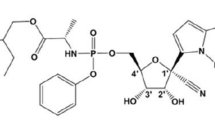
Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2, has resulted in acute respiratory distress, fatal systemic manifestations (extrapulmonary as well as pulmonary), and premature mortality among many patients. Therapy for COVID-19 has focused on the treatment of symptoms and of acute inflammation (cytokine storm) and the prevention of viral infection. Although the mechanism of COVID-19 is not fully understood, potential clinical targets have been identified for pharmacological, immunological, and vaccinal approaches.
Area covered
Pharmacological approaches including drug repositioning have been a priority for initial COVID-19 therapy due to the time-consuming nature of the vaccine development process. COVID-19 drugs have been shown to manage the antiviral infection cycle (cell entry and replication of proteins and genomic RNA) and anti-inflammation. In this review, we evaluated the interaction of current COVID-19 drugs with two ATP-binding cassette transporters [P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP)] and potential drug-drug interactions (DDIs) among COVID-19 drugs, especially those associated with P-gp and BCRP efflux transporters.
Expert opinion
Overall, understanding the pharmacodynamic/pharmacokinetic DDIs of COVID-19 drugs can be useful for pharmacological therapy in COVID-19 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pandemic coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020), has resulted in approximately 18.3 million infectees and 24,488 deaths in Korea and 541 million infectees/6.32 million deaths worldwide (Our World in Data, accessed on 22 June 2022) since the first outbreak occurred in Wuhan on 1 December 2019 (Ritchie et al 2021). The disease presents as acute respiratory distress (ARD) and can develop into fatal systemic manifestations (extrapulmonary as well as pulmonary) and premature mortality (Yan et al. 2020; Gupta et al. 2020). Clinical management of COVID-19 patients is focused on improving symptoms, supporting lung function, remedying acute inflammation (cytokine storm), and preventing infection (Yan et al. 2020).
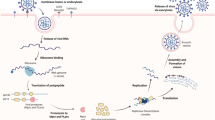
SARS-CoV-2, the novel coronavirus (65–125 nm in size), an enclosed and single-stranded positive-sense ribonucleic acid (RNA) (26–32 kbs) virus (Fig. 1a), belongs to the genus Beta-coronavirus, like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) (Zhang and Holmes 2020; Shereen et al. 2020). SARS-CoV-2 has a similar infection cycle to SARS-CoV and MERS-CoV (V’Kovski et al. 2021), as shown in Fig. 1b. The spike glycoprotein (S-gp) of the virus binds to the human cell membrane receptor angiotensin-converting enzyme 2 (ACE2) and cell entry is enabled by priming of the S-gp by the cellular transmembrane serine protease 2 (TMPRSS2) (Hoffman et al. 2020). Once the host cell is entered, the viral infection cycle proceeds with replication of the nucleocapsid (N), envelope (E), membrane (M), S-gp proteins, RNA-dependent RNA polymerase, 3-chymotrypsin-like protease (3CLpro), papain-like protease (PLpro), and the RNA genome; followed by assembly; and exocytosis of mature SARS-CoV-2. Viral infection induces pathology in the respiratory tissue, liver, intestine, and kidney, among other locations, through viral toxicity, endothelial cell damage, and immunological/inflammatory responses (Gupta et al 2020; Chen et al. 2021a). Although the mechanism of COVID-19 pathology is not fully understood, potential clinical targets have been explored for pharmacological, immunological, and molecular approaches (Chen et al. 2021a; Ghareeb et al. 2021) (Fig. 1b). The pharmacological approach to COVID-19 therapy covers most steps of the viral infection cycle (cell entry, replication of proteins and genomic RNA, and assembly), while immunological approaches (antibodies and vaccines) mainly focus on the initial step (cell entry) (Fig. 1b).
SARS-CoV-2 infection. a SARS-CoV-2 structure. S-gp, spike glycoprotein; M, membrane protein; E, envelope protein; N, nucleocapsid protein. b Viral infection cycle and molecular interventions are comprised of cell entry, host cell entry by endocytosis or membrane fusion; Replication (of viral proteins and genomic RNA); Assembly; Exocytosis (of mature SARS-CoV-2); Cytokine storm, SARS-CoV-2 infection induces pro-inflammatory pathways (IL-6, TNF, CXCL 10, and cytokines) (Chen et al. 2021a, c). Blue, orange, yellow, and green boxes represent interventions of vaccines, drugs, antibodies, and traditional Chinese medicine plus and vitamins, respectively. CP, convalescent plasma
Pharmacological intervention in COVID-19 therapy has investigated various possible candidates such as antiviral and anti-inflammatory drugs (Chen et al. 2021a; Ghareeb et al. 2021; Saleem et al. 2021). In pharmacological therapy, drug pharmacokinetic/pharmacodynamic issues are of great importance. P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which are apical membrane efflux transporters, are a crucial reason for these issues. These transporters are also one of the key factors in multidrug resistance (MDR) and affect the absorption, distribution, metabolism, and excretion of drugs in patients (Choi and Yu 2014). Many drugs have been identified as substrates and/or inhibitors of efflux transporters. Moreover, drug-drug interactions (DDIs), which lead to a reduction in a therapeutic effect or an increase in the adverse effect of a drug, should be considered in pharmacological therapy for patients with co-medications (Lazarou et al. 1998; Skvrce et al. 2011). In this review, we summarized therapeutic strategies for COVID-19 patients, especially those focused on pharmacotherapy and evaluated interactions of the current COVID-19 drugs with ATP-binding cassette (ABC) transporters P-gp and BCRP. We further examined the potential DDIs of COVID-19 drugs, especially those associated with P-gp and BCRP efflux transporters.
COVID-19
COVID-19 pathology
COVID-19 patients have various symptoms; fever, cough, labored breathing, muscle aches, dizziness, headache, sore throat, rhinorrhea, chest pain, diarrhea, abdominal pain, anorexia, nausea/vomiting, fever, and cough are the most common (Yan et al. 2020). Viral infection, morbidity, and mortality are influenced by intrinsic (sex, age, etc.) and extrinsic (lifestyle, underlying disease, etc.) factors. There are fewer SARS-CoV-2 infections among women than men (Conti and Younes 2020). ARD syndrome, an established COVID-19 pathology, is diagnosed at all ages, but the symptoms are more severe in adults than pediatric cases (Guan et al. 2020; Dudley and Lee 2020).
COVID-19 manifestations include not only pulmonary diseases such as pneumonia and ARD, but extrapulmonary indications including neurologic illnesses, ocular symptoms, thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, acute kidney injury, gastrointestinal symptoms, hepatocellular injury, hyperglycemia and ketosis, and dermatologic complications (Gupta et al. 2020; Moreira et al. 2021; Chen et al. 2021c; Mohamadi Yarijani and Najafi 2021).
Therapeutic strategies
Despite an incomplete understanding of the mechanisms of COVID-19 pathology, potential therapeutic strategies have been developed with various medical interventions (drugs, antibodies, vaccines, etc.) against fatal viral infection (Fig. 2).
Pharmacological therapy (drugs)
Since the initial COVID-19 outbreak, drug repositioning to target SARS-CoV-2 has included many potential drugs that are antiviral, anti-inflammatory, or have other functions (Chen et al. 2021a, b; Ghareeb et al. 2021). These COVID-19 drugs target most steps of the viral infection cycle (host cell entry, replication of proteins and genomic RNA, and assembly [Fig. 1b]) and are used for the treatment of acute inflammation (cytokine storm) and various clinical symptoms. More details about COVID-19 drugs will be presented in the next section.
Immunological therapy (neutralizing antibodies and convalescent plasma [CP])
The generation of monoclonal antibodies against vulnerable sites on viral surface proteins provides a path to neutralize the COVID-19 virus. The SARS-CoV specific antibody CR3022 can bind to the SARS-CoV-2 S-gp receptor-binding domain (RBD) (Tian et al. 2020; Wang et al. 2020). Many other monoclonal antibodies are also used for intervention with COVID-19 infection, especially those targeting viral protein-host receptor interaction (Fig. 1b); 47D11, m396, CR3014, S230, S304, S309, and S315 (Zhang and Liu 2020; Yuan et al. 2020; Chen et al. 2021a). In addition, recombinant human ACE2-Fc fusion protein (rc-ACE2-Ig) can block viral entry into the host cell (Kruse 2020). The recombinant fusion protein provides a potential treatment avenue for COVID-19 infection with cross-reactivity and high binding affinity to RBD of SARS-CoV and SARS-CoV-3 (Lei et al. 2020).
Convalescent patient plasma containing antibodies (immunoglobulins G, A, M, E, and D) to SARS-CoV-2 can be used for hospitalized COVID-19 patients (Roback and Guarner 2020). CP therapy has been recommended by the United State Food and Drug Administration (US FDA) for the treatment of certain hospitalized COVID-19 patients (21 April 2021) issued as an Emergency Use Authorization (National Institution of Health 2021).
Vaccinal therapy (COVID-19 vaccines)
Vaccines, the most effective control for acquiring immunity and preventing massive outbreaks of infectious diseases, have been developed against SARS-CoV-2a using inactivated virus, mRNA, viral vector, and other forms: SinoVac (Corona Vac, inactivated virus, 50% efficacy, China), Pfizer-BioNTech (BNT162B1, mRNA, 95% efficacy, Germany), Moderna (mRNA1273, mRNA, 94% efficacy, USA), Janssen COVID-19 Vaccine (Ad26COVS1, viral vector, 73% efficacy, The Netherlands), AstraZeneca (ChAdOx1 nCoV-19, viral vector, 70% efficacy, England), Sputnik V (Gram Covid Vac, viral vector, 91% efficacy, Russia), Novavax (NVX-CoV2373, recombinant protein, 96% efficacy, USA), Sinopharm (BBIBP-CorV, inactivated virus, 79% efficacy, China), Covaxin (BBV152, inactivated virus, 81% efficacy, India) and CanSino (Ad5-nCoV, viral vector, 66% efficacy, China) (Saleem et al. 2021).
The mRNA vaccine system that translated the S-pg protein of SARS-CoV-2 after injection was first used in the COVID-19 pandemic (Pardi et al. 2018). Studies of the vaccines/vaccinations are continuing, and current COVID-19 vaccines such as those developed by Moderna, Pfizer-BioNTech, and AstraZeneca have shown poor safety data in aged and sensitive individuals (Soiza et al. 2021).
Other therapeutic approaches (medicinal plants and vitamins)
Many studies have provided evidence that medicinal plants and phytocomponents, consisting of traditional Chinese, Indian Ayurvedic, and homeopathic medicines are effective in the prophylaxis and treatment of COVID-19 (Reche et al. 2020; Tallei et al. 2020; Chu et al. 2021; Saleem et al. 2021). The natural compounds of flavonoids (quercetin, baicalin, luteolin, hesperidin, kaempferol, and curcumin), phenols (glycyrrhizic acid and lignan), quinones (emodin and skonin), alkaloids (matrine), and diterpenoids (andrographolide) have potent activity for inhibiting the viral infection cycle and for improving clinical symptoms (Fig. 1b) (Saleem et al. 2021). However, the therapeutic effects of Ayurvedic and homeopathic treatments were exhibited in asymptomatic COVID-19 patients rather than symptomatic patients (Reche et al. 2020).
Several studies suggested that vitamins, used for antioxidative and immune responses, can also target SARS-CoV-2 infection (Fig. 1b) (Ghareeb et al. 2021). Vitamin D reduces pro-inflammatory cytokines and increases anti-inflammatory cytokines, while its deficiency increases the severity of ARD (Grant et al. 2020). Vitamin C, vitamin B3, and vitamin B12 also help to improve the symptoms of COVID patients (Rosa and Santos 2020; Kandeel and Al-Nazawi 2020).
COVID-19 drugs
Due to the time-consuming nature of vaccine development, repurposed drugs have been a top priority for initial COVID-19 treatments (Table 1). Antiviral (affecting the steps of the viral infection cycle) and anti-inflammatory activities are major targets for COVID-19 drugs. Various therapeutic drugs, their classical uses, and their target sites and actions in SARS-CoV-2 infection are illustrated in Fig. 1b and Table 1.
Antiviral drugs
Many potent COVID-19 drugs focus on antiviral activity.
Camostat, chloroquine, EK1C4, nafamostat, umifenovir, nelfinavir, quinacrine dihydrochloride and hydroxychloroquine prevent host cell entry of SARS-CoV-2 by blocking the interaction of the viral protein with the host cell receptor and blocking membrane fusion or endocytosis (Chen et al. 2021a, b; Ghareeb et al. 2021). Cyclosporin, disulfiram, favipiravir, lopinavir, remdesivir, ribavirin and ritonavir inhibit viral replication, including replication of viral protein and genomic RNA (Chen et al. 2021a, b; Ghareeb et al. 2021). Chloroquine and cyclosporin also target pro-inflammatory pathways induced by SARS-CoV-2 infection (Saleem et al. 2021; Chen et al. 2021a).
Chloroquine and hydroxychloroquine, classical antimalaria drugs, have potential actions for inhibition of virus post-translational modification (protein glycosylation), production of non-functional ACE2, as well as inhibition of viral cell entry (Oscanoa et al. 2020; Chen et al. 2021a). Moreover, they can be used not only as antivirals, but also as anti-inflammatories and anticoagulants (Oscanoa et al. 2020). These drugs are widely used for COVID-19 patients; however, their side effects include gastrointestinal (GI) disturbance, cardiac toxicity, hyperglycemia, and neuropsychiatric toxicity (Saleem et al. 2021).
Lopinavir and ritonavir, an amide analogue and a thiazole analogue, respectively, are protease inhibitors and antiviral drugs for the treatment and prevention of HIV/AIDS (Chen et al. 2021b; Ghareeb et al. 2021). These drugs are used in combination for HIV/AIDS treatment (Chandwani and Shuter 2008; Cattaneo et al. 2020; Biswas 2021). The combination drug has been broadly used for COVID-19 therapy but has induced multiple adverse effects such as GI disturbance, cardiac conduction abnormality, and hepatotoxicity (Ghareeb et al. 2021; Saleem et al. 2021).
Remdesivir, a broad-spectrum antiviral drug and an adenosine analogue, is used for COVID-19 therapy in adults and pediatric (> 12 years old and weighing > 40 kg) patients and has been approved by the US FDA (Saleem et al. 2021). The drug inhibits viral RNA replication (RNA polymerase function), but transaminase elevation and kidney damage have been reported as the adverse effects of this drug (Stebbing et al. 2020).
Among the above 15 drugs, chloroquine, cyclosporin A, disulfiram, hydroxychloroquine, lopinavir, nelfinavir, remdesivir, ribavirin and ritonavir have been approved by the US FDA for other uses, while camostat and nafamostat were designated orphan drugs by the US FDA (Table 1). Only remdesivir (Veklury) has been approved by US FDA for COVID-19 treatment (the first approved drug on 22 Oct. 2020), while ritovanir packaged with nirmatrevir (Palxlovid) has an emergency use authorization (EUA). With the exception of EK1C4, nelfinavir, and quinacrine dihydrochloride, the others are under the clinical trials (Table 1).
Anti-inflammatory drugs
Due to the acute inflammation (cytokine storm) generated by viral infection, anti-inflammatory drugs are also used for COVID-19 therapy. Baricitinib, dexamethasone, ruxolitinib and tocilizumab are used to inhibit pro-inflammatory pathways (Chen et al. 2021a; Ghareeb et al. 2021). Indomethacin and mycophenolic acid target SARS-CoV-2 replication and cell entry, respectively, and have potential anti-inflammatory effects as well (Chen et al. 2021a; Ghareeb et al. 2021).
Tocilizumab, a recombinant human monoclonal anti-interlukine-6 antibody, is used in COVID-19 patients (Luo et al. 2020). This antibody effectively inhibits cytokine storm and stabilizes the condition of severe or urgent patients (Ghareeb et al. 2021; Saleem et al. 2021). GI disturbance, skin/subcutaneous infections, and altered liver enzymes have been reported as adverse effects (Luo et al. 2020).
All the drugs mentioned above are US FDA-approved for other purposes, while baricitinb (Olumiant) has been approved specifically for COVID-19 treatment (10 May 2022) and tocilizumab has EUA (Table 1). Dexamethasone and ruxolitinib are under clinical trials (Table 1).
Anticancer drugs and antibiotics
Imatinib (an anticancer drug) and teicoplanin (an antibiotic) are also potent COVID-19 drugs. Both drugs are thought to inhibit COVID-19 infection by interfering with host cell entry of the virus in vitro (Chen et al. 2021a; Ghareeb et al. 2021).
Imatinib is a US FDA-approved drug and under the recruiting state for COVID-19 trials (Table 1). Teicoplanin (Targocid) was approved by the European Medicine Agency (30 Jul. 1988) and is available in many countries around the world (Vimberg 2021), but has not been approved by the US FDA.
Traditional Chinese medicines
Traditional Chinese medicines have been used for COVID-19 therapy from the early stage of the COVID-19 outbreak in China. COVID-19 medicines were developed based on the medicines used for the treatment of pandemic SARS, MERS and H1N1 influenza (Chu et al. 2021). Table 1 shows several Chinese medicines used to treat COVID-19 that were clinically trialed in China. These medicines are composed of various herb plants such as Isatis tinctoria L. (Banlagen), Lonicera japonica Thunb. (**yinhua), Paeonia lactiflora Pall. (Chishao), and Ephedia sinica Stapf (Ma huang); GI disturbance (nausea or diarrhea), abnormal liver function, heart palpitation and skin problems (itchiness or rash) have been reported as adverse effects (Chu et al. 2021).
Chinese medicines, which generally have few side effects, are being used clinically as well as in clinical trials in China (Table 1) (Chu et al. 2021).
P-gp and BCRP substrate and inhibitor drugs
ABC transporters, one of the largest transporter families, act as active efflux pumps related to pathogenic conditions as well as in normal physiological roles in living organisms (Trowitzsch and Tampe 2018; Liu 2019). P-gp (ABC subfamily B member 1 [ABCB1]; Fig. 3a) and BCRP (ABCG2; Fig. 3b), are widely expressed in important tissues such as the intestinal epithelium, the biliary canaliculi of hepatocytes, and the proximal tubules of the kidney, and are well-characterized MDR transporters among ABC transporters (Juliano and Ling 1976; Doyle et al. 1998; Robey et al. 2018; Dei et al. 2019). P-gp and BCRP are different in size and structure but are similar in their ATP-dependent function (dimerization) (Fig. 3c) (Robey et al. 2018; Dean et al. 2001). Numerous pharmacological agents are substrates and/or inhibitors of the transporters, and many drugs including antiviral and anticancer drugs are common substrates and/or inhibitors (Durmus et al. 2015; Chen et al. 2016). Efflux transporters affect drug pharmacokinetic parameters as well as MDR, and the US FDA has published guidelines for evaluation of transporter-mediated drug interactions (US FDA 2020).
ABC transporters. a P-gp consists of 12 transmembrane domains (TMDs) and 2 nucleotide-binding domains (NBDs) (approximately 150 kDa in size). b BCRP consists of 6 TMDs and 1 NBD as a monomer (approximately 72 kDa). c Drug efflux function of the transporters. ① A drug (substrate/inhibitor [green triangle] of the transporters) permeates into the cell membrane and binds to the affinity site in the TMD of the transporters. ② ATP binds to NBD and then it is hydrolyzed by ATP-hydrolyses. ③ The inward structure of the transporters changes to outward, and transporters efflux the drug to the extracellular region. The green arrows indicate the path of the drug
It is important understand the association of current COVID-19 drugs, which are administered by an oral route (except remdesivir, nafamostat, teicoplanin and tocilizumab, which are administered via an intravenous route), with ABC transporters (P-gp and BCRP) located in the GI tract (Dei et al. 2019). We evaluated interactions of the COVID-19 drugs in Table 1 (except the traditional Chinese medicines) with P-gp and BCRP (Table 2). Thomas et al. predicted potential binding of repurposed COVID-19 drugs to P-gp via in silico studies, induced-fit docking and binding free energy calculation (Thomas et al. 2021). Remdesivir (− 2,687.21 (net binding energy)), baricitinib (− 2,471.25), indomethacin (− 2,441.04), arbidol (− 2,410.27), hydroxychloroquine (− 2,148.34), ritonavir (− 2,095.04), chloroquine (− 1,965.43), lopinavir (− 1,735.14), ruxolitinib (− 1,647.90) and favipiravir (− 636.90) can be substrates or inhibitor of P-gp (Thomas et al. 2021). According to a US FDA report, ‘Drug Development and Drug Interaction’, cyclosporin A and ritonavir are in vitro inhibitors for P-gp (26 Sep. 2016), lopinavir and ritonavir are clinical inhibitors for P-gp (26 Sep. 2016) and cyclosporin A is a clinical inhibitor for BCRP (26 Sep. 2016). Moreover, many in vitro studies (apical to basolateral penetration assay, substrate efflux/accumulation assays, and ATP hydrolysis assay) have demonstrated that potential COVID-19 drugs are substrates and/or inhibitors of P-gp and/or BCRP (Table 2) (Fricker et al. 1996; Tiberghien et al. 2000; Romiti et al. 2002; Vishuvardhan et al. 2003; Burger et al. 2004; Gupta et al. 2004; Houghton et al. 2004; Li et al. 2004; Sauna et al. 2004; Anglicheau et al. 2006; Elahian et al. 2010; Doshe et al. 2010; Rijpma et al. 2014; Elbert et al. 2016; Nayak et al. 2020; Buks et al. 2021; Telbisz et al. 2021; Weiss et al. 2021). Furthermore, in vivo studies (pharmacokinetic study, brain distribution study) also support that these drugs interact with P-gp and/or BCRP transporters as substrates and/or inhibitors of transporters (Table 2) (Schinkel et al. 1995; Kim et al. 1998; Breedveld et al. 2005; Huang et al. 2006; Wang et al. 2008). The oral bioavailability of nelfinavir (Kim et al. 1998) and quinacrine (Huang et al. 2006) increased approximately fivefold (a dose of 6 mg/kg) and 49-fold (multiple doses of 10 mg/kg/day for 7 days) in P-gp-knockout (Mdr1a−/−) mice (vs. wild-type mice), respectively. In the same animal model, brain penetration of cyclosporin A (Schinkel et al. 1995), nelfinavir (Kim et al. 1998), quinacrine (Huang et al. 2006) and dexamethasone (Schinkel et al. 1995) also increased 17-fold (a dose of 1 mg/kg), 36-fold (a dose of 3 mg/kg), 6–ninefold (a dose of 2 mg/kg) and 2.5-fold (a dose of 0.2 mg/kg), respectively, after intravenous injection. Mycophenolic acid levels in the plasma and brain after oral administration of mycophenolate mofetil (60 mg/kg), a prodrug of mycophenolic acid, were markedly increased in Mdr1a/1b−/− mice compared with wild-type mice (Wang et al. 2008). The area under the curve (AUC) of plasma imatinib after IV administration (12.5 mg/kg) was higher in Bcrp1 knockout mice and Mdr1a/1b knockout mice than in wild-type mice, and clearance of the drug significantly decreased 1.6-fold in Bcrp1 knockout mice and 1.25-fold in Mdr1a/1b knockout (vs. wild type), respectively. In addition, co-administration of pantoprazole (40 mg/kg) or elacridar (100 mg/kg), P-gp and BCRP inhibitors, significantly decreased imatinib clearance by 1.7-fold and 1.5-fold, respectively, in wild-type mice. Moreover, the brain penetration of imatinib following IV administration was also increased 2.5-fold in Bcrp1 knockout mice (vs. the wild type), and co-administration with inhibitors also significantly increased brain penetration of the drug (1.8-fold by pantoprazole and 4.2-fold by elacridar) (Breedveld et al. 2005). Table 3 presents pharmacokinetic information for COVID-19 drugs that possess potential or reported interactions with P-gp and BCRP transporters.
Most drugs (19 of 23) showed interactions or potential interactions with P-gp (predominantly) and BCRP as substrates and/or inhibitors (Table 2). Among the drugs interacting with these efflux transporters, chloroquine, cyclosporin A, lopinavir, nelfinavir, quinacrine dihydrochloride, remdesivir, ritonavir, baricitinib, dexamethasone, ruxolitinib, and imatinib (11 drugs) can bind to both P-gp and BCRP concomitantly. Remdesivir, baricitinib and ritonavir, which are US FDA-approved and FDA EUA drugs for COVID-19 treatment, require to pay a care this point when the drugs are used to treat the patients, especially, who take multiple medications. Indeed, the Liverpool Drug Interaction Group (COVID-19 Drug Interaction) recommended that ripamficin (an antibiotic), a well-known P-gp inducer, should not be co-administered with remdesivir because the strong induction of P-gp potentially reduces remdesivir level (Yang 2020). Other drugs under clinical trials (except nelfinavir and quinacrine dihydrochloride (preclinic)) also need to be considered in this light. These dual-interaction drugs control the host cell entry of SARS-CoV-2 (chloroquine, nelfinavir, quinacrine dihydrochloride, and imatinib), viral replication (cyclosporine, lopinavir, remdesivir, ritonavir and ivermectin), and acute inflammation (baricitinib, dexamethasone and ruxolitinib) in COVID-19. Camostat, EK1C4, nafamostat, ribavirin, and teicoplanin might not interact with efflux transporters. In vitro efflux assays showed that camostat was not an inhibitor of P-gp or BCRP (Weiss et al. 2021), and nafamostat was not a substrate of P-gp (Li et al. 2004) (Table 2).
In addition, P-gp polymorphisms have been associated with variability in the pharmacokinetics of lopinavir and ritonavir (Rakhmanina et al. 2011; Biswas 2021). The ABCB1 C3435T single nucleotide polymorphism (SNP), one of 50 SNPs in the ABCB1 gene and one that is highly prevalent in different ethnic groups [Europe (78%), America (67%), Asia (63.5%), Africa (41.4%)], might affect the pharmacodynamics and pharmacokinetics of lopinavir and ritonavir. High expression or low expression of this SNP can lead to therapeutic failure or adverse risk of the COVID-19 drugs (Biswas 2021).
Drug-drug interactions
DDIs are another important pharmacological issue. DDIs can decrease the therapeutic effect of drug and/or increase minor or serious unexpected adverse drug reactions (Lazarou et al. 1998; Skvrce et al. 2011). COVID-19 patients, especially those who are elderly or have co-morbidities such as cancer, cardiovascular, lung, and/or kidney diseases, take multiple drugs to remedy various COVID-19 symptoms and other diseases. Therefore, COVID-19 patients with polypharmacy need to be carefully monitored.
DDIs of COVID-19 drugs with other drugs used for managing other diseases have been reported (Cattaneo et al. 2020; Baburaj et al. 2021; Thomas et al. 2021). Lopinavir/ritonavir, chloroquine, hydroxychloroquine, and dexamethasone have potential DDIs with lung cancer medications. Among these COVID-19 drugs, lopinavir/ritonavir have potentially the most severe DDIs with many anticancer drugs (afatinib, brigatinib, cabozantinib, certinib, crizotinib, cyclophosphamide, dabrafenib, docetaxel, doxorubicin, entrectinib, erlotinib, everolimus, irinotecan, larotrectinib, lorlatinib, lurbinectedin, osimertinib, selpercatinib, topotecan, vandetanib, vemurafenib, vinblastine, and vincristine) and with antitubercular drugs (rifampin, clarithromycin, levofloxacin, moxifloxacin, ofloxacin, clofazimine, delamanid, and bedaquiline) (Baburaj et al. 2021; Thomas et al. 2021). Chloroquine, hydroxychloroquine, dexamethasone, and ruxolitinib were also identified to have potential DDIs with several antitubercular drugs (rifampin, clarithromycin, levofloxacin, moxifloxacin, ofloxacin, clofazimine, delamanid and bedaquiline) (Thomas et al. 2021). Ribavirin, remdesivir, and tocilizumab might not induce DDIs with lung cancer drugs (Baburaj et al. 2021), and remdesivir, baricitinib and tocilizumab might not be related to DDIs with antitubercular drugs (Thomas et al. 2021).
Hu et al. investigated the pharmacokinetic interactions between lopinavir/ritonavir and arbidol in an animal study. An oral co-administration of lopinavir (50 mg/kg)/ritonavir (12.5 mg/kg) and arbidol (25 mg/kg) significantly enhanced the AUC of arbidol (more than twofold) and the Cmax of lopinavir in rats (Hu et al. 2021). In northern Italy, the risk of potential DDIs was analyzed in hospitalized patients with COVID-19 (n = 502, mean age 61 ± 16 years (range 15–99 years) between 21 Feb. and 30 Apr. 2020) who had co-morbidities including cardiovascular, metabolic, lung, oncological, kidney, immune, and chronic infectious diseases (Cattaneo et al. 2020). Among the patients, 68% were exposed to at least one potential DDI, and 55% of patients were exposed to at least one potentially severe DDI. DDIs of co-medications, particularly with lopinavir/ritonavir, caused various and severe adverse events, and the combination of lopinavir/ritonavir and hydroxychloroquine induced a number of potentially severe DDIs resulting in a high risk of cardiotoxicity (Cattaneo et al. 2020). Remdesivir and tocilizumab were not observed to induce any potentially severe DDIs in hospitalized patients with COVID-19.
Remdesivir and tocilizumab could be safer with regard to pharmacodynamic DDIs when given with various drugs to treat co-morbidities than other COVID-19 drugs, whereas lopinavir, ritonavir, chloroquine, and hydroxychloroquine are assumed to lead to potentially severe pharmacodynamic DDIs (Cattaneo et al. 2020; Saleh et al. 2020; Baburaj et al. 2021; Biswas 2021; Thomas et al. 2021). COVID-19 drugs interacting with the P-gp and BCRP transporters (Table 2 and 3) also lead to pharmacokinetic DDIs. These pharmacokinetic DDIs, altering the absorption, distribution, metabolism, and excretion of medications, are more common than pharmacodynamic DDIs (Peng et al. 2021).
We examined the potentially severe efflux transporter-mediated DDIs between COVID-19 drugs by consulting the website of ‘Drugs.com (https://www.drugs.com/drug_interactions.html)’ and ‘COVID-19 Drug Interactions (http://www.covid19-druginteractions.org/checker)’, ‘drug prescribing information (downloaded from FDA website)’ as well as literature data. Major DDIs were monitored when the combination treatment of chloroquine-hydroxychloroquine, chloroquine-lopinavir, chloroquine-remdesivir, cyclosporin A-nelfinavir, cyclosporin A-ritonavir, cyclosporin A-baricitinib, disulfiram-ritonavir, hydroxychloroquine-lopinavir, hydroxychloroquine-remdesivir, lopinavir-nelfinavir, lopinavir-ritonavir, nelfinavir-ruxolotinib, ritonavir-ruxolitinib, baricitinib-dexamethasone, baricitinib-mycophenolic acid, baricitinib-ruxolitinib, baricitinib-tocilizumab, baricitinib-imatinib and ruxolotinib-tocilizumab were administered (Table 4). Combinations exhibiting major DDIs were not recommended. Although many other cases showed moderate DDIs, these combinations were also not recommended. On the other hand, the combination of cyclosporin A-lopinavir, lopinavir-imatinib, ritonavir-indomethacin and ruxolitinib-imatinib appeared to lead to only minor DDIs, which has minimal clinical significance and a low risk (Drugs.com/Drug Interactions Checker).
In addition, the combination of favipiravir-chloroquine, favipiravir-cyclosporin A, favipiravir-hydroxychloroquine, favipiravir-lopinavir/ritonavir, favipiravir-remdesivir, favipiravir-dexamethasone, favipiravir-mycophenolic acid, favipiravir-ruxolitinib, favipiravir-tocilizumab, favipiravir-imatinib, remdesivir-lopinavir, remdesivir-baricitinib, remdesivir-dexamethasone, remdesivir-mycophenolic acid, remdesivir-ruxolitinib, baricitinib-lopinavir/ritonavir, baricitinib-mycophenolic acid, dexamethasone-mycophenolic acid, dexamethasone-ruxolitinib, dexamethasone-tocilizumab, ruxolitinib-mycophenolic acid, tocilizumab-lopinavir/ritonavir and tocilizumab-mycophenolic acid are not expected to lead to DDIs (COVID-19 Drug Interactions/Interaction Checkers and Drugs.com/Drug Interactions Checker). Drugs with minor or no DDIs can be considered in treatment with an adjusted regimen. Among the above DDIs, the combinations (bold and underline) of US FDA-approved and EUA drugs (remdesivir, baricitinib, ritonavir and tocilizumab) can be considered for the clinical trial. The possible or clinical mechanisms of DDIs between COVID-19 drugs are mentioned in Table 4. The mechanism of drug interaction has been mainly interpreted based on the activity of cytochrome P450 enzymes. However, the results from in silico, in vitro and non-clinical approaches to interactions with P-gp and BCRP provide the strong potential for the correlation of DDIs between COVID-19 drugs (Table 4, except tocilitinib) with these transporters (Schinkel et al. 1995; Fricker et al. 1996; Kim et al. 1998; Wang et al. 2008; Romiti et al. 2002; Vishuvardhan et al. 2003; Burger et al. 2004; Gupta et al. 2004; Houghton et al. 2004; Li et al. 2004; Sauna et al. 2004; Breedveld et al.2005; Huang et al. 2006; Anglicheau et al. 2006; Elahian et al. 2010; Doshe et al. 2010; Rijpma et al. 2014; Elbert et al. 2016; Tiberghien et al. 2000; Nayak et al. 2020; Buks et al. 2021; Telbisz et al. 2021; Weiss et al. 2021; Thomas et al. 2021). Overall, a better understanding of the pharmacokinetic and pharmacodynamic DDIs of COVID-19 drugs would be useful for managing pharmacological therapy in COVID-19 patients.
Conclusions
COVID-19, which is caused by SARS-CoV-2, results in ARD, fatal systemic manifestations (extrapulmonary as well as pulmonary), and premature mortality in patients. Clinical approaches to COVID-19 are the treatment of various symptoms and acute inflammation (cytokine storm) and the prevention of viral infection. Among various therapeutic strategies including immunological and vaccinal approaches, pharmacological therapy targets most steps of the SARS-CoV-2 infection cycle (cell entry, replication of proteins and genomic RNA) and inflammation (cytokine storm). Drug repositioning has been a priority due to the time-consuming nature of vaccine development for initial COVID-19 therapy.
We evaluated the interaction of current COVID-19 drugs with ABC transporters (P-gp and BCRP) and examined the potential DDIs of COVID-19 drugs, especially those associated with the efflux transporters. Most drugs interact with these transporters. Chloroquine, cyclosporin A, lopinavir, nelfinavir, quinacrine dihydrochloride, remdesivir, ritonavir, baricitinib, dexamethasone, ruxolitinib, and imatinib can bind to both efflux transporters, while EK1C4, ribavirin, tocilizumab and teicoplanin might not interact with any transporters. P-gp and BCRP-mediated COVID-19 drugs can lead to pharmacokinetic DDIs. Favipiravir may be safer than other COVID-19 drugs in terms of pharmacodynamic DDIs with various drugs to treat co-morbidities. Drug combinations of minor or no DDIs may be considered in clinical trials with an adjusted regimen. Moreover, combination therapy of remdesivir, baricitinib, ritonavir and tocilizumab (US FDA-approved and EUA drugs) with drugs which have little or no DDIs is expected to be widely used in the treatment of COVID-19, including favipiravir-remdesivir, favipiravir-tocilizumab, remdesivir-baricitinib, remdesivir-dexamethasone, remdesivir-mycophenolic acid, remdesivir-ruxolitinib, baricitinib-lopinavir/ritonavir, baricitinib-mycophenolic acid, dexamethasone-tocilizumab, tocilizumab-lopinavir/ritonavir and tocilizumab-mycophenolic acid. A better understanding of COVID-19 drugs and their potential pharmacokinetic and/or pharmacodynamic DDIs will be helpful in the management of pharmacological therapy for COVID-19.
References
Anglicheau D, Pallet N, Rabant M, Marquet P, Cassinat B, Me´ria P, Beaune P, Legendre C, Thervet E (2006) Role of P-glycoprotein in cyclosporine cytotoxicity in the cyclosporine–sirolimus interaction. Kidney Int 70:1019–1025
Answer MK, Iqbal M, Ahmed MM, Aldawsari MF, Ansari MN, Ezzeldin E, Khalil NY, Ali RE (2021) Improving the solubilization and bioavailability of arbidol hydrochloride by the preparation of binary and ternary β-chclodextrin complexes with poloxamer 188. Pharmaceutics 14:411
Baburaj G, Thomas L, Rao M (2021) Potential drug interactions of repurposed COVID-19 drugs with lung cancer pharmacotherapies. Arch Med Res 52:261–269
Biswas M (2021) Predictive association of ABCB1 C3435T genetic polymorphism with the efficacy or safety of lopinavir and ritonavir in COVID-19 patients. Pharmacogenomics 22:375–381
Bow D, Liu J, Kavetskaia O, Menon R, de Morais SM, Nijsen M, Sydor J, Fischer V, Shebley M (2014) A mechanistic non-clinical assessment of drug-drug interactions (metabolism and transporters) with the hepatitis C virus (HCV) regimen: ABT-450/r, ombitasvir and dasabuvir. AASLD/EASL Special Conference on Hepatitis C. New York, NY, September 12–13
Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JHM (2005) The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): Implication for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65:2577–2582
Buks R, Brusson M, Cochet S, Galochkina T, Cassinat B, Nemazanyy I, Peyrard T, Kiladjian J-J, de Brevern AG, Azouzi S, Nemer WE (2021) ABCG2 is overexpressed on red blood cells in Ph-negative myeloproliferative neoplasms and potentiates Ruxolitinib-induced apoptosis. Int J Mol Sci 22:3530
Burger H, van Tol H, Boersma AWM, Brok M, Wiemer EAC, Stoter G, Nooter K (2004) Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood 104:2940–2942
Cattaneo D, Pasina L, Maggioni AP, Giacomelli A, Oreni L, Covizzi A, Bradanini L, Schiuma M, Antinori S, Ridolfo A, Gervasoni C (2020) Drug–drug interactions and prescription appropriateness in patients with COVID-19: a retrospective analysis from a reference hospital in northern Italy. Drugs Aging 37:925–933
Chandwani A, Shuter J (2008) Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag 4:1023–1033
Chen KG, Park K, Spence JR (2021a) Studying SARS-CoV-2 infectivity and therapeutic responses with complex organoids. Nat Cell Biol 23:822–833
Chen R, Wang T, Song J, Pu D, He D, Li J, Yang J, Li K, Zhong C, Zhang J (2021b) Antiviral drug delivery system for enhanced bioactivity, better metabolism and pharmacokinetic characteristics. Int J Nanomedicine 16:4959–4984
Chen ZR, Liu J, Liao ZG, Zhou J, Peng HW, Gong F, Hu JF, Zhou Y (2021c) COVID-19 and gastroenteric manifestations. World J Clin Cases 9:4990–4997
Chen Z, Shi T, Zhang L, Zhu P, Deng M, Huang C, Hu T, Jiang L, Li J (2016) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett 370:153–164
Choi YH, Yu A-M (2014) AC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des 20:793–807
Chu L, Huang F, Zhang M, Huang B, Wang Y (2021) Current status of traditional Chinese medicine for the treatment of COVID-19 in China. Chin Med 16:63
Conti P, Younes A (2020) Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents 34:339–343
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses (2020) The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5:536–544
Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11:1156–1166
Dei S, Braconi L, Romanelli MN, Teodori E (2019) Recent advances in the search of BCRP-and dual P-gp/BCRP-based multidrug resistance modulators. Cancer Drug Resist 2:710–743
Dohse M, Scharenberg C, Shukla S, Robey RW, Volkman T, Deeken JF, Brendel C, Ambudkar SV, Neubauer A, Bates SE (2010) Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos 38:1371–1380
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. P N A S USA 95:15665–15670
Dudley JP, Lee NT (2020) Disparities in age-specific morbidity and mortality from SARS-CoV-2 in China and the Republic of Korea. Clin Infect Dis 71:863–865
Durmus S, Hendrikx JJ, Schinkel AH (2015) Apical ABC transporters and cancer chemotherapeutic drug disposition. Adv Cancer Res 125:1–41
Ebert C, Perner F, Wolleschak D, Schnöder TM, Fischer T, Heidel FH (2016) Expression and function of ABC-transporter protein ABCB1 correlates with inhibitory capacity of Ruxolitinib in vitro and in vivo. Haematologica 101:e81–e85
Elahian F, Kalalinia F, Behravan J (2010) Evaluation of indomethacin and dexamethasone effects on BCRP-mediated drug resistance in MCF-7 parental and resistant cell lines. Drug Chem Toxicol 33:113–119
Fricker G, Drewe J, Huwyler J, Gutmann H, Beglinger C (1996) Relevance of P-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br J Pharmacol 118:1841–1847
Ghareeb DA, Saleh SR, Nofal MS, Kaddah MMY, Hassan SF, Seif IK, El-Zahaby SA, Khedr SM, Kenawy MY, Masoud AA, Soudi SA, Sobhy AA, Sery JG, El-Wahab MGA, Elmoneam AAA, Al-Mahallawi AM, El-Demellawy MA (2021) Potential therapeutic and pharmacological strategies for SARS-CoV2. J Pharm Investig 5:1–16
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP (2020) Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12:988
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui DSC, Du B, Li L, Zeng G, Yuen K-Y, Chen R, Tang C, Wang T, Chen P, **ang J, Li S, Wang J, Liang Z, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Wang J, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zhong N (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26:1017–1032
Gupta A, Zhang Y, Unadkat JD, Mao Q (2004) HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther 310:334–341
Hashimoto N, Nakamichi N, Yamazaki E, Oikawa M, Masuo Y, Schinkel AH, Kato Y (2017) P-glycoprotein in skin contributes to transdermal absorption of topical corticosteroids. Int J Pharm 521:365–373
Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P (2004) Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Res 64:2333–2337
Hu Y, Zuo M, Wang X, Wang R, Lu X, Jiang S (2021) Pharmacokinetic interactions between the potential COVID-19 treatment drugs lopinavir/ritonavir and arbidol in rats. J Zhejiang Univ Sci B 18:725–726
Huang K, Zhang P, Zhang Z, Youn JY, Wang C, Zhang H, Cai H (2021) Traditional Chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther 225:107843
Huang Y, Okochi H, May BCH, Legname G, Prusiner SB, Benet LZ, Guglielmo BJ, Lin ET (2006) Quinacrine is mainly metabolized to mono-desethyl quinacrine by CYP3A4/5 and its brain accumulation is limited by P-glycoprotein. Drug Metab Dispos 34:1136–1144
Huisman MT, Smit JW, Wiltshire HR, Hoetelmans RM, Beijen JH, Schinkel AH (2001) P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol Pharmacol 59:806–813
Juliano RL, Ling V (1976) A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta Biomembr 455:152–162
Kandeel M, Al-Nazawi M (2020) Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci 251:117627
Kim RB, Fromm MF, Wandel C, Leake B, Wood AJJ, Roden DA, Wilkinson GR (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294
Kumar S, Kwei GY, Poon GK, Iliff SA, Wang Y, Chen Q, Franklin RB, Didolkar V, Wang RW, Yamazaki M, Lee Chiu S-H, Lin JH, Pearson PG, Bailie TA (2003) Pharmacokinetics and interactions of a novel antagonist of chemokine receptor 5 (CCR5) with ritonavir in rats and monkeys: role of CYP3A and P-glycoprotein. J Pharmacol Exp Ther 304:1161–1171
Kruse RL (2020) Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res 9:72
Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205
Lei C, Fu W, Qian K, Li T, Zhang S, Ding M, Hu S (2020) Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig. bioRxiv. https://doi.org/10.1101/2020.02.01.929976
Li Q, Sai Y, Kato Y, Muraoka H, Tamai TA (2004) Transporter-mediated renal handling of nafamostat mesilate. J Pharm Sci 93:262–272
Liu X (2019) ABC family transporters. Adv Exp Med Biol 1141:13–100
Liu X, Huang Y-W, Li J, Li X-B, Bi K-S, Chen X-H (2007) Determination of arbidol in human plasma by LC-ESI-MS. J Pharm Biomed Anal 43:371–375
Low ZY, Yip AJW, Lal SK (2021) Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim Chophys Acta Mol Basis Dis. https://doi.org/10.1016/j.bbadis.2021.166294
Lown KS, Mayo RR, Leichtman AB, Hsiao H-I, Turgeon DK, Schmiedlin-Ren P, Brown MB, Guo W, Rossi SJ, Benet LZ, Watkins PB (1997) Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther 62:248–260
Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J (2020) Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 92:814–818
Maruyama Y, Yoshida H, Uchino S, Yokoyama K, Yamamoto H, Takinami M, Hosoya T (2011) Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: a three-year retrospective cohort study. Int J Artif Organs 34:571–576
Mishima Y, Terui Y, Takeuchi K, Yokoyama M, Nagasaki E, Fukunaga T, Yamamoto J, Yamaguchi T, Mizunuma N, Kato Y, Hatake K (2004) Simultaneous suppression of CML and GIST by Imatinib in single patient. Blood 104:4660
Mohamadi Yarijani Z, Najafi H (2021) Kidney injury in COVID-19 patients, drug development and their renal complications: review study. Biomed Pharmacother 142:111966
Moreira JLS, Barbosa SMS, Júnior JG (2021) Pathophysiology and molecular mechanisms of liver injury in severe forms of COVID-19: an integrative review. Clin Res Hepatol Gastroenterol 45:101752
Nayak D, Tripathi N, Kathuria D, Siddharth S, Nayak A, Bharatam PV, Kundu C (2020) Quinacrine and curcumin synergistically increased the breast cancer stem cells death by inhibiting ABCG2 and modulating DNA damage repair pathway. Int J Biochem Cell Biol 119:105682
NIH (2021) ‘Convalescent plasma’ in COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/convalescent-plasma/. Accessed 26 May 2022
Oostendorp RL, Buckle T, Heijnen JH, van Tellingen O, Schellens JHM (2009) The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs 27:31–40
Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A (2020) A pharmacological perspective of chloroquine in SARS-CoV-2 infection: An old drug for the fight against a new coronavirus? Int J Antimirob Agents 56:106078
Pandey S, Tripathi P, Gupta A, Yadav JS (2021) A comprehensive review on possibilities of treating psoriasis using dermal cyclosporine. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-021-01059-5
Pardi N, Hogan M, Porter FW, Weissman D (2018) mRNA vaccines—A new era in vaccinology. Nat Rev Drug Discov 17:261–279
Peng Y, Cheng Z, **e F (2021) Evaluation of pharmacokinetic drug–drug interactions: a review of the mechanisms, in vitro and in silico approaches. Metabolites 11:75
Perry CM, Frampton JE, McCormack PL, Siddiqui MAA, Cvetković RS (2005) Nelfinavir: a review of its use in the management of HIV infection. Drugs 65:2209–2244
Posada MM, Cannady EA, Payne CD, Zhang X, Bacon JA, Pak YA, Higgins JW, Shahri N, Hall SD, Hillgren KM (2017) Prediction of transporter-mediated drug-drug interactions for Baricitinib. Clin Transl Sci 10:509–519
Preston SL, Drusano GL, Glue P, Nash J, Gupta SK, Mcnamara P (1999) Pharmacokinetics and absolute bioavailability of ribavirin in healthy volunteers as determined by stable-isotope methodology. Antimicrob Agents Chemother 43:2451–2456
Rakhmanina NY, Neely M, van Schaik RHN, Dressma HG, Williams KD, Soldin SJ, van der Anker JN (2011) CYP3A5, ABCB1 and SLCO1B1 polymorphisms and pharmacokinetics and virologic outcome of lopinavir/ritonavir in HIV-infected children. Ther Drug Minit 33:417–424
Rasmussen HB, Thomasen R, Hansen PR (2022) Nucleoside analog GS-441524: pharmacokinetics in different species, safety, and potential effectiveness against Covid-19. Pharmacol Res Perspect 10:e00945. https://doi.org/10.1002/prp2.945
Reche A, Kolse R, Gupta S, Ingle A, Chhabra KG, Nimbulkar G (2020) Therapeutic options for Covid–19: Pandemic—A review. Int J Res Pharm Sci 11:420–424
Rijpma SR, van den Heuvel JMW, van der Velden M, Sauerwein RW, Russel FGM, Koenderink JB (2014) Atovaquone and quinine anti-malrials inhibit ATP binding cassette transporter activity. Malar J 13:359
Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, MacDonald B, Beltekian D, Dattani S, Roser M (2021) Coronavirus pandemic (COVID-19) – the data. Our world in data https://ourworldindata.org/coronavirus-data. Accessed 22 June 2022
Roback JD, Guarner J (2020) Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA 323:1561
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18:452–464
Romiti N, Tramonti G, Chieli E (2002) Influence of different chemicals on MDR1 P-glycoprotein expression and activity in the HK-2 proximal tubular cell line. Toxicol Appl Pharmacol 183:83–91
Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. Rev Panamer Salud Pub 44:e40–e40
Saleh M, Gabriels J, Chang D, Kim BS, Mansoor A, Mahmood E, Makker P, Ismail H, Goldner B, Willner J, Beldner S, Mitra R, John R, Chinitz J, Skipitaris N, Mountantonakis S, Epstein LM (2020) Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol 13:e008662
Sauna ZE, Peng X-H, Nandigama K, Tekle S, Ambudkar SV (2004) The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1). Mol Pharmacol 65:65–684
Saleem A, Akhtar MH, Haris M, Abdel-Daim MM (2021) Recent updates on immunological, pharmacological, and alternative approaches to combat COVID-19. Inflammopharmacology 29:1331–1346
Schinkel AH, Wagenaar E, van Deemter L, Mol CAAM, Borst P (1995) Absence of the mdr1 P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96:1698–1705
Shereen MA, Khan S, Kazmi A, Kazmi A, Bashir N, Siddique R (2020) COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res 24:91–98
Skvrce NM, Sarinic VM, Mucalo I, Krnic D, Bozina N, Tomic S (2011) Adverse drug reactions caused by drug-drug interactions reported to Croatian agency for medicinal products and medical devices: a retrospective observational study. Croat Med J 52:604–614
Soiza RL, Scicluna C, Thomason EC (2021) Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 50:279–283
Stebbing J, Phelan A, Grifn I, Tucker C, Oechsle O, Smith D, Richardson P (2020) COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 20:400–402
Tallei TE, Tumilaar SG, Niode NJ, Fatimawali KBJ, Idroes R, Effendi Y, Sakib SA, Emran TB (2020) Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: a molecular docking study. Scientifica (cairo) 2020:6307457
Telbisz Á, Ambrus C, Mózner O, Szabó E, Vá rady G, Bakos É, Sarkadi B, Özvegy-Laczka C (2021) Interactions of potential anti-COVID-19 compounds with multispecific ABC and OATP drug transporters. Pharmaceutics 13(1):81
Tiberghien F, Loor F (1996) Rnaking of P-glycoprotein substrates and inhibitors by a calcein-AM fluorometry screening assay. Anticancer Drugd 7:568–578
Tiberghien F, Wenandy T, Loor F (2000) The potent immunosuppressive cyclosporin FR901459 inhibits the human P-glycoprotein and formyl peptide receptor functions. J Antibiot (tokyo) 53:509–515
Thomas L, Birangal SR, Ray R, Miraj SS, Munisamy M, Varma M, Chidananda SVC, Banerjee M, Shenoy GG, Rao M (2021) Prediction of potential drug interactions between repurposed COVID-19 and antitubercular drugs: an integrational approach of drug information software and computational techniques data. Ther Adv Drug Saf 12:1–29
Tian X, Li C, Huang A, **a S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T (2020) Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microb Infect 9:382–385
Trowitzsch S, Tampe R (2018) ABC transporters in dynamic macromolecular assemblies. J Mol Biol 430:4481–4495
US FDA, In Vitro Drug interaction studies-cytochrome P450 enzymes- and transporter-mediated drug interactions guidance for industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidancedocuments/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions (Accessed on 10 November 2021)
van Waterschoot R, ter Heine R, Wagnaar E, van der Kruijssen CMM, Rw R, Huitema ADR, Beijnen JH, Schinkel AH (2010) Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br J Pharmacol 160:1224–1233
Vimberg V (2021) Teicoplanin-a new for an old drug in the COVID-19 era? Pharmaceuticals 14(12):1227
Vishuvardhan D, Moltke LL, Richert C, Greenblatt DJ (2003) Lopinavir: acute exposure inhibits P-glycoprotein; extended exposure induces P-glycoprotein. AIDS 17:1092–1094
V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19:155–170
Wang C, Li W, Drabek D, Okba NMA, van Haperen R, Osterhaus ADME, van Kuppeveld FJM, Haagmans BL, Grosveld F, Bosch B-J (2020) A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 11:2251
Wang J, Figurski M, Shaw LM, Burckart GJ (2008) The impact of P-glycoprotein and Mrp2 on mycophenolic acid levels in mice. Transpl Immunol 19:192–196
Weiss J, Bajraktari-Sylejmani G, Haefeli WE (2021) Low risk of the TMPRSS2 inhibitor camostat mesylate and its metabolite GBPA to act as perpetrators of drug-drug interactions. Chem Biol Interact 338:109428
**a S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30:343–355
Yang K (2020) What do we know about remdesivir drug interactions? Clin Transl Sci 13:842–844
Yan Y, Shin WI, Pang YX, Meng Y, Lai J, You C, Zhao H, Lester E, Wu T, Pang CH (2020) The first 75 days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Int J Environ Res Public Health 17:2323
Yuan M, Wu NC, Zhu X, Lee C-CD, So RTY, Lv H, Mok CKP, Wilson IA (2020) A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 368:630–633
Zhang L, Liu Y (2020) Potential interventions for novel coronavirus in China: a systematic review. J Med Virol 92:479–490
Zhang YZ, Holmes EC (2020) A genomic perspective on the origin and emergence of SARS-CoV-2. Cell 181:223–227
Funding
This study was supported by a National Research Foundation of Korea grant from the Korean government (Grant No. 2020R1A2B5B01002489) and a Korea Basic Institute (Grant No. National Research Facilities and Equipment Center) grant from the Ministry of Education (Grant No. 2021R1A6C101A442). J Lee was supported by a Research Professor-Grant 2021 from Ewha Womans University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors (J Lee, J Kim, J Kang and HJ Lee) have potential conflicts of interest to declare.
Research involving human and animal rights
This article does not contain any studies with humans or animals performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., Kim, J., Kang, J. et al. COVID-19 drugs: potential interaction with ATP-binding cassette transporters P-glycoprotein and breast cancer resistance protein. J. Pharm. Investig. 53, 191–212 (2023). https://doi.org/10.1007/s40005-022-00596-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-022-00596-6